Abstract
Tuberous sclerosis (TSC) is an autosomally dominant neurocutaneous disease notable for its high comorbidity with autism in human patients. Studies of murine models of tuberous sclerosis have found defects in cognition and learning, but thus far have not uncovered deficits in social behaviors relevant to autism. To explore social communication and interaction in TSC2 heterozygous mice, we recorded ultrasonic vocalizations (USV) and found that although both wild-type (WT) and heterozygous pups born to WT dams showed similar call rates and patterns, baseline vocalization rates were elevated in pups born to heterozygous dams. Further analysis revealed several robust features of maternal potentiation in all but WT pups born to heterozygous dams. This lack of potentiation is suggestive of defects in mother–pup social interaction during or before the reunion period between WT pups and heterozygous dams. Intriguingly, male pups of both genotypes born to heterozygous dams showed particularly heightened call rates and burst patterns. Because our maternal retrieval experiments revealed that TSC2+/− dams exhibited improved defensive reactions against intruders and highly efficient pup retrieval performance, the alterations in their pups’ USVs and maternal potentiation do not appear to result from poor maternal care. These findings suggest that a pup's interaction with its mother strongly influences the pup's vocal communication, revealing an intriguing dependence of this social behavior on TSC2 gene dosage of both parties involved. Our study of this murine model thus uncovers social abnormalities that arise from TSC haploinsufficiency and are suggestive of autism.
Keywords: USV, TSC, mTOR, maternal care, social communication
Autism afflicts roughly 0.2% of persons worldwide, often for unknown reasons and almost always without definitive or curative treatment (1). Although many cases are of completely unknown etiology, certain environmental or genetic factors are thought to dramatically increase the risk for autism. Tuberous sclerosis (TSC) is an autosomally dominant neurocutaneous disorder that exhibits a high comorbidity with autism. Roughly 20% of tuberous sclerosis patients fall ill to autism, posing anywhere from a 50- to a 100-fold increased risk for autism compared with that in the normal population (2).
The greatly increased risk for autism in TSC patients positions TSC as a superb candidate for understanding the genetic basis and pathophysiology of this disease. Verification of murine models bearing mutations in the tuberous sclerosis complex as models for autism could yield important insights into the molecular pathway defects that render increased susceptibility to autism and provide a testbed for exploring therapeutic options (3). The genetic etiology of TSC has been traced to two molecules that form a complex to regulate the mammalian target of rapamycin (mTOR) pathway, a major player in transcriptional and translational regulation. Normally, mTOR phosphorylates critical regulators of protein synthesis and promotes translation of a wide variety of proteins involved in cell growth and proliferation, including neuronal proteins that play key roles in axon pathfinding and synaptic plasticity (4). Two proteins, TSC1 and TSC2, combine to form a complex known as the tuberous sclerosis complex, a GTPase activating protein that negatively regulates mTOR (5). The complex, however, is prone to mutation, and mutations in either TSC1 or TSC2 inactivate the complex, resulting in unchecked cellular proliferation (6).
The absence of detectable social phenotypes in TSC2+/− mice in a previous study (7) could either imply the lack of such autistic features in this mouse model or simply reflect limitations in detecting mouse behavioral abnormalities. Crucial to the diagnosis of autism are impairments in communication, including delays in verbal language and stereotyped, repetitive language patterns (1). Hidden from normal human detection, mouse communicative strategies include numerous olfactory cues as well as ultrasonic vocalizations (USVs) (8, 9). Mice begin vocalizing shortly after birth, peak in vocalization rates around postnatal day 8 (P8), and continue vocalizing, albeit at reduced rates, throughout adulthood (10). Many potential murine models bearing mutations in genes associated with autism have been reported to exhibit changes in USVs, where either a decrease or an increase in vocalization rate may reflect communication impairments (3). Recent advances in computational analysis of vocalizations in BTBR mice, a murine model of autism, enabled classification of 10 distinct call types and revealed differences between BTBR and other mouse strains in the distribution of call types and mean durations of individual calls (11).
TSC2+/− mice have thus far failed to show any detectable deficits in sociability by standard assays of social behavior. However, here we show that changes in USVs of mouse pups upon separation from their mothers is dependent on the genotypes of not only the pup, but the mom as well. Thus we reveal abnormalities in the social communication of TSC2+/− mice.
Results
Maternal Potentiation in TSC2+/− Pups.
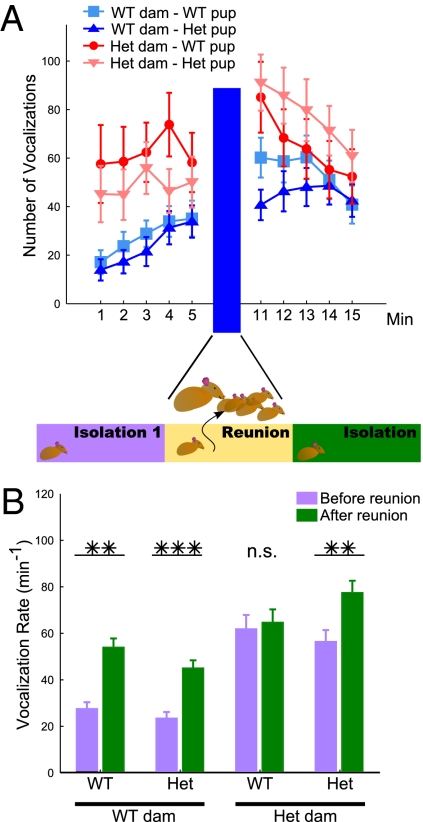
Pups were individually removed from the nest and placed directly in an isolation chamber, then returned directly to the nest before undergoing a second isolation period identical to the first (Fig. 1A). As expected, WT pups from WT dams underwent robustly potentiated vocalization responses following reunion with the dam, with vocalization rates during the second isolation period double that of the first [F(1, 92) = 13.95, P < 0.001] (Fig. 1B). Similarly, heterozygous pups from both WT and heterozygous dams also potentiated [F(1, 92) = 10.04, P = 0.002 and F(1, 92) = 16.34, P < 0.001, respectively]. Only one group, WT pups born to heterozygous dams, failed to potentiate [F(1, 92) = 0.11, P = 0.745], with a correspondingly significant three-way statistical interaction of the isolation period, dam genotype, and pup genotype [F(1, 88) = 6.107, P = 0.015]. Whereas heterozygous pups from heterozygous dams underwent the expected potentiation, all pups born to heterozygous dams exhibited dramatic increases (up to 2-fold more calls at baseline) in USV call rates both before and after reunion with their mothers. This increase yielded a significant main effect of dam genotype on call rate [F(1, 88) = 13.899, P < 0.001]. Thus the rate of pup vocalizations upon isolation varied with the genotype of the mother, whereas the extent of maternal potentiation seemed to be governed by the interaction between maternal and pup genotypes.
Fig. 1.
Vocalization rates were elevated in pups born to heterozygous dams. (A) Offspring from both wild type (WT) and heterozygous (het) dams (blue and red, respectively) were isolated, reunited, and reisolated for 5 min per session. Although WT and heterozygous pups from WT dams did not differ significantly in call rate or temporal pattern, pups from heterozygous dams were more vocal than pups from WT dams. (B) All groups but WT pups born from heterozygous dams exhibited maternal potentiation. Note the overall increase in calls from the heterozygous dam group (P < 0.001), and a three-way statistical interaction among isolation period × dam genotype × pup genotype (P = 0.015). *P < 0.05, **P < 0.01, ***P < 0.001.
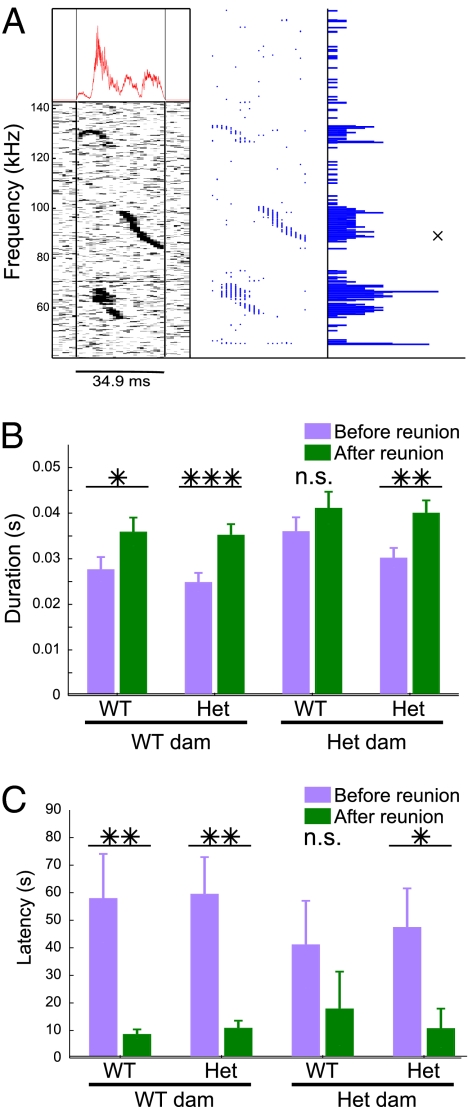
Maternal potentiation is usually quantified by call counts, typified by higher call rates in the reisolation period. However, examination of additional parameters may reveal other call features relevant to communication and maternal potentiation. To explore these features, we measured the impact of reunion on call duration, latency to call, and median sound frequency. Calls were segmented from noise by measuring and thresholding the intensity envelope. Call durations could then be precisely calculated from the identification of the start and end point of the segmented call (Fig. 2A). Across all genotype groups, the mean call durations were found to be significantly higher in the second isolation period [F(1, 86) = 40.344, P < 0.001], indicating that potentiation is a matter not merely of increased call rate, but also increased average call duration (Fig. 2B). Although duration increased on average across all groups, there was a main effect of dam genotype, in which calls emitted by pups from heterozygous dams were longer on average [F(1, 86) = 6.645, P = 0.012]. WT pups from these heterozygous dams were also the only group that failed to significantly increase call duration during reisolation. Thus the same group that did not undergo potentiation in terms of call rate also did not potentiate in call duration. Pups from heterozygous dams exhibited both higher call rates and longer call durations, meaning that these pups spent a longer total amount of time vocalizing than did pups born to WT dams.
Fig. 2.
Maternal potentiation was exhibited in call duration and latency. (A) Representative spectrogram of a multicomponent call and its segmentation. (Left) Call with segment lines, calculated from amplitude envelope shown in overlay. (Center) Extracted instantaneous frequencies. (Right) Histogram of frequency counts. (B) Mean duration of each call rose on average from isolation 1 to isolation 2 (P < 0.001), and call durations were longer on average across isolation periods from pups born to heterozygous dams (P = 0.012). (C) Call latency strongly decreased following reunion on average across all genotypes (P < 0.001). Only the WT pup, heterozygous dam group did not statistically significantly increase in call duration or decrease in latency.
Latency to call was measured as the time from the start of recording until the first call. Latencies decreased dramatically on average across all groups during the second isolation period [F(1, 86) = 24.703, P < 0.001], thus revealing yet another measure of maternal potentiation (Fig. 2C). No main effect of dam or pup genotype in latency was found; however, the WT pups from heterozygous dam were again notable as the only group that did not exhibit a decrease in call latency at a statistically significant level.
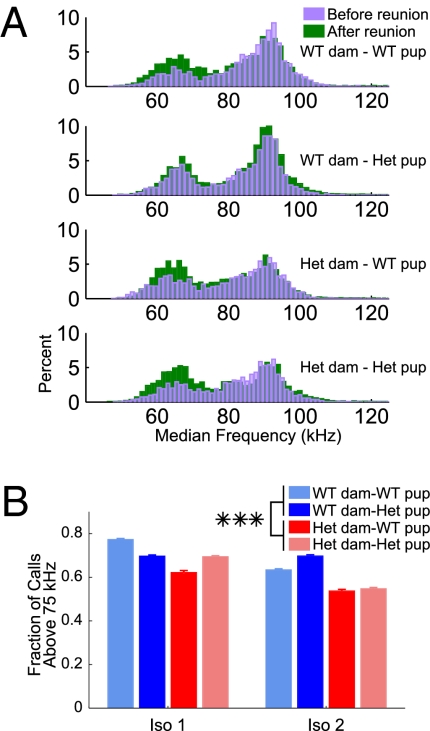
To assess individual sound frequencies and call type classification of pup USVs, we developed a technique for extracting calls by identifying instantaneous frequencies within a call throughout the ultrasonic range of 40–180 kHz. This call extraction allowed us to accurately measure median frequencies even in calls with many different frequency components in the same time window. Median frequency probability density plots revealed a bimodal distribution of calls, with a population above and below 75 kHz. During the first isolation period, calls with median frequencies above 75 kHz were predominant for pups born to both heterozygous and WT dams. However, during the second isolation period, pups born to heterozygous dams tended to equalize the number of calls with frequencies above and below 75 kHz (Fig. 3).
Fig. 3.
Proportion of low-frequency calls was elevated in pups from heterozygous dams. (A) Probability density plots of median sound frequencies of individual calls overlaid from before and after reunion (purple and green, respectively) reveal a bimodal distribution of call frequencies. (B) Fraction of calls above and below 75 kHz became almost equal to one another in pups from heterozygous dams after reunion. These pups’ fraction of high frequency calls was significantly lower than that of pups from WT dams across isolation period (P < 0.001).
Increased Emission of Multicomponent Calls.
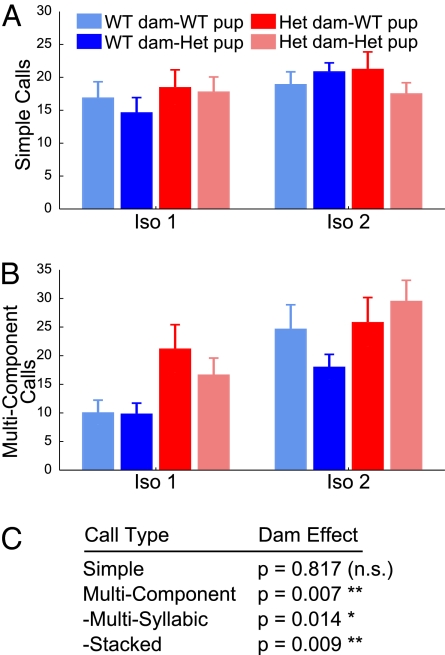
Informed by Scattoni et al. (11), who classified individual pup calls into 10 types, we categorized pup vocalizations based on properties of their subcalls. At the broadest level, our categorization identifies simple calls, containing a single component, and multicomponent calls. We further subdivided multicomponent calls into multisyllabic calls, where components are separated in time, and stacked calls, where components are separated in frequency space (Fig. S1).
When we examined rates of simple calls alone, we saw no differences across isolation period or dam genotype [F(1, 73)iso = 0.831, P = 0.370; F(1, 73)dam = 0.054, P = 0.817], suggesting that any increase in calls mostly derived from an increase in multicomponent calls (Fig. 4A). Indeed, the fraction of emitted multicomponent calls increased (potentiated) after reunion in pups from WT dams [F(1, 77)WT dam/WT pup = 26.04, P < 0.001; F(1, 77)WT dam/het pup = 6.28, P = 0.014] (Fig. 4B). TSC2+/− pups from TSC2+/− dams also potentiated in terms of multicomponent calls [F(1, 77) = 12.14, P = 0.001]. Again, WT pups from TSC2+/− dams was the only group that failed to potentiate in terms of this call type [F(1, 77) = 1.39, P = 0.242]. Interestingly, all pups born to heterozygous dams demonstrated a strong preferences for multicomponent calls [F(1, 73) = 7.751, P = 0.007], indicating that the higher vocalization rate in pups born to heterozygous dams also results from a selective preference for multicomponent calls.
Fig. 4.
Multicomponent calls were selectively elevated. (A) There were no significant differences in numbers of simple calls across isolation period or genotype group. (B) Multicomponent call numbers increased after reunion in both WT (P < 0.001) and TSC2+/− (P = 0.014) pups from WT dams as well as TSC2+/− pups from TSC2+/− dams (P = 0.001), but not from WT pups born to TSC2+/− dams (P = 0.242). Pups from heterozygous dam emitted a significantly higher fraction of multicomponent calls across both periods (P = 0.007). (C) Multivariate statistics summary shows a significant main effect of dam genotype on both multisyllabic and stacked subgroupings of multicomponent calls.
USV Burst Patterns.
Having seen changes in individual call parameters, we were curious as to whether TSC2 heterozygosity altered the temporal organization of calls. Inspection of intercall interval (ICI) distributions revealed two peaks: a larger peak at shorter ICIs corresponding to intraburst calls, followed by a much smaller peak at longer ICIs that corresponded to interburst intervals (Fig. S2A). To automatically detect bursts in the record of call times, we set a burst threshold by finding the minimum of the ICI distribution between these two peaks. We were then able to measure mean burst durations as well as call counts per burst and burst rates. Similar to call potentiation, bursts showed potentiation (increases) in duration and burst rate as well as an increased number of calls per burst following reunion (Fig. S2 B–D). Again, there was a main effect of dam genotype, whereby pups born to heterozygous dams emitted longer bursts at faster rates and with more calls per burst [F(1, 73) = 5.526, P = 0.021 for duration; F(1, 73) = 8.737, P = 0.004 for rate; and F(1, 73) = 5.913, P = 0.017 for calls per burst].
Influence of Gender on USV Call Intensity.
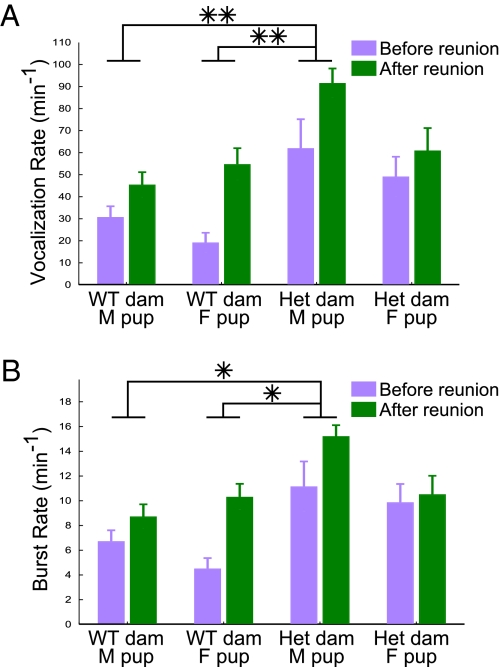
To examine possible gender-dependent vocalization differences in TSC2+/− mutant mice, all groups were split along dam genotype and pup gender (pooling across pup genotype), revealing a three-way statistical interaction of isolation period, dam genotype, and pup gender for call rates [F(1, 88) = 8.267, P = 0.005]. Males from heterozygous dams were found to express particularly heightened rates of both individual calls as well as call bursts, regardless of pup genotype (Fig. 5 and Fig. S3). Although not statistically different from other groups, WT females from heterozygous dams were the only pups to actually decrease in absolute value of call rates, mean call duration, calls per burst, burst rate, and stacked calls (Fig. S4). Thus pup vocalization patterns vary not only with dam genotype, but also with dam genotype combined with pup genotype or gender.
Fig. 5.
Males from heterozygous dams were particularly susceptible to changes in rate. (A) Male pups born to heterozygous dams were found to vocalize at significantly higher rates than either male (P = 0.002) or female pups (P = 0.003) born to WT dams. (B) Burst analysis revealed that this same group also vocalized at higher burst rates than did male (P = 0.023) or female pups from WT dams (P = 0.021).
Measures of Maternal Care.
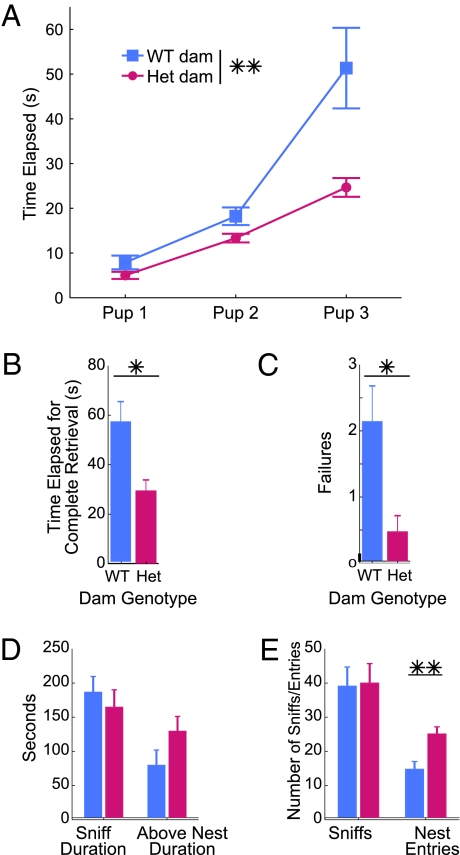
Dam genotype exhibits a strong main effect on maternal potentiation, raising the question of whether differences in maternal care during development, the reunion, or both have an impact on pups’ behavior. To further dissect the impact of maternal care on pup behavior, we measured latency to retrieve pups from a scattered nest. Both WT and TSC2+/− dams retrieved their first pup with no trouble and at equal latencies. Retrieval latency was similar for the second pup but differed dramatically during retrieval of the third and final pup (Fig. 6 A and B; Movies S1 and S2). Although WT dams often deposited pups away from the nest or even “deretrieved” pups by taking them out of the nest, heterozygous dams methodically and consistently retrieved and returned all three pups back to their nest (P = 0.012 for misretrievals; Fig. 6C). Overall, heterozygous dams responded to pups more quickly [F(1, 16) = 10.094, P = 0.006].
Fig. 6.
TSC2+/− dams exhibited improved maternal care. (A) Heterozygous dams demonstrated more efficient retrieval scores, bringing all three pups back to the nest in less than half the time as WT dams (P = 0.006 for dam effect). (B) Total time for final collection of all pups back to the nest was significantly lower for TSC2+/− dams (P = 0.011). (C) WT dams exhibited significantly higher failure rate to deposit pup in nest or to keep pup there (P = 0.012). (D) Resident intruder tests showed no significant difference in total duration of sniffs or attacks and time over nest. (E) Although numbers of sniffs or attacks did not differ between dam groups, heterozygous dams entered nest significantly more often (P = 0.003).
As another measure of maternal care, male intruders were placed into dams’ otherwise undisturbed home cages. Dams typically defend their nest by sniffing and nipping at the intruder and spending time hovering over the nest. Although both WT and heterozygous dams sniffed and bit intruders equally well (P = 0.528 for sniff duration, P = 0.912 for sniff count), heterozygous dams showed a trend toward increased duration above their nest and a significantly higher number of entries into their nest (P = 0.003; Fig. 6 D and E). The increased number of nest entries as well as the shorter pup retrieval latencies suggest that heterozygous dams show greater attentiveness to caring for and protecting their pups.
Discussion
TSC2 heterozygosity has been shown to have an impact on learning and memory, but no changes in social behavior have previously been reported. Here we show that TSC2 heterozygosity is sufficient to alter ultrasonic vocalizations in a behavioral assay of social interaction between mother and pup.
TSC2 Dam Haploinsufficiency Alters Baseline Vocalization Rates.
USV recordings in TSC2+/− pups and their WT siblings from both TSC2+/− and WT dams show that TSC2 heterozygosity is sufficient to induce changes in social communication. Isolation-induced vocalizations have long been thought to serve a communicative role by eliciting search-and-retrieval behavior in lactating dams. Indeed, vocalizations produced from isolated speakers induce similar maternal retrieval behaviors as do isolated vocalizing pups (12). The baseline rate of isolation-induced vocalizations in pups may serve as an early biomarker of communicative function in mice, and a large number of mouse lines with mutations in autism candidate genes show changes in baseline vocalization rates (3). Although both WT and TSC2+/− pups born to WT dams expressed no difference in baseline rates compared with each other or with C57BL/6J mice in other studies (11, 13), pups born to TSC2+/− dams showed baseline rates roughly 3-fold that of their WT-dam counterparts. This dramatic increase in overall vocalizations may reflect a particularly heightened anxiety state, as vocalization rates are known to be susceptible to anxiolytic drugs, whereas anxiogenics increase call number and amplitude (14).
Several murine models of autism with known vocalization defects express decreased vocalization rates, whereas others vocalize at higher than normal rates, which may correspond to an increase in anxiety or disturbances in the use of language. The almost 2-fold increase in baseline vocalization rates we observed in pups born to TSC2+/− dams is similar to the increased rates in the BTBR and the 2-fold rate increase in both the MECP2 and patDp/+ murine models of autism (11, 15, 16). Interestingly, there was no difference in baseline rates between WT and heterozygous pups born to heterozygous dam, suggesting that maternal genotype plays a dominant role in influencing baseline call rates in the TSC2 mouse line. As vocalization experiments are typically set up with solely heterozygous mating pairs or solely WT dams, it would be intriguing to compare baseline vocalization rates in these lines from WT, heterozygous, and homozygous mutant dam genotypes, when available.
Changes in Maternal Potentiation Are Dependent on Genotype of Both Dam and Pup.
Although baseline vocalizations provide insight into the pups’ immediate response to isolation, maternal potentiation highlights the influence of mother–pup interaction on vocalization as social communication. Baseline vocalizations have typically been interpreted as distress calls, susceptible to changes in temperature and other environmental cues, whereas maternal potentiation is robust in rats and mice, independent of temperature and specific to reunion with a familiar parent (13). During the reunion period, the pup may associate maternal retrieval as a reward for cries, and thus on a subsequent isolation, the pup cries out even more vehemently. Indeed, mice lacking the μ-opioid receptor gene, which has been shown to mediate reward pathways, are deficient in maternal potentiation (17), suggesting that potentiation is dependent on a learning process by which the dams’ responsiveness to cries reinforces the pups’ crying behavior (18). TSC2+/− mice were recently shown to exhibit cognitive and learning deficits in spatial memory tasks (7), which may also impinge on social learning and communication.
In our experiments, only one group failed to undergo potentiation: WT pups born to heterozygous dams. This failure to potentiate could arise from a deficit in reward pathways in the pup, the reward itself as provided by the dam, or both—the mother's and pup's mutual response to one another. An inborn deficit of the pup is an unlikely explanation because those pups that show no potentiation have no TSC2 mutation, and WT pups from WT dams potentiate normally. Maternal care per se also cannot account for our findings, given the faster pup retrieval times and improved defense against resident intruders by TSC2+/− dams. It thus appears more likely that the USV phenotypes reflect differences in mother–pup interaction. It would be interesting to further elucidate the genetic influence of both dam and pup through audio and visual recordings of mother–pup interactions before pup isolation and during the reunion period.
TSC Heterozygosity Influences Additional Vocalization Parameters.
Typically, maternal potentiation is measured as an increase in rates of call. In addition to call rates, we found that maternal potentiation is also expressed as an increase in call duration and decrease in latency to call. Together, the increased call rate, increased duration of individual calls, and reduced latency to begin calling constitute a dramatic increase in total time of calling during maternal potentiation as well as a much earlier vocalization response. Intriguingly, the only genotype combination that did not increase in rate of call also failed to undergo a statistically significant alteration in mean call duration or latency to call. Thus these three parameters appear to correlate strongly with one another, and WT pups born to heterozygous dams appear to be robustly deficient in maternal potentiation by all criteria. Although it is possible that this lack of maternal potentiation results from ceiling effects, the fact that heterozygous pups from the same dams vocalize at higher rates after reunion than do any other genotype group makes ceiling effects less likely.
The pitch of infants’ cries has been shown to alter adults’ perception of distress. Esposito and Venuti (19, 20) showed that when cries were artificially manipulated to change their pitch, ICI, or speed, both higher and lower pitched cries as well as cries with shorter pauses increased the perception of distress in both parents and nonparents. Subjects who were later diagnosed to have autism emitted higher pitched cries than those subjects with typical or delayed development, and autistic children's cries elicited greater negative states in their listeners. Intrigued by the altered pitch of cries in autistic children, we examined the sound frequencies emitted by TSC2+/− mice and found an altered distribution of high- versus low-frequency calls after reunion in pups born to heterozygous dams. The altered pitch may have conveyed a heightened level of distress to the dams and contributed to the increased maternal response rates during retrievals and defense against intruders.
Although total time of calling reflects the total quantity of calls, we also explored the organizational structure of USVs into call patterns and types. Burst analysis revealed that calls were not a series of isolated events but, rather, clustered into discrete bouts. Consistent with the individual call parameters, the burst durations, calls per burst, and rates were all increased in pups born to heterozygous dams. The increase in call and burst durations, as well as calls per burst during the second isolation, may convey a different message from the pup to the dam, perhaps one of greater anxiety or urgency. It would be interesting to see whether speaker playback of preferentially longer calls or bursts elicits faster retrieval times by dams.
Preverbal autistic children have been shown to produce a higher proportion of atypical vocalizations, which include squeals, growls, and yells (21). The BTBR mouse model of autism likewise emits a higher proportion of multicomponent calls. When we examined the numbers of simple and multicomponent calls separately, we found that at baseline, pups born to WT dams emitted an ≈2:1 ratio of simple calls to multicomponent calls, suggesting that multicomponent calls are “atypical” vocalizations. Both the increased calls during potentiation and the higher rates of calls from pups born to heterozygous dams are due to a selective increase in multicomponent calls. Thus pups from heterozygous dams exhibit a higher proportion of multicomponent or atypical vocalizations consistent with that seen in autistic infants. Further acoustical analysis of infants’ crying may identify prognosticators of language development and early biomarkers of communication impairments.
Males from Heterozygous Dams Are Particularly Susceptible to Changes in Vocalizations.
Autism shows a strong genetic preference for males, exhibiting a 4:1 ratio of male to female in the human population, although autism in TSC is closer to 1:1 and has been reported to show no differences in behavioral features between genders (2, 22). Males from heterozygous dams vocalized at much higher rates both at baseline and following reunion compared with WT dam groups at either period, both in individual calls and several burst parameters. Independently of pup genotype, male gender thus appears to lend susceptibility to autistic-like features in pups from heterozygous dams. The differences seen here based on pup gender raise the question of whether, despite the report that autism in human TSC patients are closer to parity between males and females (23), different genders may display features of autism in different ways. Although the end diagnoses might be the same, it may be worthwhile to look for gender-dependent qualitative differences in expression of autism and other comorbid behavioral disorders with TSC to see whether this approach might help to improve treatment plans for TSC patients of different genders.
TSC2+/− Dams Demonstrate Improved Maternal Care.
The possible maternal impacts on vocalization may be broadly classified into prenatal versus postnatal effects. Prenatal effects include possibilities as diverse as imprinting of genes inherited maternally, metabolic defects that affect gestational development, or immune responses to the pups during pregnancy (24). Environmental stimuli are known to influence vocalization rates, including changes in handling, litter size, and fostering (25, 27), and differences in maternal care might affect the environment in which pups are raised and subsequently influence their vocalization rates. To explore the impact of postnatal effects, we assayed pup retrieval performance and maternal defense against a resident intruder as a measure of responsiveness to both internal and external cues. Remarkably, TSC2+/− dams demonstrated striking improvements in pup retrieval performance and entries to their nest.
These behaviors may be reminiscent of the repetitive, idiosyncratic behavior evident in autistic patients, who are often extremely focused on and perhaps extremely capable of performing a particular task. The behavior may also reflect a heightened anxiety state similar to that seen in the increased vocalization rate of their pups, although the absence of anxious behaviors in open field and elevated plus maze assays of anxiety from a previous study of adult male TSC2+/− mice makes this explanation less likely (7). Further assays such as licking and grooming measurements remain to be explored to assess whether other aspects of heterozygous dams’ maternal behavior influence their ability to care for their pups in ways that might alter the vocalization response. In earlier studies of oxytocin, 5HT1A, and 5HT1B receptor knockout mice, correlations were found between maternal genotype and pups’ vocalization responses (27, 28). Testing these mouse lines for deficits in maternal potentiation might help us to better understand the signaling pathways involved in mother–pup social interactions. As previous studies of TSC2 heterozygosity have demonstrated defects in axonal connectivity as well as L-LTP (7, 29), it would be interesting to track these cellular and physiological defects across development to explore their behavioral impact at different stages of life, including any correlations with maternal behavior.
Taken together, our findings reveal that TSC2 haploinsufficiency impinges on social communication in a manner dependent on the interaction of genotype of both dam and pup. Further experiments to dissect the molecular underpinnings of this complex relationship may identify new targets for intervention in TSC patients, whether pharmaceutical or behavioral.
Materials and Methods
Animals.
TSC2+/− mice that were generated as described previously (30) were back-crossed for 10 generations to C57BL/6J background. Matings were set up with one WT and one heterozygous mouse to achieve approximately equal numbers of offspring by genotype (half TSC2+/+, half TSC2+/−), with either the mother or the father bearing the TSC2 mutation so as to compare TSC2+/− and WT dams.
Isolation-Induced Vocalizations and Maternal Potentiation.
Pups on P10 were isolated one by one from their home cage and placed in a cardboard recording box situated in an anechoic chamber. During a 5-min isolation period, vocalizations were recorded from an Avisoft UltraSoundGate CM16/CMPA microphone. Each pup was next reunited with its dam and littermates by placing it at the end farthest from the nest to allow the dam to retrieve the pup. After a 5-min reunion, that pup was reisolated and recorded for an additional 5 min.
Pup Retrievals.
On P6, pups were removed from their nest, and three pups were returned, one to each corner of the cage away from the nest. The dam was then returned to the nest area facing away from the pups and allowed to retrieve pups under video recording.
Resident Intruder.
On P10, a male C57BL/6J adult (3–4 mo old) intruder mouse was introduced into the home cage while recorded by video camera. The intruder was left in the cage for 15 min before removal, after which video files were analyzed for maternal defense of her nest and home cage.
Statistics.
All effects are reported as significant at P < 0.05, and error bars are given as SEM. For USV experiments, n = 26 (WT dam, WT pup; 11 female, 15 male), n = 28 (WT dam, heterozygous pup; 13 female, 15 male), n = 17 (heterozygous dam, WT pup; 9 female, 8 male), n = 25 (heterozygous dam, heterozygous pup; 17 female, 8 male); n = 6 WT dam litters, n = 5 heterozygous dam litters (Fig. S5). 46,497 calls were segmented and analyzed from USV experiments. For maternal care assays and resident intruder assays, n = 9 dams per genotype.
Supplementary Material
Acknowledgments
We thank S. Bonasera, E. Storm, E. Goulding, R. Liu, J. Rubenstein, B. Cheyette, L. Tecott, and F. Huang for helpful conversations, code, and support; N. Shao and M. Wu for guidance in maternal care experiments; and A. Rowson-Baldwin and Y. Shu for excellent technical assistance. This study was supported by National Institutes of Health Grant R37 MH 06334 (to L.Y.J.). Y.N. Jan and L.Y. Jan are Howard Hughes Medical Institute investigators.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005620107/-/DCSupplemental.
References
- 1.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. Eur J Paediatr Neurol. 2004;8:327–332. doi: 10.1016/j.ejpn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Moy SS, Nadler JJ. Advances in behavioral genetics: Mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 4.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 5.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Holmes GL, Stafstrom CE. Tuberous Sclerosis Study Group Tuberous sclerosis complex and epilepsy: Recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 7.Ehninger D, et al. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci. 2002 doi: 10.1002/0471142301.ns0814s17. Chapter 8:Unit 8.14. [DOI] [PubMed] [Google Scholar]
- 9.Ehret G. Infant rodent ultrasounds—a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 10.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu A, et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 2007;1163:91–99. doi: 10.1016/j.brainres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Scattoni ML, et al. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel TR, Hill JL, Mayor RB. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakatani J, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picker JD, Yang R, Ricceri L, Berger-Sweeney J. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport. 2006;17:541–544. doi: 10.1097/01.wnr.0000208995.38695.2f. [DOI] [PubMed] [Google Scholar]
- 17.Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 18.Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: Relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Esposito G, Venuti P. How is crying perceived in children with autistic spectrum disorder. Res Autism Spectr Disord. 2007;2:371–384. [Google Scholar]
- 20.Esposito G, Venuti P. Understanding early communication signals in autism: A study of the perception of infants’ cry. J Intellect Disabil Res. 2010;54:216–223. doi: 10.1111/j.1365-2788.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 21.Sheinkopf SJ, Mundy P, Oller DK, Steffens M. Vocal atypicalities of preverbal autistic children. J Autism Dev Disord. 2000;30:345–354. doi: 10.1023/a:1005531501155. [DOI] [PubMed] [Google Scholar]
- 22.de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): A postal survey of UK families. Eur Child Adolesc Psychiatry. 2007;16:16–24. doi: 10.1007/s00787-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 23.Curatolo P, Napolioni V, Moavero R. Autism spectrum disorders in tuberous sclerosis: Pathogenetic pathways and implications for treatment. J Child Neurol. 2010 doi: 10.1177/0883073810361789. 10.1177/0883073810361789. [DOI] [PubMed] [Google Scholar]
- 24.Wöhr M, et al. Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: An embryo-transfer study. Behav Genet. 2008;38:579–595. doi: 10.1007/s10519-008-9221-4. [DOI] [PubMed] [Google Scholar]
- 25.Schwarting RKW, Jegan N, Wöhr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav Brain Res. 2007;182:208–222. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Wöhr M, Houx B, Schwarting RKW, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93:766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller A, et al. Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev Psychobiol. 2003;42:194–205. doi: 10.1002/dev.10079. [DOI] [PubMed] [Google Scholar]
- 29.Nie D, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13:163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/-) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.