Abstract
Elevating serotonin (5-HT) levels with selective serotonin reuptake inhibitors (SSRIs) is the most widely used treatment for depression. However, current therapies are ineffective, have delayed benefit, or cause side effects in many patients. Here, we define a mechanism downstream of 5-HT1A receptors that mediates antidepressant-like behavior and is profoundly and selectively enhanced by genetic disruption of regulators of G protein signaling (RGS) activity at Gαi2. Animals rendered insensitive to RGS protein regulation through a mutation in Gαi2 (G184S) exhibited spontaneous antidepressant- and anxiolytic-like behaviors. Mice expressing RGS-insensitive Gαi2 also exhibited increased cortical and hippocampal phosphorylation of glycogen synthase kinase-3β, a constitutively active proapoptotic kinase that is inhibited through phosphorylation in response to serotonin, SSRIs, and 5-HT1 receptor agonists. Both behavioral and biochemical phenotypes were blocked by treatment with WAY 100635, a 5-HT1A–selective antagonist. RGS-insensitive mice were also 5–10 times more responsive to the antidepressant-like effects of the SSRI fluvoxamine and 5-HT1A–selective agonist 8-hydroxy-2-dipropylaminotetralin. In contrast, the antidepressant potency of agents acting through nonserotonergic mechanisms was unchanged as was 5-HT1A action on body temperature. The findings point to a critical role for endogenous RGS proteins to suppress the antidepressant-like effects of 5-HT1A receptor activation. By selectively enhancing the beneficial effects of serotonin, inhibition of RGS proteins represents a therapeutic approach for the treatment of mood disorders.
Keywords: 5-HT1A receptors, depression, G protein, regulator of G protein signaling proteins, transgenic mouse
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed antidepressants (1). Their use is based on a strategy of augmenting the effects of endogenous serotonin by increasing synaptic concentrations. However, the therapeutic benefit of SSRI administration is delayed by weeks or even months. In addition, these drugs are ineffective in many patients and often cause unpleasant and harmful side effects (2). SSRI therapy causes general increases in synaptic serotonin that activate many types of serotonin (5-HT) receptors with a diverse distribution that couple to a wide array of signaling pathways. Manipulation of 5-HT1 receptor activity is generally thought to underlie the beneficial as well as the unfavorable effects of SSRIs. The 5-HT1 receptors are members of the superfamily of G protein-coupled receptors that activate the adenylyl cyclase-coupled inhibitory class of heterotrimeric G proteins. Although multiple lines of evidence suggest that 5-HT1 receptors preferentially couple to Gαi2 in vitro (3), the relative contribution of different Gαi/o-family proteins to these beneficial and unfavorable effects of 5-HT1A receptor activation is not known.
Regulator of G protein signaling (RGS) proteins are a family of more than 30 members defined by a characteristic 120-amino acid RGS homology (RH) domain. RGS proteins primarily function as GTPase accelerating proteins (GAPS) that bind to and rapidly deactivate Gα subunits, thereby greatly limiting the lifetime of active Gα and Gβγ signaling molecules. Thus, RGS proteins are the principle negative regulators of Gαi/o-mediated signaling (4–6), and evidence suggests that they participate in shaping behavioral responses stemming from several types of dopaminergic, opioidergic, and serotonergic neurotransmission (7–10). However, because of the large number of RGS proteins, defining the regulatory contribution of each has proven difficult, in part because of functional redundancy. To examine the combined regulatory role of endogenous RGS proteins, we used a knock-in mouse in which the GAP effects of endogenous RGS proteins to Gαi2 are silenced by a point mutation in the switch I region of Gαi2(G184S) that disrupts its association with RGS proteins (11, 12). Thus, all GAP-related RGS protein regulation is prevented without altering intrinsic functions of Gα, such as receptor interaction, association with βγ subunits, or coupling to downstream effectors (13). In the RGS-insensitive Gαi2(G184S) knock-in animals, there is no detectable alteration of endogenous expression or distribution of the RGS-protein family members and Gαi2(G184S) protein (13).
Here, we examine the hypothesis that serotonin physiology at 5-HT1 receptors is enhanced in Gαi2(G184S) knock-in mice. Given the established role for serotonin in the regulation of mood, we evaluated the behavior of these mice in several tests that are predictive of depression or anxiety. We found that genetic disruption of RGS activity produces an antidepressant and anxiolytic phenotype and selectively enhances the antidepressant-like effects of the SSRI fluvoxamine and direct 5-HT1A receptor activation.
Results
Antidepressant drugs decrease immobility in rodent models of learned helplessness, such as the tail-suspension test and forced-swim test. Animals homozygous [Gαi2(G184S/G184S)] and heterozygous [Gαi2(G184S/+)] for RGS insensitivity at Gαi2 showed antidepressant-like behavior in the tail-suspension test in that they exhibited less immobility (106 ± 14 and 142 ± 19 s, respectively) compared with wild-type C57BL/6 littermates (189 ± 11 s) (Fig. 1A). In the forced-swim test (Fig. 1B), homozygous [Gαi2(G184S/G184S)] and heterozygous [Gαi2(G184S/+)] RGS-insensitive mice also exhibited decreased immobility times (164 ± 10 and 183 ± 8 s, respectively) compared with wild-type animals (223 ± 9 s). Previous studies with Gαi2(G184S/G184S) mice showed an increase in generalized activity measured over an 8-d period in the home cage (12). Despite this, we observed no differences between genotypes in measures of acute spontaneous activity in a new environment (Fig. S1), suggesting that reduced immobility in the tail-suspension and forced-swim tests was not caused by an increase in locomotor activity. The importance of Gαi2 in contributing to antidepressant-like behavior was highlighted by the finding that mice lacking Gαi2 (Gαi2−/−) (14) exhibited increased immobility (185 ± 22 s) in the tail-suspension test compared with their wild-type littermates (132 ± 10 s), indicating that a loss of Gαi2 function produces a pro- rather than antidepressant phenotype (Fig. 1C).
Fig. 1.
A role for endogenous RGS proteins and Gαi2 in the antidepressant-like phenotype of Gαi2 mutant mice. Heterozygous (GS/+) and homozygous (GS/GS) mice lacking RGS protein regulation at Gαi2 [Gαi2(G184S/+) and Gαi2(G184S/G184S), respectively] exhibited decreased immobility times compared with wild-type littermates (+/+) in the (A) tail-suspension test or (B) forced-swim test. Data are presented as the mean ± SEM (n = 9–12) and were analyzed by one-way ANOVA with Tukey's posthoc test with *P < 0.05 and **P < 0.01 compared with wild-type mice. (C) Untreated mice null for Gαi2 (−/−) exhibited increased immobility in the tail-suspension test compared with wild-type (+/+) control animals. Immobility times between untreated Gαi2(G184S/+) knock-in mice and Gαi2(−/−) mice vary because of behavioral differences inherent to the distinct genetic background (C57BL/6J or 129SvEv, respectively) of the mouse strains. Data are the mean ± SEM (n = 6–8); *P < 0.05 versus wild-type (+/+) control animals by Student t test. (D) WAY 100635 (0.1 mg/kg s.c. 30 min before testing) reversed the decreased immobility exhibited by heterozygous (GS/+) and homozygous (GS/GS) mice expressing RGS-insensitive Gαi2. Data are mean ± SEM (n = 8–11) and were analyzed by two-way ANOVA with the Bonferroni posthoc test. There was a significant effect of genotype [F(2,51) = 6.50; P = 0.0031], with Gαi2(G184S/+) (*P < 0.05) and Gαi2(G184S/G184S) mice (**P < 0.01) significantly different from untreated Gαi2 (+/+) animals. There was also a significant effect of WAY 100635 treatment [F(1,51) = 27.92; P < 0.0001], with Gαi2(G184S/+) (**P < 0.01) and Gαi2(G184S/G184S) mice (***P < 0.001) significantly different from untreated Gαi2(G184S/+) and Gαi2(G184S/G184S) mice, respectively.
The antidepressant-like phenotype could have a number of underlying causes. However, multiple lines of evidence suggest that 5-HT1 receptors preferentially couple to Gαi2 relative to Gαo (3). Therefore, we hypothesized that inhibition of RGS protein GAP activity at Gαi2 leads to enhanced 5-HT1A receptor activity, which, in turn, results in exaggerated behavioral responses that use this signaling pathway. To test this hypothesis, mice expressing RGS-insensitive Gαi2 were treated with the 5-HT1A receptor antagonist WAY 100635 at a dose (0.1 mg/kg s.c.) that inhibits the activity of the selective 5-HT1A agonist 8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT) in the tail-suspension test without altering locomotor activity (Fig. S2 A and B). Treatment resulted in a reversal of the antidepressant-like phenotype (Fig. 1D), with immobility scores of WAY 100635-treated Gαi2(G184S/+) and Gαi2(G184S/G184S) mice increased to levels not significantly different from those of untreated and WAY 100635-treated wild-type animals. Similar findings were obtained after administration of the general 5-HT1/2 antagonist metergoline, which partially reversed the reduction in immobility by the SSRI fluvoxamine in wild-type mice (Fig. S2C) and increased immobility times in Gαi2(G184S/+) and Gαi2(G184S/G184S) mice (Fig. S2D).
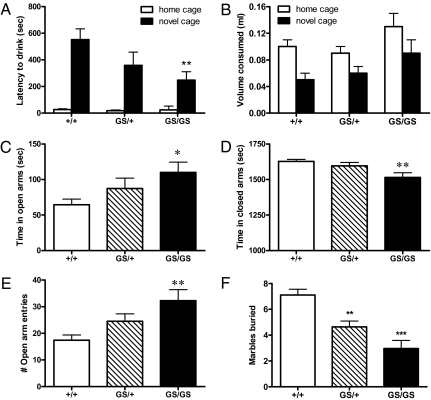
The tail-suspension test and forced-swim test are widely used to assess antidepressant activity in rodent animal models, particularly transgenic mice (15), and noted for showing predictive validity for known antidepressants with therapeutic efficacy in humans (refs. 16, 17 have reviews). In addition, the novelty-induced hypophagia (NIH) test is a behavioral model of mood, wherein animals trained to drink a sweetened solution in the home cage environment exhibit hyponeophagia or delayed/reduced consumption when placed in a novel cage (18). Hyponeophagia behaviors are diminished after chronic but not acute or subacute administration of antidepressants. RGS-insensitive Gαi2(G184S/G184S) mice exhibited a drinking latency in the novel cage, one-half of that observed in wild-type littermates (247 ± 63 vs. 552 ± 81 s, respectively) (Fig. 2A), with no significant changes in consumption in either cage environment (Fig. 2B). These behaviors are similar to those observed in mice treated with chronic but not acute fluoxetine (19, 20).
Fig. 2.
Decreased anxiety-related behaviors in mice with RGS-insensitive Gαi2. Heterozygous (GS/+) and homozygous (GS/GS) mice lacking RGS protein regulation at Gαi2 [Gαi2(G184S/+) and Gαi2(G184S/G184S), respectively] exhibited decreased drinking latencies in novel but not home cage environments compared with wild-type littermates (+/+) in (A) the novelty-induced hyponeophagia test with (B) no significant changes in the volume consumed across genotype. Data are presented as the mean ± SEM (n = 7–8) and were analyzed by two-way ANOVA with the Bonferroni post hoc test. There was a significant effect of genotype on latency to drink [F(2,38) = 3.49; P = 0.0406], with Gαi2(G184S/G184S) mice (**P < 0.01) significantly different from wild-type littermates. In the elevated plus maze, homozygous (GS/GS) and heterozygous (GS/+) RGS-insensitive mice exhibited reduced anxiety-related behaviors compared with wild-type littermates with (C) more time spent in open arms, (D) less time spent in closed arms, and (E) a greater number of entries into open arms. Data are presented as the mean ± SEM (n = 12) and were analyzed by one-way ANOVA with Tukey's post hoc test with *P < 0.05 and **P < 0.01 compared with wild-type mice. (F) In the marble-burying test, both homozygous (GS/GS) and heterozygous (GS/+) RGS-insensitive mice buried significantly fewer marbles compared with wild-type mice. Data are presented as the mean ± SEM (n = 8) and were analyzed by one-way ANOVA with Tukey's post hoc test with **P < 0.01 and ***P < 0.01 compared with wild-type mice.
Anxiety disorders are often comorbid with depression, and antidepressant drugs can be used in the treatment of anxiety. Tests for anxiety in rodents make use of the aversion of the animals for exposed locations (21). In the elevated plus maze homozygous Gαi2(G184S/G184S), but not heterozygous RGS-insensitive mice, showed a significant anxiolytic phenotype with more time spent in open arms (Fig. 2C), less time spent in closed arms (Fig. 2D), and a greater number of entries into open arms (Fig. 2E) than wild-type littermates. Obsessive compulsive disorder is an anxiety disorder that can be managed with antidepressants, including SSRIs, and is modeled by marble burying in rodents (ref. 22 has a review). Both homozygous Gαi2(G184S/G184S) and heterozygous Gαi2(G184S/+) RGS-insensitive mice buried significantly fewer marbles (3.0 ± 0.6 and 4.6 ± 0.5, respectively) compared with wild-type mice (7.1 ± 0.5) (Fig. 2F). No differences in time in center or rearing were observed between genotypes in the open field test (Fig. S3 A and B), which may represent a less anxiogenic environment (21).
If removal of RGS regulation of Gαi2 signaling enhances 5-HT1A activation, then agonists acting directly at 5-HT1A receptors and SSRIs indirectly leading to 5-HT1A receptor activation should exhibit increased antidepressant potency and/or efficacy. Homozygous Gαi2(G184S/G184S) mice exhibit little immobility behavior (~100 s) in the tail-suspension test, thereby limiting the possibility of seeing an antidepressant drug effect. Therefore, to increase the window for drug effects, heterozygous Gαi2(G184S/+) mice were compared with wild-type littermates. The 5-HT1A agonist 8-OH-DPAT afforded a dose-dependent decrease in immobility in both genotypes; however, the potency of 8-OH-DPAT was 10-fold greater in heterozygous animals compared with wild-type controls (Fig. 3A). In contrast, 8-OH-DPAT–induced hypothermia occurred with an identical potency, similar maximum response, and similar time course in wild-type and heterozygous Gαi2(G184S/+) animals (Fig. 3B and Fig. S4). The SSRI fluvoxamine also produced a dose-dependent decrease in immobility in both heterozygous [Gαi2(G184S/+)] and wild-type mice, with a 5-fold greater potency in heterozygous [Gαi2(G184S/+)] mice (Fig. 3C).
Fig. 3.
Enhanced antidepressant potency of serotonin-related agents in mice expressing RGS-insensitive Gαi2 as measured using the tail-suspension test. (A) The 5-HT1A–selective agonist 8-OH-DPAT showed greater potency to reduce immobility time in heterozygote Gαi2(G184S/+) mice (GS/+) compared with wild-type (+/+) littermates. Data are presented as mean ± SEM (n = 8–11) and were analyzed by two-way ANOVA with the Bonferroni posthoc test. There was a significant effect of genotype [F(1,72) = 24.04; P < 0.0001], with a significant difference at 1 mg/kg (***P < 0.001). (B) Core body temperature in wild-type (+/+) and Gαi2(G184S/+) mice (GS/+) 30 min after administration of increasing doses of 8-OH-DPAT. Maximum temperature losses of 3.5 ± 0.6 °C and 3.8 ± 0.7 °C were observed in wild-type and heterozygous animals, respectively. Data, presented as the mean ± SEM (n = 4–5), were analyzed by nonlinear regression (Graphpad Prism). ED50 values of 8-OH-DPAT were similar in animals of either genotype: 0.38 mg/kg (0.1–17 mg/kg) in wild-type versus 1.1 mg/kg (0.1–11 mg/kg) in heterozygous mice. Time course data are provided in Fig. S4. (C) The SSRI fluvoxamine showed greater potency to reduce immobility times in Gαi2(G184S/+) heterozygotes (GS/+) compared with wild-type (+/+) littermates, with a significant main effect for genotype [F(4,75) = 12.78; P = 0.0006] with significant differences seen at doses of fluvoxamine (0.3 and 1 mg/kg;*P < 0.05). Also in the tail-suspension test, (D) the norepinephrine uptake inhibitor desipramine and (E) the D2 dopamine receptor agonist pramipexole were equipotent at reducing immobility times in Gαi2(G184S/+) heterozygous (GS/+) and wild-type (+/+) littermates; (F) the dopamine/ norepinephrine uptake inhibitor bupropion was ineffective in both genotypes. Data are presented as mean ± SEM (n = 8–11) and were analyzed by two-way ANOVA with Bonferroni posthoc test.
Agents that increase synaptic levels of the nonserotonin neurotransmitters norepinephrine and dopamine are also recognized for both clinical efficacy in depression and an ability to decrease immobility times in animal models of learned helplessness (23, 24). However, the norepinephrine reuptake inhibitor desipramine (Fig. 3D) exhibited similar antidepressant potencies in heterozygous Gαi2(G184S/+) and wild-type animals as did the direct dopamine D2 receptor agonist pramipexole (Fig. 3E). The dopamine/norepinephrine reuptake inhibitor buproprion was ineffective in both genotypes (Fig. 3F). These results show a profound selectivity in that endogenous RGS protein control of Gαi2 signaling is specific for 5-HT1A–mediated antidepressant-like effects but not thermoregulatory actions of 5-HT1A agonists or antidepressant agents acting through nonserotonergic mechanisms. Moreover, the augmented behavioral responses seem specific to 5-HT1A receptors, because 5-HT2A/C–induced head twitching was not altered in Gαi2(G184S/+) mice compared with wild-type littermates (Fig. S5).
Glycogen synthase kinase-3β (GSK3β) has been identified as a putative biochemical mediator of the mood-related actions of serotonin (25, 26). GSK3β is a constitutively active, proapoptotic kinase that is inhibited by phosphorylation at Ser-9 in response to an array of agents with antidepressant-like activity, including brain-derived neurotrophic factor (BDNF) (27, 28), SSRIs (29), and 5-HT1A receptor agonists (29). Consequently, we examined whether this enzyme was altered in the Gαi2(G184S/+) mice. Mice homozygous or heterozygous for RGS-insensitive Gαi2 exhibited increased basal phosphorylation at Ser-9 of GSK3β in the cortex (Fig. 4 A and B) and hippocampus (Fig. 4 A and C). After WAY 100635 administration at a dose (0.1 mg/kg s.c.) that reversed the antidepressant-like behavioral phenotype, homozygous and heterozygous animals showed a progressive loss of phospho-GSK3β in the cortex (Fig. 4 A and B), whereas, in the hippocampus, WAY 100635 completely blocked increases in phospho-GSK3β in either genotype (Fig. 4 A and C).
Fig. 4.
5-HT1A receptor-mediated phosphorylation of GSK3β in mice expressing RGS-insensitive Gαi2. (A) Phospho- and total GSK3β levels in the cortex and hippocampus of wild-type (+/+) and RGS-insensitive Gαi2 heterozygous (GS/+) and homozygous (GS/GS) animals treated with and without the 5-HT1A receptor antagonist WAY 100635 (0.1 mg/kg s.c. 30 min before testing). Western blots shown are representative of four to six experiments. Densitometric analyses for (B) cortex and (C) hippocampus are shown as the relative optical density (ROD) of phospho-GSK3β normalized to total GSK3β and are the mean ± SEM (n = 4–6). The data were analyzed by two-way ANOVA with the Bonferroni posthoc test. In the cortex, there was a significant effect of genotype [F(2,23) = 11.21; P = 0.0004] with Gαi2(G184S/+) and Gαi2(G184S/G184S) mice (**P < 0.01 significantly different from wild-type animals). There was also a significant effect of WAY 100635 treatment [F(1,23) = 9.75; P = 0.0048] in Gαi2(G184S/G184S) mice [*P < 0.05 compared with untreated Gαi2(G184S/G184S) mice]. Similarly, in the hippocampus, there was a significant effect of genotype [F(2,26) = 6.35; P = 0.0057] with Gαi2(G184S/+) and Gαi2(G184S/G184S) (***P < 0.001 significantly different from wild-type animals). There was also a significant effect of WAY 100635 treatment [F(1,26) = 33.49; P < 0.0001] with Gαi2(G184S/+) and Gαi2(G184S/G184S) [***P < 0.001 significantly different from untreated Gαi2(G184S/+) and Gαi2(G184S/G184S) mice, respectively].
Discussion
Optimizing antidepressant therapeutics requires a clearer understanding of the receptors and signaling pathways involved. The present findings show spontaneous antidepressant and anxiolytic effects from the genetic ablation of RGS action at Gαi2 in mice. The findings also provide strong evidence that a discrete population of 5-HT1A receptors that signal through Gαi2 are involved in these behaviors. Removing RGS modulation of Gαi2 activity provides sufficient gain of function, such that endogenous serotonin evokes an antidepressant-like state, decreases anxiety-related behavior, and maintains increased levels of Ser-9 phosphorylation of GSK3β. Dramatic increases in the potency of the SSRI fluvoxamine and the 5-HT1A agonist 8-OH-DPAT to produce antidepressant-like behavior with no differences observed for agents acting at norepinephrine or dopamine receptors suggest a profound selectivity of effect of the RGS-insensitive Gαi2 mutation. One caveat to our findings is that homozygous Gαi2(G184S/G184S) mice with complete RGS insensitivity at Gαi2 have a complex phenotype with abnormalities including low birth rate and low body weight (12). However, heterozygote Gαi2(G184S/+) animals with partial RGS insensitivity at Gαi2 do not show similar physiological abnormalities (12), yet they exhibit this 5-HT1A–dependent antidepressant phenotype and improved sensitivity to serotonin-related antidepressants. These observations are consistent with previous biochemical studies showing a semidominant effect of this heterozygous gain-of-function mutation (12) and suggest that endogenous RGS proteins play a major role in regulating the antidepressant/anxiolytic effects of endogenous serotonin and serotonergic agents acting at 5-HT1A receptors.
Despite the large excess of Gαo in the CNS relative to Gαi2 (30, 31), several lines of evidence point to a critical role for Gαi2 protein signaling and RGS regulation as determinants of 5-HT1A receptor coupling to antidepressant and anxiolytic effects. First, in vitro evidence indicates that 5-HT1A receptors preferentially couple to Gαi2 relative to Gαo (3). Second, our findings show that mice lacking Gαi2 exhibit prodepressant-like behaviors, although it should be emphasized that the tail-suspension and forced-swim tests have only been validated as predictors of antidepressant-like but not prodepressant-like effects in humans. Third, we observe increased levels of GSK3β phosphorylation in mice expressing RGS-insensitive Gαi2 that are sensitive to 5-HT1A receptor antagonism. Finally, we show spontaneous antidepressant-like and anxiolytic behaviors resulting from the blockade of RGS action at Gαi2, implicating the involvement of a population of Gαi2-coupled 5-HT1A receptors.
It has been postulated that increased activity of postsynaptic 5-HT1A receptors in the hippocampus and frontal cortex is required for antidepressant behaviors (1, 32), whereas enhanced activity of somatodendritic 5-HT1A autoreceptors in the raphe nucleus decreases synaptic levels of serotonin, leading to pro- rather than antidepressant-like effects. Indeed, a major component of the antidepressant action of SSRIs is decreased function of 5-HT1A autoreceptors in the raphe nucleus, ultimately reducing autoinhibition and so, increasing synaptic serotonin in the hippocampus and frontal cortex. Therefore, it is possible that, in mice expressing RGS-insensitive Gαi2, increased signaling at 5-HT1A autoreceptors could lead to receptor down-regulation, causing increased serotonin release. However, altered signaling at 5-HT1A autoreceptors does not seem to be responsible for the observed behavioral effects in mice expressing RGS-insensitive Gαi2 for several reasons; 5-HT1A autoreceptors have been shown to be responsible for the hypothermic actions of 8-OH-DPAT (33), whereas we see no phenotypic change in this response. In addition, the fact that we see enhanced activity of fluvoxamine and 8-OH-DPAT indicates the 5-HT1A receptor system is more, not less, efficient, suggesting that postsynaptic rather than presynaptic 5-HT1A signaling is augmented by RGS insensitivity at Gαi2. Certainly, there are reports that postsynaptic densities in the cortex and hippocampus are enriched in Gαi2 but not Gαo (34, 35). Finally, it has been suggested that presynaptic 5-HT1A autoreceptors in the dorsal and medial raphe nuclei have large receptor reserves and therefore, exhibit efficient receptor/effector coupling, whereas postsynaptic 5-HT1A receptors located primarily in the hippocampus and prefrontal cortex have little or no receptor reserve (36–38). Notably, we have shown that RGS proteins have more regulatory control over receptor systems weakly coupled to G protein activation (39).
Taken together, the present findings imply that postsynaptic 5-HT1A receptors likely located in the hippocampus and frontal cortex are responsible for the mood-altering effects of endogenous serotonin and the increased activity of endogenous serotonin and serotonergic drugs in mice expressing RGS-insensitive Gαi2. Thus, specificity of Gα protein coupling and/or sensitivity to RGS protein regulation may provide a means to segregate and potentially target populations of 5-HT1A receptors associated with the mood-altering effects of serotonin. These results suggest two approaches to developing more selective antidepressants. First, functionally selective 5-HT1A agonists that activate Gαi2 but not other Gαi/o family G proteins may be beneficial. Second, identification of the specific RGS protein(s) involved in modulating the activity of 5-HT1A receptor-coupled Gαi2 and development of inhibitors against these proteins (4, 10, 40) may provide either antidepressants in their own right or a means of selectively enhancing antidepressant actions of serotonin-related drugs.
Materials and Methods
Transgenic Mice.
Wild-type Gαi2(+/+), heterozygous Gαi2(G184S/+), and homozygous Gαi2(G184S/G184S) RGS-insensitive Gαi2 knock-in animals were derived from heterozygous breeding as previously described (12). All animals were backcrossed into the C57BL/6J strain for four generations and were matched for age and gender. In some antidepressant experiments, later generations were used (up to F8), but control experiments showed no differences in behavior between the F4 and F8 generations. Wild-type littermates were used as controls for Gαi2(G184S/+) and Gαi2(G184S/G184S) animals. Mice null for Gαi2 (Gαi2−/−) were derived as previously described (14) on a 129SvEv background. At weaning, mice were group-housed (six animals per cage) with same-sex littermates in a temperature- and humidity-controlled vivarium under a 12-h light/dark cycle (lights on at 7:00 AM). Testing was conducted during the light phase of the light/dark cycle between 9:00 AM and 12:00 PM. Animals used in behavioral studies were typically between 8 and 16 wk of age and were naive to all behavioral treatments before testing. Room luminance was maintained between 70- and 75-ft candles for all behavioral studies. Unless specified otherwise, all drugs were administered i.p. 30 min before behavioral testing. All experimental procedures were approved by the local Institutional Animal Care and Use Committee and followed the National Institute of Health guidelines outlined in “Using Animals in Intramural Research.”
Tail-Suspension Test.
Mice were individually suspended by the tail from a metal bar elevated 30 cm using adhesive tape (41). Behavior was videotaped for 6 min, and videos were later scored for immobility time (seconds) by a blinded observer.
Forced-Swim Test.
Mice were tested in the Porsolt forced-swim test similar to that described in Hirani et al. (42). Twenty-four hours before testing, mice were subjected to a 15-min preswim in ~10 cm of 25 °C water in a 20 × 13-cm glass cylinder. On the day of testing, mice were tested for 5 min, and behavior was videotaped and scored for immobility time (seconds) by a blinded observer.
Measurements of Core Body Temperature.
Mice were implanted with radio-telemetric probes (E-4000 E-Mitter; Mini-Mitter) as previously described (43) and allowed at least 5 d to recover. On the day of testing, animal cages were placed onto a receiving pad for real-time recording of body temperature. Temperature measurements were taken every minute for 45 min to provide baseline temperature data. After agonist or vehicle injections, body temperature was recorded for an additional 60 min.
Novelty-Induced Hypophagia.
The novelty-induced hypophagia paradigm was performed according to Dulawa et al. (20), with hypophagia behaviors assessed in nonfasted animals in home and novel cage environments using Ensure as a sweetened solution. In this assay using C57BL/6 mice, imipramine (30 mg/kg i.p.) decreased the latency to drink (from 500 ± 30 s to 381 ± 20 s) and increased the volume consumed (from 0.04 ± 0.01 mL to 0.09 ± 0.01 mL).
Elevated Plus Maze.
The elevated plus maze was used to measure anxiety-like behavior. The maze consisted of four Plexiglas arms (30 × 5 cm) extending from a common center (5 × 5 cm). Two enclosed arms had 13-cm opaque walls, whereas the center and two open arms had no walls. The maze was positioned 53 cm above the floor. Mice were placed in the center of the maze facing an open arm, and their location (center, open arms, or enclosed arms) was recorded in the absence of investigators by a video camera positioned above the maze. The time spent in each portion of the maze was later measured by an observer who was blinded to the mouse genotype. An entry was defined as having all four paws within the same arm. As a control, administration of diazepam (1 mg/kg i.p.) to C57BL/6 mice increased the time spent in the open arms from 39.0 ± 12 s to 131 ± 20 s.
Marble Burying.
The marble-burying assay is commonly used as a rodent model of obsessive compulsive disorder (22). Each mouse was individually placed in a clear polycarbonate box (18 × 28 × 13 cm) containing 5 cm of corncob bedding and 15 marbles (three rows of five marbles each). The number of buried marbles was counted after 30 min. Marbles were considered buried if they were at least two-thirds covered. The assay was validated with imipramine (10 mg/kg i.p.), which decreased the number of marbles buried by C57BL/6 mice from 7.5 ± 0.5 to 4.8 ± 0.5. Imipramine at 30 mg/kg i.p. completely prevented marble-burying behavior.
Phosphorylation of GSK3β.
Methods were performed according to Li et al. (29), with modifications. Mice were killed by decapitation, and brain regions were quickly dissected (~90 s) on an ice-cold surface, homogenized in solubilization buffer containing protease and phosphatase inhibitors, and boiled for 5 min. Standard immunoblotting techniques were used to visualize levels of phospho- and total GSK3β using commercially available antibodies (Cell Signaling Technology).
Supplementary Material
Acknowledgments
S.M.G. was a student on the University of Michigan Undergraduate Research Opportunities Program. This work was supported by National Institutes of Health Grants (to R.R.N. and J.R.T.); J.N.T. was funded by a National Institutes of Health postdoctoral training grant (to J.R.T.), the American Association of Colleges of Pharmacy New Investigator Program Grant, and the Bower, Bennett, and Bennett Endowed Chair.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000003107/-/DCSupplemental.
References
- 1.Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- 2.Whittington CJ, Kendall T, Pilling S. Are the SSRIs and atypical antidepressants safe and effective for children and adolescents? Curr Opin Psychiatry. 2005;18:21–25. [PubMed] [Google Scholar]
- 3.Raymond JR, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhong H, Neubig RR. Regulator of G protein signaling proteins: Novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837–845. [PubMed] [Google Scholar]
- 5.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Talbot JN, et al. Differential modulation of mu-opioid receptor signaling to adenylyl cyclase by regulators of G protein signaling proteins 4 or 8 and 7 in permeabilised C6 cells is Galpha subtype dependent. J Neurochem. 2010;112:1026–1034. doi: 10.1111/j.1471-4159.2009.06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachariou V, et al. Essential role for RGS9 in opiate action. Proc Natl Acad Sci USA. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyer CE, et al. Regulators of G-protein signaling 4: Modulation of 5-HT1A-mediated neurotransmitter release in vivo. Brain Res. 2004;1022:214–220. doi: 10.1016/j.brainres.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 9.Rahman Z, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 10.Traynor JR, Neubig RR. Regulators of G protein signaling & drugs of abuse. Mol Interv. 2005;5:30–41. doi: 10.1124/mi.5.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Lan KL, et al. A point mutation in Galphao and Galphai1 blocks interaction with regulator of G protein signaling proteins. J Biol Chem. 1998;273:12794–12797. doi: 10.1074/jbc.273.21.12794. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, et al. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol. 2006;26:6870–6879. doi: 10.1128/MCB.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, et al. RGS-insensitive G-protein mutations to study the role of endogenous RGS proteins. Methods Enzymol. 2004;389:229–243. doi: 10.1016/S0076-6879(04)89014-1. [DOI] [PubMed] [Google Scholar]
- 14.Jain M, et al. Targeted inactivation of Galpha(i) does not alter cardiac function or beta-adrenergic sensitivity. Am J Physiol Heart Circ Physiol. 2001;280:H569–H575. doi: 10.1152/ajpheart.2001.280.2.H569. [DOI] [PubMed] [Google Scholar]
- 15.Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Cryan JF, Holmes A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 18.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 20.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 21.Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- 22.Witkin JM. Animal models of obsessive-compulsive disorder. Curr Protoc Neurosci. 2008;45:9.30.1–9.30.9. doi: 10.1002/0471142301.ns0930s45. [DOI] [PubMed] [Google Scholar]
- 23.Brunello N, et al. Noradrenaline in mood and anxiety disorders: Basic and clinical studies. Int Clin Psychopharmacol. 2003;18:191–202. doi: 10.1097/00004850-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Stern WC, Harto-Truax N. Two multicenter studies of the antidepressant effects of bupropion HCl versus placebo. Psychopharmacol Bull. 1980;16:43–46. [PubMed] [Google Scholar]
- 25.Beaulieu JM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu JM. Not only lithium: Regulation of glycogen synthase kinase-3 by antipsychotics and serotonergic drugs. Int J Neuropsychopharmacol. 2007;10:3–6. doi: 10.1017/S1461145706006857. [DOI] [PubMed] [Google Scholar]
- 27.Mai L, Jope RS, Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem. 2002;82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaltiel G, Chen G, Manji HK. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol. 2007;7:22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Li X, et al. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 31.Milligan G, Streaty RA, Gierschik P, Spiegel AM, Klee WA. Development of opiate receptors and GTP-binding regulatory proteins in neonatal rat brain. J Biol Chem. 1987;262:8626–8630. [PubMed] [Google Scholar]
- 32.Matsuda T, Somboonthum P, Suzuki M, Asano S, Baba A. Antidepressant-like effect by postsynaptic 5-HT1A receptor activation in mice. Eur J Pharmacol. 1995;280:235–238. doi: 10.1016/0014-2999(95)00254-i. [DOI] [PubMed] [Google Scholar]
- 33.Richardson-Jones JW, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, et al. Occurrence of the alpha subunits of G proteins in cerebral cortex synaptic membrane and postsynaptic density fractions: Modulation of ADP-ribosylation by Ca2+/calmodulin. Proc Natl Acad Sci USA. 1992;89:8686–8690. doi: 10.1073/pnas.89.18.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki C, Go CG, Wu K, Siekevitz P. Light and electron microscopic localization of alpha subunits of GTP-binding proteins, G(o) and Gi, in the cerebral cortex and hippocampus of rat brain. Brain Res. 1992;596:189–201. doi: 10.1016/0006-8993(92)91547-r. [DOI] [PubMed] [Google Scholar]
- 36.Meller E, Goldstein M, Bohmaker K. Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: Possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol. 1990;37:231–237. [PubMed] [Google Scholar]
- 37.Meller E, Li H, Carr KD, Hiller JM. 5-Hydroxytryptamine(1A) receptor-stimulated [(35)S]GTPgammaS binding in rat brain: Absence of regional differences in coupling efficiency. J Pharmacol Exp Ther. 2000;292:684–691. [PubMed] [Google Scholar]
- 38.Cox RF, Meller E, Waszczak BL. Electrophysiological evidence for a large receptor reserve for inhibition of dorsal raphe neuronal firing by 5-HT1A agonists. Synapse. 1993;14:297–304. doi: 10.1002/syn.890140407. [DOI] [PubMed] [Google Scholar]
- 39.Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR. Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem. 2003;278:9418–9425. doi: 10.1074/jbc.M208885200. [DOI] [PubMed] [Google Scholar]
- 40.Roman DL, et al. Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol. 2007;71:169–175. doi: 10.1124/mol.106.028670. [DOI] [PubMed] [Google Scholar]
- 41.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 42.Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: Modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 43.Fantegrossi WE, et al. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.