Abstract
In methanogenic Archaea, the final step of methanogenesis generates methane and a heterodisulfide of coenzyme M and coenzyme B (CoM-S-S-CoB). Reduction of this heterodisulfide by heterodisulfide reductase to regenerate HS-CoM and HS-CoB is an exergonic process. Thauer et al. [Thauer, et al. 2008 Nat Rev Microbiol 6:579–591] recently suggested that in hydrogenotrophic methanogens the energy of heterodisulfide reduction powers the most endergonic reaction in the pathway, catalyzed by the formylmethanofuran dehydrogenase, via flavin-based electron bifurcation. Here we present evidence that these two steps in methanogenesis are physically linked. We identify a protein complex from the hydrogenotrophic methanogen, Methanococcus maripaludis, that contains heterodisulfide reductase, formylmethanofuran dehydrogenase, F420-nonreducing hydrogenase, and formate dehydrogenase. In addition to establishing a physical basis for the electron-bifurcation model of energy conservation, the composition of the complex also suggests that either H2 or formate (two alternative electron donors for methanogenesis) can donate electrons to the heterodisulfide-H2 via F420-nonreducing hydrogenase or formate via formate dehydrogenase. Electron flow from formate to the heterodisulfide rather than the use of H2 as an intermediate represents a previously unknown path of electron flow in methanogenesis. We further tested whether this path occurs by constructing a mutant lacking F420-nonreducing hydrogenase. The mutant displayed growth equal to wild-type with formate but markedly slower growth with hydrogen. The results support the model of electron bifurcation and suggest that formate, like H2, is closely integrated into the methanogenic pathway.
Keywords: energy conservation, Archaea, formate dehydrogenase, formylmethanofuran dehydrogenase, F420-nonreducing hydrogenase
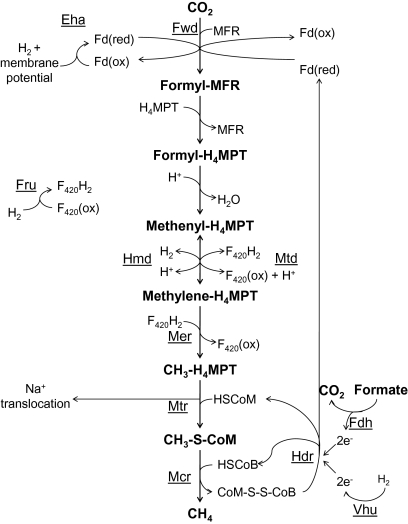
The biochemical steps in methanogenesis from CO2 are well known, but the interactions that lead to net energy conservation are not well understood. The steps in the pathway are diagrammed in Fig. 1 (1). The first step involves the reduction of CO2 and covalent attachment to a unique cofactor, methanofuran (MFR), via the action of formylmethanofuran dehydrogenase (Fwd) to generate formyl-MFR. This represents an energy-consuming step in the pathway and is dependent on reduced ferredoxin, thought to be produced at the expense of a chemiosmotic membrane potential via the energy-conserving hydrogenase, Eha. Next, the formyl group is transferred to another carrier, tetrahydromethanopterin (H4MPT), and is then reduced to generate methyl-H4MPT. The methyl group is then transferred to yet another carrier, coenzyme M (HS-CoM), by methyl-H4MPT-CoM methyltransferase (Mtr) to generate methyl-S-CoM. At this point, Na+ ions are translocated across the cell membrane. The final step involves reduction of the methyl group to CH4 and capture of HS-CoM by coenzyme B (HS-CoB) to form a CoM-S-S-CoB heterodisulfide. To regenerate HS-CoM and HS-CoB, another enzyme is used, heterodisulfide reductase (Hdr).
Fig. 1.
The methanogenic pathway with hypothesized roles for the heterodisulfide reductase complex. Electron flow from formate or hydrogen to Hdr may drive the reduction of the CoM-S-S-CoB heterodisulfide, as well as the reduction of ferredoxin via flavin-mediated electron bifurcation as outlined by Thauer et al. (1). Eha, energy-conserving hydrogenase; Fdh, formate dehydrogenase; Fru, F420-reducing hydrogenase; Fwd, formyl-MFR dehydrogenase; Hdr, heterodisulfide reductase; Hmd, H2-dependent methylene-H4MPT dehydrogenase; Mcr, methyl-CoM reductase; Mer, methylene-H4MPT reductase; Mtd, F420-dependent methylene-H4MPT dehydrogenase; Mtr, methyl-H4MPT-CoM methyltransferase; Vhu, F420-nonreducing hydrogenase.
Most methanogens can use H2 as the electron donor, and many can also use formate. Reduced coenzyme F420 (F420H2) is a required intermediate, and can be generated from H2 by F420-reducing hydrogenase (Fru) or by a cycle involving the enzymes H2-dependent methylene-H4MPT dehydrogenase and F420-dependent methylene-H4MPT dehydrogenase (2). When formate is the electron donor, it is oxidized to CO2 by a formate dehydrogenase (Fdh) that yields F420H2.
How net energy is conserved in most methanogens is not well understood, because the membrane potential generated during the methyl transfer from H4MPT to HS-CoM would appear to be depleted by Eha to fuel the reduction of CO2 to formyl-MFR. The solution to this dilemma apparently resides in the exergonic heterodisulfide reduction step. Methanogens from the order Methanosarcinales, known as the methylotrophic methanogens, have a membrane-bound electron transport chain involving the quinone-like methanophenazine and a cytochrome-containing Hdr complex that translocates protons across the cell membrane concomitant with CoM-S-S-CoB reduction, resulting in net energy conservation (1). However, all other methanogens (the hydrogenotrophic methanogens) lack methanophenazine and cytochromes, have a cytoplasmic Hdr, and are not known to generate a membrane potential at this step (1). Nevertheless, these organisms grow rapidly and are found in numerous anaerobic environments. It was recently proposed that methanogens without cytochromes use flavin-based electron bifurcation from Hdr to simultaneously reduce CoM-S-S-CoB and reduce ferredoxin for Fwd to generate formyl-MFR (1). If this were to occur, then ferredoxin reduction by Eha would not be required and net energy conservation would result.
It was our intention to find protein–protein interactions involving Hdr that may indicate if there are potential pathways for energy conservation that have eluded prior characterization in methanogens without cytochromes. To this end, we performed experiments with the hydrogenotrophic methanogen, Methanococcus maripaludis. M. maripaludis is ideal for such an undertaking because of its rapid growth under laboratory conditions, a well-developed set of genetic tools (3, 4), the ability to grow in continuous culture under conditions of defined nutrient limitation (5), and the availability of an exhaustive dataset from quantitative measurements of the proteome (6, 7).
Results
Hdr Complexes with F420-Nonreducing Hydrogenase, Fdh, and Fwd.
To characterize the protein interactions that take place between Hdr and associated proteins, the β subunits of Hdr were C-terminally tagged with a 10-amino acid extension containing a 6×-His tag. The β subunits were used for purification as this subunit has been demonstrated to contain the active site for heterodisulfide reduction (8). The M. maripaludis genome encodes two Hdrs (9), and either HdrB1 (strain MM1263) or HdrB2 (strain MM1264) was tagged to determine if there were differences between the protein interactions of each. In addition, we had preliminary evidence (based on early purification experiments) that one of two Fdhs encoded in the M. maripaludis genome might be included in protein complexes with Hdr. Therefore, we also constructed a strain (MM1265) in which FdhA1 was C-terminally tagged with a 13-amino acid extension containing a 6×-His tag. To avoid any confounding influence of the second Fdh, Fdh2, the FdhA1 His-tag was constructed in a background strain that contained an in-frame deletion of the fdh2 gene cluster. In each case, the His-tagged version of the protein replaced the wild-type gene in the genome. Growth experiments showed that each His-tagged protein was functional (Fig. S1). Thus, a strain containing His-tagged HdrB1 and a null mutation in hdrB2 grew normally, as did a strain containing His-tagged HdrB2 and a null mutation in hdrB1. Because Hdr is essential, each His-tagged protein must be functional. Similarly, MM1265 containing His-tagged FdhA1 and a deletion of fdh2 grew normally on formate. Because Fdh is required for growth on formate, His-tagged FdhA1 must be functional.
For protein preparations, three experimental strains were grown: MM1263 containing His-tagged HdrB1, MM1264 containing His-tagged HdrB2, and MM1265 containing His-tagged FdhA1. Two control strains were also grown: the parental strain MM901 containing no His-tagged proteins, and strain MM1262 containing the deleted fdh2 and no His-tagged proteins. All five strains were grown under three conditions: hydrogen excess or limitation in a chemostat and batch culture with formate as the sole electron donor. This process was followed because several genes are regulated in response to hydrogen availability in M. maripaludis (10), and there could be differences in the composition of the Hdr complex in response to different growth conditions. Cell extracts were made from all 15 cultures and protein purifications were done anaerobically using Ni-affinity columns. Purified samples were analyzed by mass spectrometry. Spectral counts (SC) were tabulated for any protein that returned ≥10 SC in any of the three growth conditions with the three experimental strains (Table S1).
For each protein, SCs were compared. From an initial inspection of the data it appeared that there were two groups: those that consistently had similar SCs in all five strains (background proteins), and those that had markedly greater SCs in MM1263 and MM1264 (the experimental strains) compared with MM901 (the control strain) or in MM1265 (experimental) to MM1262 (control). To distinguish clearly between these groups, three proteins among the background proteins (reference proteins) were used as the basis for the calculation of normalized SC ratios (Methods). The results are presented in Table S2. A protein was considered enriched by copurification with the His-tagged protein if the normalized SC ratio was greater by at least three SDs than the average ratio for the 13 background proteins, or if more than five SCs were detected in the experimental sample and none was seen in the control. The results are summarized in Table 1. Subunits from five different proteins copurified with both of the His-tagged HdrBs and with His-tagged FdhA1; these were Hdr1, Hdr2, Fdh1, the selenocysteine-containing F420-nonreducing hydrogenase (Vhu), and the tungsten-containing formylmethanofuran dehydrogenase (Fwd). The findings supported these conclusions regardless of which of the three reference proteins was used for the normalization calculation. None of these proteins was observed to bind nonspecifically to His-tagged constructs in M. maripaludis (11). In general, multiple subunits of a given protein were enriched in the experimental samples, although a few subunits were not detected. Generally, the relative abundances of subunits from each protein were in agreement with those found in proteomic studies of whole-cell extracts from M. maripaludis (6, 7), and those subunits that were not detected here were detected at low levels in the whole proteome. The five proteins were enriched in samples from all three growth conditions, except for Fdh1 under H2 excess and all proteins under H2 excess with His-tagged FdhA1. The absence of Fdh in these samples is not surprising because fdh expression is markedly down-regulated when cells are grown under H2 excess (10).
Table 1.
Proteins enriched in purified complexes
| H2-excess* |
H2-limited |
Formate |
|||||||
| MM1263† | MM1264 | MM1265 | MM1263 | MM1264 | MM1265 | MM1263 | MM1264 | MM1265 | |
| HdrAu | + | + | − | + | + | + | + | + | + |
| HdrB1 | + | + | ND | + | + | + | + | + | + |
| HdrB2 | + | + | ND | + | + | + | + | + | + |
| HdrC1 | + | + | ND | + | ND | ND | + | + | + |
| HdrC2 | + | + | ND | + | + | + | + | + | + |
| FdhA1 | − | − | ND | + | + | + | + | + | + |
| FdhB1 | ND | ND | ND | + | + | + | + | + | + |
| FwdA | + | + | − | + | + | + | + | + | + |
| FwdB | + | + | − | + | + | + | + | + | + |
| FwdC | + | + | ND | + | + | + | + | + | + |
| FwdD | + | + | ND | + | + | + | ND | + | + |
| FwdF | + | + | ND | + | + | + | + | + | + |
| VhuA | + | + | ND | + | + | + | + | + | + |
| VhuD | + | + | ND | + | + | + | + | + | + |
| VhuG | + | + | ND | ND | ND | + | + | + | + |
| VhuU | + | ND | ND | ND | ND | ND | ND | ND | ND |
+, SC values relative to control samples support enrichment in protein complex (unshaded squares in Table S2). −, SC values relative to control samples do not support enrichment in protein complex (shaded squares in Table S2). ND, not detected (SC ≤ 5 in experimental sample).
*Growth condition.
†Experimental strain. MM1263, His-tagged HdrB1; MM1264, His-tagged HdrB2; MM1265, His-tagged FdhA1.
Each of the four enzymes represented in the complex is encoded in the genome in two different forms, but only in the case of the HdrB and HdrC subunits were both purified in the complexes. Thus, both HdrB1 and HdrB2 and both HdrC1 and HdrC2 were generally present in the complex; however, both are not required to make a functional Hdr (Fig. S1). Hdr is a tetramer of trimers in the α4β4γ4 configuration in methanogens (12), and evidently the B1 and B2 subunits are interchangeable, as are the C1 and C2 subunits. In the case of the F420-nonreducing hydrogenase and HdrA, the two forms of the enzyme contain selenocysteine (Vhu and HdrAu) or cysteine (Vhc and HdrAc). Only the selenocysteine forms were detected here, consistent with previous studies of regulation by selenium, predicting that only the former should be expressed in our selenium-containing medium (13). Indeed, in two studies of the whole proteome of M. maripaludis, the selenocysteine proteins were detected at least 100-fold more frequently than the cysteine proteins (6, 7). Two formylmethanofuran dehydrogenases are represented in the genome, tungsten-containing (Fwd) and molybdenum-containing (Fmd). Only Fwd was detected here, and in the whole proteome the Fwd subunits were detected at least 10-fold more frequently. Of the two Fdhs, Fdh1 found in the complex with Hdr was also detected at least 10-fold more frequently in the proteome than Fdh2. In contrast, the HdrB and C subunits were detected in more similar amounts in the proteome, with HdrB2 and C2 detected only 2- to 4-fold more frequently than HdrB1 and C1. Hence, the enzyme forms detected in the complexes with Hdr and Fdh1 were consistently those that were more frequently detected in the proteome.
F420-Nonreducing Hydrogenase Is Not Essential for Growth on Formate.
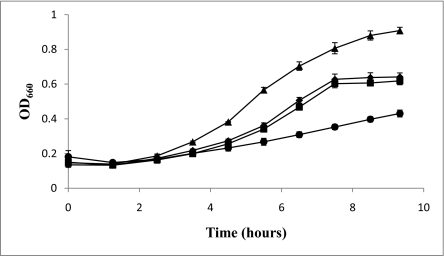
The presence of Fdh in a complex with Hdr suggested the possibility of direct electron flow between these two enzymes. If this is the case, then F420-nonreducing hydrogenase might not be needed for growth on formate because H2 generated from formate (via F420H2) (2) would not be necessary for heterodisulfide reduction. To test this hypothesis, we constructed in-frame deletions that eliminated the genes encoding the putative active subunits for hydrogen oxidation in both copies of the F420-nonreducing hydrogenase, vhuAU and vhcA. M. maripaludis was transformed with these constructs and a ΔvhuAU ΔvhcA mutant (MM1272) was successfully obtained after growth on formate. The mutant grew on formate similarly to the wild-type strain, but grew poorly on H2 (Fig. 2). The slight growth on H2 might be explained via a poorly understood F420H2:heterodisulfide oxidoreductase activity that has been demonstrated for the closely related Methanococcus voltae (14). In any case, F420-nonreducing hydrogenase clearly plays the major role in heterodisulfide reduction with H2 but is unnecessary for heterodisulfide reduction with formate.
Fig. 2.
Growth of Δvhu Δvhc strain vs. wild-type strain on formate or H2. OD660, optical density at 660 nm. (■) MM1272 (Δvhu Δvhc) grown on formate; (◆) MM901 (wild type) grown on formate; (●) MM1272 grown on H2; (▲) MM901 grown on H2. Data are from three independent cultures and error bars represent one SD around the mean.
Discussion
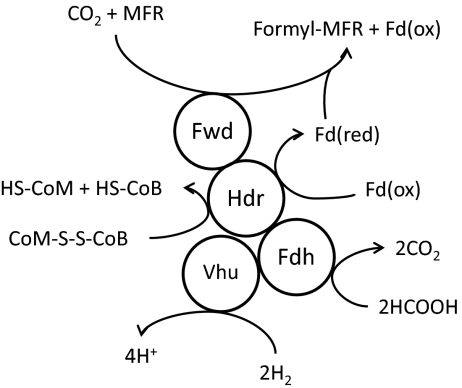
We have reported here evidence for a protein complex that comprises four separate enzymes: heterodisulfide reductase, F420-nonreducing hydrogenase, formate dehydrogenase, and formylmethanofuran dehydrogenase. The coelution of these proteins with three separate protein subunits under three different growth conditions strongly suggests that the observed complex exists in vivo and may be involved in energy conservation. The interaction of Hdr with F420-nonreducing hydrogenase was suggested previously in studies of these enzymes in Methanothermobacter marburgensis (15, 16). Indeed, in some methanogens the α subunit of Hdr is fused to the δ subunit of the F420-nonreducing hydrogenase (16). However, the presence of Fdh and Fwd in the complex with Hdr is unique. The results provide insight into electron flow during methanogenesis with formate, and supports the electron bifurcation mechanism for energy conservation during methanogenesis. A model for the role of the protein complex is presented in Fig. 3.
Fig. 3.
Model of complex of proteins that interact with Hdr. When H2 is used as the electron donor for methanogenesis, electrons are transferred to Hdr via Vhu. Flavin-mediated electron bifurcation at HdrA then results in reduction of the CoM-S-S-CoB heterodisulfide and a ferredoxin that is used by Fwd for the first step in methanogenesis. When hydrogen is limiting or is replaced by formate, Fdh is highly expressed (10) and incorporates into the complex. When formate is used as the electron donor for methanogenesis, electrons are transferred to Hdr from formate via Fdh. Fd(red), reduced ferredoxin; Fd(ox), oxidized ferredoxin.
Fdh–Hdr Interaction.
Previously, only the F420-nonreducing hydrogenase was thought to deliver electrons to Hdr. Hdr purified from M. marburgensis was observed to associate with an F420-nonreducing hydrogenase, but not with Fdh (15, 16). However, this organism is not known to grow with formate as the sole electron donor (17). The association of Fdh with Hdr suggests a pathway of electron flow to Hdr that does not involve H2. Electrons may flow from formate to Hdr via Fdh. In support of this hypothesis, we were able to eliminate Vhu and Vhc and retain rapid growth on formate, although growth was markedly decreased on H2 (Fig. 2). This finding contrasts with an unsuccessful attempt to delete vhuU in M. voltae (18), even under conditions of selenium starvation where this organism would theoretically generate a truncated VhuU peptide. However, in that study only H2, not formate, was used as the electron donor. To our knowledge, thus far our ΔvhuAU ΔvhcA mutant is unique among hydrogenotrophic methanogens in having a growth defect specifically on H2. Its phenotype supports a recent suggestion that H2 may not be a required intermediate for methanogenesis from formate in M. maripaludis (19), although it may still be needed for biosynthesis. Interestingly, most members of the Methanomicrobiales lack genes encoding the F420-nonreducing hydrogenases yet still grow well on H2; this suggests that there may still be other proteins that interact with Hdr and mediate electron transfer from H2 to Hdr for reduction of CoM-S-S-CoB in these organisms (perhaps the F420-reducing hydrogenase) (20).
It should be noted that in addition to providing electrons for Hdr during growth on formate, Fdh must also provide F420H2, which is required for at least one step in methanogenesis (methylene-H4MPT reductase), and which is also used by F420-dependent methylene-H4MPT dehydrogenase. It has been suggested that F420H2 could also donate electrons to Hdr, based on work in M. voltae that observed an interaction between Hdr and Fru in purified membrane fractions (14). However, deletion of Fru in M. maripaludis had no effect when cells were grown on formate, suggesting that F420H2:heterodisulfide oxidoreductase activity is not important under these conditions (2).
Hdr–Fwd Interaction.
Observations made over 30 years ago demonstrated that CH3-S-CoM addition to cell extracts stimulated CO2 reduction to methane (21). This phenomenon, termed the “RPG effect,” is only observed in methanogens that lack cytochromes. The Hdr–Fwd interaction found here can explain the RPG effect by invoking electron transfer to Fwd through the action of Hdr. In fact, an interaction between Hdr and Fwd was first proposed in 1988 (22) and was later implicated in potential flavin-based electron bifurcation by Hdr to drive the first step in methanogenesis (1). Because previous Hdr purifications did not show any interaction between Hdr and Fwd (15, 16), it is likely that the physical interaction between these two proteins is weak, and our method of purification, which involved minimal manipulation, was able to retain Fwd bound to Hdr. HdrA is known to contain one mole of FAD per mole of HdrA (15), and it is likely that, through flavin-based electron bifurcation, HdrA can reduce ferredoxin that is then used by Fwd. The FwdF subunit of Fwd is purified as part of the complex and is a predicted polyferredoxin (23); this may be the ferredoxin that mediates electron transfer to Fwd after bifurcation.
Conventional models of methanogenesis hold that reduced ferredoxin used to power the endergonic reduction of CO2 to formyl-MFR is generated via the action of Eha. Studies of formylmethanofuran dehydrogenase activity in M. marburgensis demonstrated that it copurified with a hydrogenase activity, but the identity of the hydrogenase, and any other interacting peptides, was not determined (24). However, Eha consumes membrane potential to generate reduced ferredoxin, leaving little or no net energy for ATP synthesis. CO2 reduction to formyl-MFR using electrons bifurcated by Hdr would retain membrane potential for ATP synthesis (1). The in vivo action of Eha may, therefore, be to generate reduced ferredoxin for anabolism, as is the case for the paralogous Ehb (25). However, it is tempting to speculate that under certain conditions Eha can still play a role in CO2 reduction to formyl-MFR. There is a well-characterized phenomenon in methanogens without cytochromes where growth is decoupled from methanogenesis under certain conditions (26). This may be ecologically beneficial in that rapid utilization of nutrients such as H2 can result in methanogens without cytochromes outcompeting other organisms in the environment. Electron flow from the Eha hydrogenase would result in a futile cycle where methane production and hydrogen consumption rates would increase. Reduction of ferredoxin by Eha may dominate at high partial pressures of H2 while reduction of ferredoxin by Hdr may dominate at low partial pressures.
Methods
Strain Construction.
Strains used in this study are described in Table 2. PCR primers and plasmids can be found in Table S3. MM901 was used as the background strain for all genetic manipulations. To construct MM901, which contains a deletion of the uracil phosphoribosyltransferase gene (Δupt), pBLPrt (4) was digested with MluI-XhoI followed by extension with Klenow (New England Biolabs) and blunt-end ligation to generate pBLPrtsmhpt. This vector was transformed into M. maripaludis S2 as described in ref. 4 and selected in McCas medium (4) containing 1 mg/mL neomycin followed by selection for a mutant containing the in-frame deletion of upt on medium containing 250 μg/mL 6-azauracil to resolve the merodiploid. MM901 was transformed with constructs derived from the suicide vector pCRUptNeo to make other markerless gene replacements. pCRUptNeo was constructed exactly as described for the suicide vector pCRPrtNeo (4), except the upt gene was amplified with Easy-A polymerase (Stratagene) and ligated into the appropriate vectors. To create genomic copies of HdrB1 or HdrB2 with a C-terminal 6×-His tag, the 3′ region of the gene for HdrB1 or HdrB2 was PCR amplified using Phusion DNA polymerase (Finnzymes) with primers encoding a 10-amino acid extension and blunt-end ligated to a PCR fragment derived from the downstream genomic region of the gene to place the primer-encoded His-tag at the 3′ end of the ORF. The fragment was ligated to XbaI-NotI digested PCRUptNeo. The resulting vector was transformed into strain MM901 as described (4), with selection of the mutant on McCas plates containing the 250 μg/mL 6-azauracil in place of 8-azahypoxanthine, to make strains MM1263 and MM1264. A genomic copy of FdhA1 was created with a 13-amino acid extension containing a 6×-His-tag in a deletion strain of the fdh2 locus. First, fdhA2B2 was deleted to generate MM1262 by PCR amplifying genomic regions flanking the genes with Herculase DNA polymerase (Stratagene), digesting the products with AscI and ligating them together. This construct was transferred to XbaI-NotI-digested pCRUptNeo and transformed into MM901, as described above. The FdhA1 gene was PCR-amplified and ligated into SpeI-AscI-digested pLCW40neo (11) upstream of a 6×-His tag to make pLCW40fdhA1. The region downstream of fdhA1 in S2 genomic DNA was PCR-amplified and blunt-end ligated to the 3′ end of the gene encoding the His-tag (PCR amplified from pLCW40fdhA1) and the construct was transferred into XbaI-NotI-digested pCRUptNeo. The resulting plasmid construct was then transformed as above into strain MM1262 to generate MM1265. The region encoding the vhuA and vhuU genes was deleted following the same procedure as the deletion of fdh2, except mutants with reduced growth on H2 were enriched once as described (27) in McCas medium (4), with 2.5 μg/mL puromycin and a headspace of H2/CO2, and colonies screened for the mutation were grown under a N2:CO2 atmosphere on formate medium (see below) containing 7.5 g/L noble agar and 250 μg/mL 6-azauracil. Deletion of vhcA in this background was done as described for deletion of fdh2, except cells were transformed on formate medium and merodiploids were selected with 5 mg/mL neomycin. The vhu vhc double mutant was designated MM1272. Deletion and His-tag constructs were verified by DNA sequencing and mutations were verified by PCR screens and Southern blot.
Table 2.
Strains
| Strain | Notes |
| S2 | Wild type M. maripaludis (34) |
| MM901 | S2 with an in frame deletion of the uracil phosphoribosyltransferase gene (Mmp0680) |
| MM1262 | MM901 with an in frame deletion of fdhA2B2 (Mmp0138 and Mmp0139) |
| MM1263 | MM901 with a 6x C-terminal Histidine tag on HdrB1 (Mmp1155) |
| MM1264 | MM901 with a 6x C-terminal Histidine tag on HdrB2 (Mmp1053) |
| MM1265 | MM1262 with a 6x C-terminal Histidine tag on FdhA1 (Mmp1298) |
| MM1272 | MM901 with an in frame deletion of the vhuAU and vhcA regions (Mmp1694, Mmp1693, and Mmp0823) |
Growth of Strains with Hydrogen Excess, Hydrogen Limitation, or Formate.
Cultures for each strain were grown in a chemostat under conditions of H2 limitation/phosphate excess or H2 excess/phosphate limitation as described in refs. 5 and 10 and modified in ref. 7. Cultures were grown until steady state was reached and OD660 remained stable at ~0.6 for >48 h. Cultures were then collected as described below. For growth with formate as the sole electron donor, cultures were grown in 400-mL batch culture at 37 °C with agitation at 100 rpm (Jeio Tech SK-600 shaker) with described medium (2) with 20 mM NH4Cl in place of casamino acids (formate medium). Cultures were grown for ~24 h to a final OD660 of ~0.4 to 0.5 and collected as described below.
Affinity Purification of Tagged Proteins.
Four-hundred milliliters from each chemostat culture was collected anaerobically as described (11), brought into an anaerobic chamber (Coy Laboratory Products), and transferred into a 0.5-L centrifuge bottle. Formate-grown cultures were brought directly into an anaerobic chamber and transferred to a 0.5-L centrifuge bottle. Samples were then centrifuged anaerobically at 4 °C at 12,800 × g for 25 min. The resulting cell pellet was suspended in 1 to 2 mL residual growth medium and placed in 5 mL glass tubes with an atmosphere of N2/H2 (95:5) and stored at −80 °C for up to 2 months. All purifications were done under anaerobic conditions in an atmosphere of N2/H2 (95:5) in an anaerobic chamber. Cells were thawed and sonicated on ice using a Microson ultrasonic cell disrupter at setting 8. Cell lysate was centrifuged at 16,000 × g for 10 min. The crude protein extract was collected and combined with 25 mM Hepes pH 7.5, 10 mM sodium dithionite, 100 mM NaCl, and 10 mM imidazole as binding buffer. Finally, 0.5 mL of Ni2+ resin (Novagen Inc.) was added and the mixture was incubated anaerobically at 37 °C while shaking for 1 h. After incubation, sample was placed in a Poly-Prep chromatography column (Bio-Rad) under the same atmosphere and the supernatant was allowed to run through the column. The resin was washed three times with 5 mL binding buffer, then eluted with the same buffer containing 100 mM (for HdrB1 and HdrB2) or 200 mM (for FdhA1) imidazole. After elution, samples were stored in elution buffer at −80 °C until ready for mass spectrometric analysis. SDS/PAGE of the purified proteins was done with 4 to 20% gradient gels (Pierce) and is shown in Fig. S2.
Mass Spectrometry of Purified Protein Samples.
After thawing, 100 μL of the sample was diluted with 100 μL of 10% acetonitrile in Millipore water containing sufficient trypsin (Promega sequencing grade) to make the final trypsin-protein ratio ≈1:3–1:10. A larger-than-normal quantity of trypsin was used because of the presence of dithionite in the elution buffer. After digestion at 37 °C for 12 h, samples were placed in a Speed-Vac (Jouan RTC60) to bring final volumes to 50 μL. Capillary HPLC/tandem mass spectrometry was performed in a data-dependent manner using a single dimension separation with a Michrom Magic 2002 HPLC modified in-house (6) for capillary operation and interfaced to a Thermo LTQ linear ion-trap mass spectrometer. The samples were loaded on a 10 cm × 75 μm intradermal Aqua C18 reversed-phase capillary column fabricated in-house, flushed 15 min with Millipore water for desalting, then eluted with a binary gradient as reported previously for the reversed phase portion of a 2D separation (6, 7). The raw data files were searched against the M. maripaludis inferred protein database (6, 7, 9) using Sequest (28) and peptide level results were organized at the protein level using DTASelect (29), such that all redundant identifications were saved, thus allowing a summation of the spectral counts associated with each protein-encoding ORF in the database. All Sequest and DTASelect adjustable parameters were set as described (6, 7).
Analysis of Mass Spectral Data.
SCs [numbers of peptide detections for a given protein, i.e., spectral counts (30–32)] have been established as an accurate method for measuring relative protein abundance in M. maripaludis (7). For each protein sample analyzed, SCs were tabulated for all proteins that had a SC of at least 10 in at least one sample. Three proteins that were detected in all samples and that did not appear enriched in the experimental strains relative to the control strains were chosen as a basis for normalization (reference proteins). For each protein detected, ratios of SCs in each experimental sample (MM1263, MM1264, or MM1265) to SCs in the control sample (MM901 or MM1262) grown under the same condition were then calculated using each reference protein as follows: normalized ratio = (SCproteinexp SCproteincont)/(SCrefexp SCrefcont).
Analysis of the vhuAU vhcA Mutant During Growth on H2/CO2 or Formate.
MM901 or MM1272 was grown to OD660 ~0.6 in formate medium. Cultures were washed once with 5 mL N-free medium (33) and ~0.5 mL was transferred to tubes containing 5 mL either McCas with a headspace of H2/CO2 (80:20) at 40 psi or formate medium with 0.2% casamino acids and a headspace of N2/CO2 (80:20) at 30 psi and grown at 37 °C at 100 rpm agitation (Jeio Tech SK-600 shaker). Cell density (OD660) was monitored.
Supplementary Material
Acknowledgments
We thank Dan Park, Sujung Lim, and Brian Moore for assistance in the laboratory, and Ulf Neiss and Erik Hendrickson for helpful advice and discussion. This work was funded by the US Department of Energy Office of Basic Energy Sciences, Basic Research for the Hydrogen Fuel Initiative, Grant DE-FG02-05ER15709. K.C.C. was supported by the University of Washington National Science Foundation Integrative Graduate Education Research Traineeship program in Astrobiology, Grant DGE-0504219.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003653107/-/DCSupplemental.
References
- 1.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic Archaea: Ecologically relevant differences in energy conservation. Nat Rev Microbiol. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson EL, Leigh JA. Roles of coenzyme F420-reducing hydrogenases and hydrogen- and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J Bacteriol. 2008;190:4818–4821. doi: 10.1128/JB.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumbula DL, Whitman WB. Genetics of Methanococcus: Possibilities for functional genomics in Archaea. Mol Microbiol. 1999;33:1–7. doi: 10.1046/j.1365-2958.1999.01463.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore BC, Leigh JA. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J Bacteriol. 2005;187:972–979. doi: 10.1128/JB.187.3.972-979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haydock AK, Porat I, Whitman WB, Leigh JA. Continuous culture of Methanococcus maripaludis under defined nutrient conditions. FEMS Microbiol Lett. 2004;238:85–91. doi: 10.1016/j.femsle.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Xia Q, et al. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol Cell Proteomics. 2006;5:868–881. doi: 10.1074/mcp.M500369-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Q, et al. Quantitative proteomics of nutrient limitation in the hydrogenotrophic methanogen Methanococcus maripaludis. BMC Microbiol. 2009;9:149. doi: 10.1186/1471-2180-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann N, et al. A cysteine-rich CCG domain contains a novel [4Fe-4S] cluster binding motif as deduced from studies with subunit B of heterodisulfide reductase from Methanothermobacter marburgensis. Biochemistry. 2007;46:12875–12885. doi: 10.1021/bi700679u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickson EL, et al. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J Bacteriol. 2004;186:6956–6969. doi: 10.1128/JB.186.20.6956-6969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrickson EL, Haydock AK, Moore BC, Whitman WB, Leigh JA. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc Natl Acad Sci USA. 2007;104:8930–8934. doi: 10.1073/pnas.0701157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodsworth JA, Leigh JA. Regulation of nitrogenase by 2-oxoglutarate-reversible, direct binding of a PII-like nitrogen sensor protein to dinitrogenase. Proc Natl Acad Sci USA. 2006;103:9779–9784. doi: 10.1073/pnas.0602278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedderich R, Berkessel A, Thauer RK. Purification and properties of heterodisulfide reductase from Methanobacterium thermoautotrophicum (strain Marburg) Eur J Biochem. 1990;193:255–261. doi: 10.1111/j.1432-1033.1990.tb19331.x. [DOI] [PubMed] [Google Scholar]
- 13.Berghöfer Y, Agha-Amiri K, Klein A. Selenium is involved in the negative regulation of the expression of selenium-free [NiFe] hydrogenases in Methanococcus voltae. Mol Gen Genet. 1994;242:369–373. doi: 10.1007/BF00281785. [DOI] [PubMed] [Google Scholar]
- 14.Brodersen J, Gottschalk G, Deppenmeier U. Membrane-bound F420H2-dependent heterodisulfide reduction in Methanococcus voltae. Arch Microbiol. 1999;171:115–121. doi: 10.1007/s002030050686. [DOI] [PubMed] [Google Scholar]
- 15.Setzke E, Hedderich R, Heiden S, Thauer RK. H2: Heterodisulfide oxidoreductase complex from Methanobacterium thermoautotrophicum. Composition and properties. Eur J Biochem. 1994;220:139–148. doi: 10.1111/j.1432-1033.1994.tb18608.x. [DOI] [PubMed] [Google Scholar]
- 16.Stojanowic A, Mander GJ, Duin EC, Hedderich R. Physiological role of the F420-non-reducing hydrogenase (Mvh) from Methanothermobacter marburgensis. Arch Microbiol. 2003;180:194–203. doi: 10.1007/s00203-003-0577-9. [DOI] [PubMed] [Google Scholar]
- 17.Wasserfallen A, Nölling J, Pfister P, Reeve J, Conway de Macario E. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int J Syst Evol Microbiol. 2000;50:43–53. doi: 10.1099/00207713-50-1-43. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer M, Bestgen H, Bürger A, Klein A. The vhuU gene encoding a small subunit of a selenium-containing [NiFe]-hydrogenase in Methanococcus voltae appears to be essential for the cell. Arch Microbiol. 1998;170:418–426. doi: 10.1007/s002030050662. [DOI] [PubMed] [Google Scholar]
- 19.Lupa B, Hendrickson EL, Leigh JA, Whitman WB. Formate-dependent H2 production by the mesophilic methanogen Methanococcus maripaludis. Appl Environ Microbiol. 2008;74:6584–6590. doi: 10.1128/AEM.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thauer RK, et al. Hydrogenases from Methanogenic Archaea, nickel, a novel cofactor, and H2 storage. Annu Rev Biochem. 2010 doi: 10.1146/annurev.biochem.030508.152103. 10.1146/annurev.biochem.030508.152103. [DOI] [PubMed] [Google Scholar]
- 21.Gunsalus RP, Wolfe RS. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977;76:790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- 22.Rouvière PE, Wolfe RS. Novel biochemistry of methanogenesis. J Biol Chem. 1988;263:7913–7916. [PubMed] [Google Scholar]
- 23.Hochheimer A, Schmitz RA, Thauer RK, Hedderich R. The tungsten formylmethanofuran dehydrogenase from Methanobacterium thermoautotrophicum contains sequence motifs characteristic for enzymes containing molybdopterin dinucleotide. Eur J Biochem. 1995;234:910–920. doi: 10.1111/j.1432-1033.1995.910_a.x. [DOI] [PubMed] [Google Scholar]
- 24.Wasserfallen A. Formylmethanofuran synthesis by formylmethanofuran dehydrogenase from Methanobacterium thermoautotrophicum Marburg. Biochem Biophys Res Commun. 1994;199:1256–1261. doi: 10.1006/bbrc.1994.1366. [DOI] [PubMed] [Google Scholar]
- 25.Porat I, et al. Disruption of the operon encoding Ehb hydrogenase limits anabolic CO2 assimilation in the archaeon Methanococcus maripaludis. J Bacteriol. 2006;188:1373–1380. doi: 10.1128/JB.188.4.1373-1380.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Poorter LM, Geerts WJ, Keltjens JT. Coupling of Methanothermobacter thermautotrophicus methane formation and growth in fed-batch and continuous cultures under different H2 gassing regimens. Appl Environ Microbiol. 2007;73:740–749. doi: 10.1128/AEM.01885-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladapo J, Whitman WB. Method for isolation of auxotrophs in the methanogenic archaebacteria: Role of the acetyl-CoA pathway of autotrophic CO2 fixation in Methanococcus maripaludis. Proc Natl Acad Sci USA. 1990;87:5598–5602. doi: 10.1073/pnas.87.15.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectra of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 29.Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: Tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Opiteck GJ, Friedrichs MS, Dongre AR, Hefta SA. Changes in the protein expression of yeast as a function of carbon source. J Proteome Res. 2003;2:643–649. doi: 10.1021/pr034038x. [DOI] [PubMed] [Google Scholar]
- 31.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 33.Lie TJ, Leigh JA. Regulatory response of Methanococcus maripaludis to alanine, an intermediate nitrogen source. J Bacteriol. 2002;184:5301–5306. doi: 10.1128/JB.184.19.5301-5306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitman WB, Shieh J, Sohn S, Caras DS, Premachandran U. Isolation and characterization of 22 mesophilic methanococci. Syst Appl Microbiol. 1986;7:235–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.