Abstract
Death by apoptosis shapes tissue homeostasis. Apoptotic mechanisms are so universal that harnessing them for tailored immune intervention would seem challenging; however, the range and different expression levels of pro- and anti-apoptotic molecules among tissues offer hope that targeting only a subset of such molecules may be therapeutically useful. We examined the effects of the drug ABT-737, a mimetic of the killer BH3 domain of the Bcl-2 family of proteins that induces apoptosis by antagonizing Bcl-2, Bcl-XL, and Bcl-W (but not Mcl-1 and A1), on the mouse immune system. Treatment with ABT-737 reduced the numbers of selected lymphocyte and dendritic cell subpopulations, most markedly in lymph nodes. It inhibited the persistence of memory B cells, the establishment of newly arising bone marrow plasma cells, and the induction of a cytotoxic T cell response. Preexisting plasma cells and germinal centers were unaffected. Notably, ABT-737 was sufficiently immunomodulatory to allow long-term survival of pancreatic allografts, reversing established diabetes in this model. These results provide an insight into the selective mechanisms of immune cell survival and how this selectivity avails a different strategy for immune modulation.
Keywords: apoptosis, immunity, memory, transplantation
The central tenet for understanding the development and activation of the immune system is that it maximizes reactivity to foreign material while minimizing reactivity to self. Immune tolerance, however, is not always established or maintained, nor is immune reactivity to foreign antigens always desirable. Autoimmune diseases, for example, are situations where tolerance of self has broken down, and an overactive immune response triggers pathology by targeting particular cell types or proteins. Conversely, immune responses to allogeneic organ grafts, although appropriate within the foreign-self dichotomy, are clinically deleterious. In both settings, autoimmune diseases and graft rejection, agents able to modulate the acquired immune system, are likely to be of considerable clinical utility. Several immuno-suppressive drugs are in use: for example, methotrexate in autoimmune diseases like rheumatoid arthritis and calcineurin inhibitors (cyclosporin, tacrolimus) and mycophenolate mofetil and mTOR inhibitors (rapamycin) in transplantation. Although very useful, many such drugs have unwanted side effects and more selective drugs are continuously being sought (1, 2).
Programmed cell death, or apoptosis, plays a central role during development of immune cells and for maintaining tissue homeostasis; it shapes the immune repertoire and refines and terminates immune responses (3). Whether a cell survives or dies by apoptosis is determined in large part by the interaction between anti- and proapoptotic proteins (Fig. S1). The anti-apoptotic proteins (e.g., Bcl-2, Bcl-XL, Bcl-W, A1, and Mcl-1) share primary sequence homology in multiple Bcl-2-homology (BH) domains, whereas the proapoptotic proteins can be further subdivided into the multidomain proteins (e.g., Bax and Bak) or ones that only bear similarity in the BH3 region. These BH3-only proteins (e.g., Bad, Bim, Noxa, and Puma) can be induced to bind the prosurvival Bcl-2 family members, thereby removing the restraints on proapoptotic Bax and Bak that, in turn, mediate apoptosis by permeabilizing the outer mitochondrial membrane (4, 5).
ABT-737, a small molecule BH-3 mimetic compound discovered and developed by Abbott Laboratories as an antitumor agent, induces apoptosis by selectively inhibiting the anti-apoptotic proteins Bcl-2, Bcl-XL, and Bcl-W (6). In vitro ABT-737 is cytotoxic as a single agent for many primary clinical samples of B lymphoid tumors and chronic lymphocytic leukemia (CLL) as well as small cell lung cancer cell lines (6). Because ABT-737 does not bind Mcl-1 or A1, it is not surprising that expression levels of Mcl-1 within cells correlates with resistance to ABT-737 (7, 8) and that A1 expression also promotes resistance (8).
Herein we report effects of ABT-737 treatment on leukocyte populations in the mouse immune system in both steady-state and model systems of activation. Treatment with ABT-737 in vivo selectively reduced peripheral leukocyte populations, inhibited humoral immunity, and dampened a CTL response. Interestingly, ABT-737 was sufficiently immunomodulatory to protect islet allografts from immune-mediated rejection, allowing reversal of established diabetes in this model. These results provide insight into the survival mechanisms of immune cells and herald the use of BH3 mimetics as a unique class of immunomodulatory drugs based on selective apoptosis for B and T cell-targeted therapeutics.
Results
The BH3 Mimetic Compound ABT-737 Reduces the Numbers in Selected Subsets of Peripheral Immune Cells.
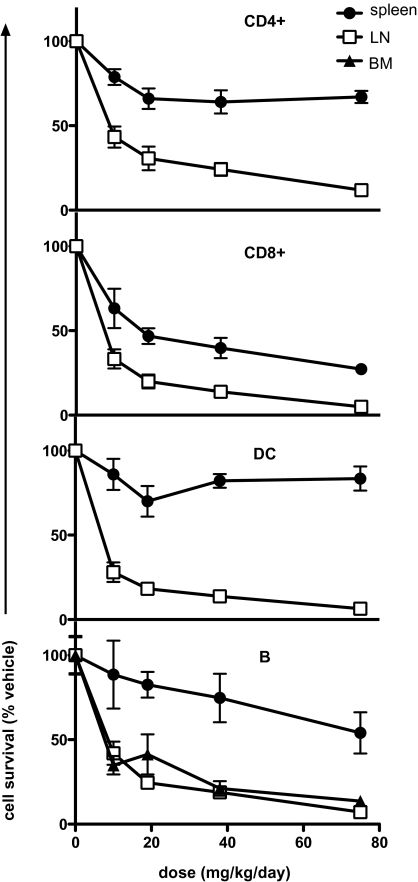
An initial examination of the in vivo response of leukocytes to extended exposure (14 d) to ABT-737 revealed significant reduction of T cells (both CD4 and CD8) and B cells in all tissues examined and DC, but only in LN (Fig. S2). NK cells and granulocytes were resistant to drug treatment (Fig. S2), consistent with their dependence on Mcl-1 and/or A1 for survival (9, 10). The sensitivity of immune cells to ABT-737 was assessed by titrating the drug by dose from 10 to 75 mg/kg per day for 14 d, which revealed that if a cell type was sensitive to ABT-737, then such sensitivity was apparent even at the lowest dose used (Fig. 1). The drug effects were less pronounced in the spleen, where CD8+ T cells, CD4+ T cells, and B cells were reduced to 30%, 60%, and 60%, respectively, of original cell numbers with the highest dosage used compared with a reduction of all cell types in lymph nodes to <10% (Fig. 1). The time-course of responsiveness to ABT-737 revealed the maximum effect in all sensitive subsets to be after 5 d of daily dosing at 75 mg/kg per day. This response was maintained throughout the course of treatment, 14 d in this instance (Fig. S2). After cessation of drug treatment, T and B lymphocyte cellularity rapidly recovered, albeit slower in LN than spleen (Fig. S2). Collectively, these results suggest substantial dependence of B, T, and DC cell types on “Bcl-2-like” (Bcl-2, Bcl-XL, Bcl-W) prosurvival proteins, with some variation depending on tissue localization.
Fig. 1.
Selective reduction in immune cell subsets induced by in vivo ABT-737 treatment is proportional to drug dosage. B6 mice were treated daily with ABT-737 (10, 19, 38, or 75 mg/kg) or vehicle (0 mg/kg) for 14 consecutive d (n = 6). After treatment, LN, spleen, and BM were recovered and the proportion of T cells (CD4+,CD8+), B cells, and DC remaining in the organs was determined by flow cytometry. All data are shown as a proportion (%) of the average number of cell subsets isolated from vehicle-treated mice, with means ± SE from individual drug-treated mice.
To discount the possibility that the effects on the immune system by ABT-737 could be due to off-target effects, we enumerated immune cells that were Bax- and Bak-deficient. Because doubly deficient mice die prenatally, we reconstituted irradiation chimeras with doubly deficient fetal liver. Leukocytes from such chimeric mice whose hemopoietic cells lacked Bax and Bak were insensitive to ABT-737 (Fig. S3), consistent with the premise that ABT-737 acts directly on wild-type cells through the Bax/Bak-induced apoptotic pathway (7, 8).
ABT-737 Differentially Affects T Cell Subsets in LN and Spleen.
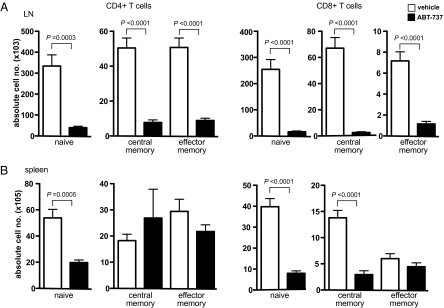
To determine whether T cell sensitivity to ABT-737 treatment was predicated on the maturation or differentiation state of a T cell, C57BL/6 mice were treated for 14 consecutive d with either ABT-737 (75 mg/kg) or vehicle control. Spleen and LN were recovered, and the numbers of naïve (CD62Lhi CD44lo), central memory (CD62Lhi CD44hi), and effector memory (CD62Llo CD44hi) cells were determined by flow cytometry. All naïve and memory T cells (CD4+ and CD8+) in LN were significantly reduced by ABT-737 treatment (Fig. 2A). In contrast, whereas all naïve cells and CD8+ central memory T cells were effectively reduced by ABT-737 in spleen, central and effector memory CD4+, and effector memory CD8+ T cell remained refractory to ABT-737 treatment (Fig. 2B).
Fig. 2.
In vivo ABT-737 treatment affects all naïve and memory T cells in LN, while sparing central memory and effector memory CD4+, and effector memory CD8+ T cell subsets in spleen. B6 mice were treated daily for 14 consecutive d with either ABT-737 (75 mg/kg) or vehicle control (n = 5–6). After treatment, LN (A) and spleen (B) were recovered and the number of naïve (CD62Lhi CD44lo), central memory (CD62Lhi CD44hi), and effector memory (CD62Llo CD44hi) CD4+ and CD8+ T cell subsets determined by flow cytometry. All data are expressed as the means ± SE from individual mice.
ABT-737 Inhibits CTL and B Cell Responses in Vivo.
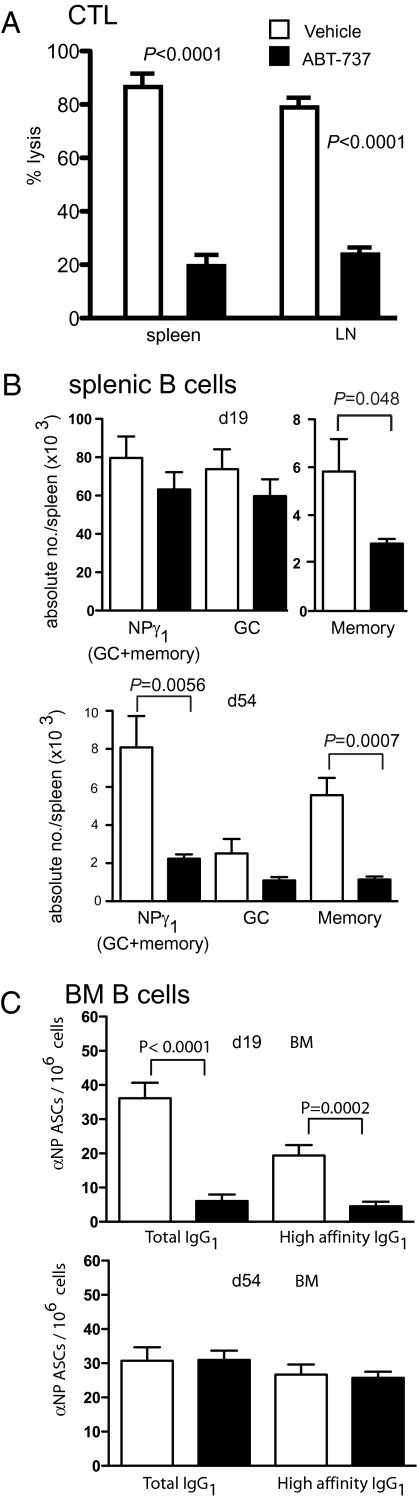
Having established the impact of ABT-737 on the steady-state immune system, we next examined its effects on the development of specific immune responses. C57BL/6 mice were treated daily for a week with either ABT-737 (75 mg/kg) or vehicle control, and on treatment day 6, mice were primed with ovalbumin (OVA) antigen in the form of irradiated OVA-coated H-2Kb−/− splenocytes (OCS), a protocol known to induce CTL. Seven days after T cell priming, in vivo CTL responses were assayed by measuring the persistence in spleen and LN of OVA peptide-pulsed target cells, CFSE labeled, and injected intravenously. Mice treated with ABT-737 showed significantly less OVA-specific CTL activity, with an approximately 4-fold reduction in specific target lysis when compared with vehicle treated controls (Fig. 3A).
Fig. 3.
In vivo CTL and B cell responses against exogenous antigen are inhibited by ABT-737 treatment. (A) B6 mice were treated daily for 14 consecutive d with either ABT-737 (75 mg/kg) (n = 8) or vehicle control (n = 8). On treatment day 6, mice were primed in vivo with irradiated, ovalbumin-coated splenocytes (OCS) and endogenous in vivo CTL responses measured in spleen and lymph node after 7 d. Means ± SE are shown. (B) B6 mice were immunized with NP-KLH in alum and then treated with ABT-737 (75 mg/kg) or vehicle control for 14 consecutive d starting either day 5 or day 40 after immunization. Spleens were analyzed for NP-specific GC or memory B cells on day 19 (Upper) or 54 (Lower). (C) Frequencies of total (NP20) and high affinity (NP2) NP-specific IgG1 ASCs in BM on day 19 (Upper) or 54 (Lower) after immunization. Data are the average means ± SE of between six to eight mice for each group at each time point.
We next assessed the ability of ABT-737 treatment to alter B cell immune responses by using the T cell dependent antigen (4-hydroxy-3-nitrophenyl acetyl coupled to carrier protein keyhole limpet hemocyanin; NP-KLH). Mice were immunized with alum-adjuvanted NP-KLH i.p. and then treated with ABT-737 (75 mg/kg) or vehicle control for 14 consecutive d, starting 5 d after immunization. On day 19 after immunization, the numbers of NP-specific B cell subsets [germinal center (GC), memory, and plasma cells] were quantified. Antigen-specific B cells were detected and partitioned into GC and memory compartments by flow cytometry on the basis of surface staining for B220, NP, IgG1, and CD38 (Fig. S4). This analysis revealed memory B cells to be susceptible to ABT-737, whereas GC B cells were refractory (Fig. 3B). To determine whether the memory cells were sensitive during formation or maintenance, mice were immunized and memory was allowed to develop before ABT-737 treatment was started at day 40 after immunization. The mice were analyzed after 14 d of treatment with ABT-737 or vehicle, i.e., day 54 after immunization. The memory B cell compartment was still affected by ABT-737, indicating that these B cells, once produced, depend on the “Bcl-2-like” survival proteins (Fig. 3B).
Antigen-specific antibody secreting cells (ASC) are also generated during the B cell response to antigen. In the later stages of T cell-dependent immune responses, ASC originate in the GC, then migrate to the bone marrow (BM), where they compete for access to survival niches to become long-lived plasma cells (11). When immunized mice were treated with ABT-737 or vehicle control starting on day 5 of the response, the frequency of antigen-specific IgG1 ASC in the spleen was significantly reduced, although interestingly not for the high affinity IgG1-secreting cells (Fig. S4). In the BM however, there was a marked reduction in the frequency of both total and high affinity NP-specific ASC (Fig. 3C). These data indicate that ABT-737 blocks formation of an antigen-specific plasma cell compartment in the BM but not the spleen. Interestingly, when the mice were treated starting day 40 after immunization, by which time a BM plasma cell compartment had formed, there was no reduction in the frequency of ASC in the BM (Fig. 3C) or the spleen (Fig. S4), suggesting that established plasma cells were resistant to ABT-737. Surprisingly, the frequency of antigen-specific IgG1 ASC in the spleens of day 40-treated mice increased (Fig. S4), a phenomenon that is underinvestigated.
Treatment with ABT-737 Protects Pancreatic Islet Allografts from Immune-Mediated Rejection.
We opined that the reduction in immune cell numbers triggered by ABT-737 might ameliorate graft rejection. To test this possibility, spontaneously diabetic (50-1/CBA; H-2k) mice were treated daily for 5 consecutive d with either ABT-737 (50 mg/kg) or vehicle control before receiving a fully allogenic pancreatic islet graft [(C57BL/6 × SJL)F1; H-2b,s]. Treatment was continued daily for 9 d after transplantation. Islet graft function was monitored by diabetes reversal, determined by measuring blood glucose levels. Whereas transplant recipients receiving vehicle treatment consistently rejected their islet grafts by 21 d after transplantation, remarkably all recipients treated with ABT-737 had superior control of their blood glucose levels than their untreated counterparts, indicating prolonged graft survival (Fig. 4).
Fig. 4.
Allogenic islet graft rejection is ameliorated by in vivo ABT-737 treatment. 50-1/CBA nonautoimmune diabetic mice were treated daily for 14 consecutive d with either ABT-737 (50 mg/kg) or vehicle control. On treatment day 6, mice received an allogenic graft of F1 (B6 × SJL) islets transplanted under the kidney capsule. Blood glucose was measured at various times after transplantation. Grafts were deemed successful if normal blood glucose (<15 mmol/L) levels were achieved within 4 d after transplantation. Islets grafts transplanted into ABT-737–treated recipients functioned significantly longer than grafts transplanted into vehicle control-treated recipients (no icon) (P < 0.002 as determined by a log-rank test).
Discussion
Mimetics of proapoptotic proteins have generated great clinical interest for treating certain cancers (12, 13); ABT-737 is prototypic of such drugs. There are several anti-apoptotic molecules, and different tissues express varying levels of each of these. Thus, the great potential of the BH3 mimetics to specifically antagonize only certain anti-apoptotic Bcl-2 proteins means that they have selective effects on differing tissues. In our case, ABT-737 appears to have selective effects not only on the immune system but also within various compartments of the immune system, presumably reflecting differential usage of anti-apoptotic proteins in these cells and locations (8, 9). As such, ABT-737 leads to a reduction of lymphocytes and DCs, especially in LN, and affects newly arising immune responses (e.g., affecting the formation of new antibody-producing cells without affecting long-term plasma cell memory in BM; affecting CTL induction in the spleen but sparing effector memory CD8 T cells in the spleen). We considered these features as auspicious for ABT-737 modulating transplantation rejection where transplantation antigens are assumed to prime immune responses in draining LN. Indeed islet allograft survival was prolonged by ABT-737 treatment. These immunological and transplantation findings indicate that BH3 mimetics with specificity against certain anti-apoptotic proteins (in this case, the Bcl-2 family members: Bcl-2, Bcl-XL, and Bcl-W) form a unique class of immunomodulatory compounds.
Whereas some immunomodulatory agents such as corticosteroids are pleiotropic in their effects, ABT-737 appears to have a selective action on the immune system that is directed primarily at cells of the adaptive immune response (T and B lymphocytes), leaving innate cell populations (granulocytes and NK cells) intact. Although all T cells are targeted by ABT-737 in the LN, CD4+ central and effector memory T cells and CD8+ central memory T cell populations within the spleen remain unaffected, allowing some acquired immunity to persist. A similar dichotomy in the B cell compartment was observed where memory B cells in the spleen were affected, as were short-lived plasma cells in transit, but long-lived plasma cells once in the BM were spared.
Modulating the immune response is advantageous in treating autoimmune disease and ameliorating transplant rejection. The advent of cyclosporin, a calcineurin inhibitor that can suppress T cell function without myelosuppression, revolutionized the field of transplantation (14, 15). Cyclosporin and other modern immunosuppressive drugs such as FK506 (another calcineurin inhibitor), mycophenolate mofetil (affecting the purine salvage pathway), and rapamycin (mTor inhibitor) nevertheless increase the risk of life-threatening or lifestyle-restricting infections and malignancy (16). Some like rapamycin can be myelosuppressive (1). Moreover, many of these drugs are toxic to tissues being transplanted (e.g., pancreatic islets, kidneys), making organ toxicity one of the key limitations for long-term graft survival (beyond 5 y) (2, 17). As such, these drugs are almost always used in combination and the optimal combinatorial regimens are still being tailored (18). Therefore, efficacious drugs with improved target specificity continue to be sought.
Our data lead us to conclude that BH3 mimetics like ABT-737, with selective effects on lymphocyte and dendritic cell populations, are a unique class of immunomodulatory drugs. They will add to the list of potentially useful transplant drugs or, indeed, may replace some of them, either as a single agent or when used in combination. Both this report and another (6) involving in vivo usage of ABT-737 show it to be well tolerated. In terms of using these compounds in organ transplantation, it is interesting to note that human pancreatic islets express high amounts of Mcl-1 and may be thus protected from BH3-mimetic-induced apoptosis (19).
In conclusion, we aver that ABT-737 is a unique class of immunomodulatory drug whose mechanism of action is antagonizing the Bcl-2 proteins and which shows selectivity to newly arising immune responses, thus cogently warranting its further clinical evaluation. ABT-737 may represent the next stage in developing efficacious and safe immunomodulatory therapy that not only prevents allograft rejection, but may also mollify autoimmune disease or immunopathology.
Methods
Mice, Reagents, and Immunization.
C57BL/6 (B6), BALB/c, SJL, B6.CD45.1, Rag.CD45.1, H-2Kb−/−, and RIP-H-2Kb (50-1/CBA) have been described (20, 21). Eight- to 10-wk C57BL/6 (B6) mice were used for all drug treatment experiments. Bax−/−Bak−/− mice were generated by reconstitution of irradiated recipients with embryonic day (E)14.5 fetal liver cells as described (22–24). All mice were housed under specific pathogen free (SPF) conditions at The Walter and Eliza Hall Institute of Medical Research and were handled according to guidelines approved by the institutional Animal Ethics Committee. ABT-737 (Abbott Laboratories) or vehicle control was prepared and administered at 75 mg/kg (unless specified) as a daily i.p. injection for up to 14 consecutive d as described (25). Immunization for B cell assays comprised a single i.p. injection of 100 μg of NP coupled to KLH at a ratio of 27:1 and precipitated onto alum (26).
Cell Subset Analysis, Antibodies, and Flow Cytometry.
Single-cell suspensions were prepared from spleen, inguinal lymph node, and femur. Spleen and lymph node suspensions were prepared by digestion in Collagenase/Dnase-1 as described (27). Blood was collected by cardiac puncture. Red cells in blood, spleen, and BM were lysed in 0.156 M NH4Cl. Anti-CD4, CD8, CD11c, Thy1.2, CD19, CD23, CD21, CD38, B220, CD45.2, IgM, IgD, IgG1, FcγR, Mac-1, NK1.1, CD62L, CD44, Streptavidin (BD Biosciences), Gr-1 (in house), and a conjugate of NP to phycoerythrein, made as described (28), were used to identify leukocyte subsets by flow cytometry, with absolute cell numbers determined by live cell count in 0.2% Trypan blue (Sigma) or addition of fluorchrome-conjugated beads (BD Biosciences) directly to samples. NP binding was detected as described (28).
Enzyme-Linked Immunospot (ELISPOT) Assay.
The frequency of ASC was determined as described (26, 29). Cells were incubated O/N at 37 °C on precoated 96-well MultiScreen-HA filter plates (Millipore). Spots were visualized with IgG1-specific goat anti-mouse antibodies conjugated to horseradish peroxidase (Southern Biotechnology Associates), and color was developed with 3-amino-9-ethyl carbazole (Sigma). Plates were washed extensively, and spots were counted with an AID ELIspot reader system (Autoimmun Diagnostika).
In Vivo CTL Assay.
The induction of CTL was determined essentially as described (30). Mice were primed with 2 × 107 irradiated, OVA-coated splenocytes isolated from MHC H-2Kb−/− mice, with 1 μg of lipopolysaccharide, IV. Seven days after T cell priming, mice were injected with 2 × 107 1:1 mix of SIINFEKL (Mimotopes) peptide-pulsed B6 splenocytes labeled CFSE high, and unpulsed control splenocytes labeled CFSE low, IV. After 18 h, spleen and inguinal lymph node were recovered and specific lysis of target cells determined by flow cytometry.
Pancreatic Islet Isolation and Transplantation.
Pancreatic islets were isolated by collagenase digestion and purified on a Histopaque-1077 (Sigma) density gradient as described (31). Viable islets were handpicked and cultured overnight in DME supplemented with 10% FCS at 37 °C; 10% CO2. Allogenic islet grafts were performed between donor and recipient mice mismatched for both class I and class II major histocompatibility complex (MHC) antigens. Four hundred donor islets (SJL × B6) (F1) were grafted under the kidney capsule of 7- to 12-wk-old nonimmune diabetic 50–1/CBA recipients. Blood glucose was measured via tail vein bleed at 1, 3, and 5 d after transplantation, and then weekly intervals to monitor initial diabetes reversal and then graft rejection. Grafts were deemed successful if normal blood glucose (<15 mM) was achieved within 4 d after transplantation.
Statistical Analysis.
Statistical analysis was performed by using GraphPad Prism software (GraphPad Software). A Student's t test was used to compare two sets of data. Graft survival was measured by a log-rank test. Data are shown as means ± SE where applicable, with a P < 0.05 considered statistically significant.
Supplementary Material
Acknowledgments
We thank Ms. Nicole Ashman for expert assistance and diligent care of mice. We thank the National Health & Medical Research Council, Juvenile Diabetes Research Foundation, Diabetes Australia, Rebecca Cooper Foundation, the Victorian State Government, the Leukemia Lymphoma Society, the Cancer Council of Victoria, and the Victorian Cancer Agency for financial support. I.B.V. is supported by a fellowship from the Olle Engkvist Byggmastare Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005256107/-/DCSupplemental.
References
- 1.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(Suppl 1):3–10. doi: 10.2165/00003495-200868001-00002. [DOI] [PubMed] [Google Scholar]
- 3.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 4.Green DR. Apoptotic pathways: Ten minutes to dead. Cell. 2005;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 6.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 7.Konopleva M, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: Regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- 11.Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Cragg MS, et al. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651–3659. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse C, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 14.Kahan BD. Cyclosporine: A revolution in transplantation. Transplant Proc. 1999;31(1-2A):14S–15S. doi: 10.1016/s0041-1345(98)02074-0. [DOI] [PubMed] [Google Scholar]
- 15.Ptachcinski RJ, Burckart GJ, Venkataramanan R. Cyclosporine. Drug Intell Clin Pharm. 1985;19:90–100. doi: 10.1177/106002808501900202. [DOI] [PubMed] [Google Scholar]
- 16.Helderman JH, Goral S. Gastrointestinal complications of transplant immunosuppression. J Am Soc Nephrol. 2002;13:277–287. doi: 10.1681/ASN.V131277. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro AM, et al. Strategic opportunities in clinical islet transplantation. Transplantation. 2005;79:1304–1307. doi: 10.1097/01.tp.0000157300.53976.2a. [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H. Calcineurin inhibitor sparing in renal transplantation. Transplantation. 2008;86:761–767. doi: 10.1097/TP.0b013e3181856f39. [DOI] [PubMed] [Google Scholar]
- 19.Kobayash H, et al. Immunohistochemical analysis of apoptosis-related proteins in human embryonic and fetal pancreatic tissues. Int J Pancreatol. 2000;27:113–122. doi: 10.1385/ijgc:27:2:113. [DOI] [PubMed] [Google Scholar]
- 20.Pérarnau B, et al. Single H2Kb, H2Db and double H2KbDb knockout mice: Peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland RM, et al. Bcl-2 protection of islet allografts is unmasked by costimulation blockade. Transplantation. 2004;77:1610–1613. doi: 10.1097/01.tp.0000132283.95107.9c. [DOI] [PubMed] [Google Scholar]
- 22.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 23.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 25.Mason KD, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 28.Ridderstad A, Tarlinton DM. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J Immunol. 1998;160:4688–4695. [PubMed] [Google Scholar]
- 29.Lalor PA, Nossal GJ, Sanderson RD, McHeyzer-Williams MG. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22:3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 30.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 31.Liu M, Shapiro ME. A new method for isolation of murine islets with markedly improved yields. Transplant Proc. 1995;27:3208–3210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.