Abstract
Regulation of the actin-myosin cytoskeleton plays a central role in cell migration and cancer progression. Here, we report the discovery of a cytoskeleton-associated kinase, pseudopodium-enriched atypical kinase 1 (PEAK1). PEAK1 is a 190-kDa nonreceptor tyrosine kinase that localizes to actin filaments and focal adhesions. PEAK1 undergoes Src-induced tyrosine phosphorylation, regulates the p130Cas-Crk-paxillin and Erk signaling pathways, and operates downstream of integrin and epidermal growth factor receptors (EGFR) to control cell spreading, migration, and proliferation. Perturbation of PEAK1 levels in cancer cells alters anchorage-independent growth and tumor progression in mice. Notably, primary and metastatic samples from colon cancer patients display amplified PEAK1 levels in 81% of the cases. Our findings indicate that PEAK1 is an important cytoskeletal regulatory kinase and possible target for anticancer therapy.
Keywords: cancer, cell migration, phosphoproteomics
All cells have the fundamental ability to change shape and to extend membrane projections from the cell surface (1). This process is mediated by the actin-myosin cytoskeleton and is critical for sensing and adapting to changes in the extracellular environment. Although proper cytoskeletal regulation is important for numerous physiological processes including axon/dendrite formation, migration, differentiation, and proliferation, its deregulation can also contribute to human diseases including cancer metastasis (1–5). Therefore, it is crucial to understand the mechanisms that control the cytoskeleton and how it contributes to cancer progression.
Tyrosine phosphorylation of cytoskeleton-associated proteins plays a central role in pseudopodium formation (6). A pseudopodium (or invadopodium in a cancer cell) is a highly specialized, actin-rich structure that protrudes from the cell surface in migrating cells. It serves to tether the extending membrane to the underlying substrate via the formation of focal adhesions and to mediate traction forces that propel the cell forward (7, 8). The pseudopodium also plays an important role in steering the cell by degrading extracellular matrix protein barriers and by sensing changes in chemokine and adhesive gradients that serve as guidance cues. Therefore, understanding how this structure is regulated is crucial to understanding how cells migrate and invade tissues during cancer progression. To this end, we described a novel method for purifying the pseudopodia from cells for signal transduction and proteomic studies (9–11). Using this fractionation method and quantitative mass spectrometry (MS), we have profiled the relative differences in the pseudopodium and cell body proteomes (10). Although this approach has identified important new pseudopodial proteins, it did not provide a robust method for detailed analysis of phosphotyrosine (pY) proteins or kinases present in this subcellular structure (10). pY proteins are difficult to detect because of their low abundance and typically require an enrichment step before MS analysis (12, 13).
In this report, we use pY immunoaffinity enrichment and Multidimensional Protein Identification Technology (MudPIT) to define the pseudopodial phosphotyrosine proteome and to search for novel kinases involved in cell migration and cancer cell invasion (14). This approach uncovered a unique 190-kDa nonreceptor atypical tyrosine kinase family member KIAA2002 (sgk269) that is enriched by 2.6-fold in the pseudopodium. We have named this protein pseudopodium-enriched atypical kinase 1 (PEAK1). Our biochemical and biological findings indicate that PEAK1 is a previously undescribed member of the canonical Src-p130Cas-Crk-II-Paxillin and Erk cytoskeletal signaling pathways. Furthermore, we observed that PEAK1 localizes to focal adhesions, strongly associates with the actin cytoskeleton, and plays an important role in cancer cell migration and proliferation in vitro and in vivo.
Results
Proteomic and Bioinformatic Analyses of the Pseudopodial pY Proteome.
The pseudopodium is highly enriched with pY proteins involved in the regulation of actin polymerization and focal adhesion dynamics (Fig. S1A) (9). To identify kinases and their substrates that spatially regulate these processes, we used a strategy that allows for the large-scale purification of pseudopodia actively extending toward an LPA gradient (9–11). The relative differences in pY proteins from pseudopodia were compared with pY proteins isolated from purified cell bodies by using pY immunoaffinity purification followed by MudPIT (14). Importantly, many tyrosine-phosphorylated proteins bind strongly to the actin cytoskeleton and focal adhesions in migratory cells and, thus, are largely insoluble in detergents like Nonidet P-40 and TX-100 (15). Therefore, to increase the yield of pY proteins, cellular extracts were prepared by using denaturing conditions that maximize protein solubility by boiling the samples in 1% SDS lysis buffer. These conditions also eliminate non-pY to pY protein–protein interactions. After dilution of the SDS/protein extract, pY proteins were then selectively purified with antiphosphotyrosine antibodies and identified by MudPIT (14). This method significantly enhances the specific purification and coverage of the pseudopodial pY proteome. Using this approach, a total of 309 pY proteins were identified in the cell body and pseudopodial fractions (Dataset S1 and Table S1). Of these, 211 proteins were enriched by at least 1.5-fold in the pseudopodium compared with the cell body (Fig. S1B). Functional annotation and statistical analysis of these pseudopodial-enriched pY proteins using the Fatigo search engine (16) and the KEGG pathway databases revealed that the highest percentage of pY proteins mapped to signaling pathways that regulate the actin cytoskeleton and focal adhesions (Fig. S1C). Protein network analysis using the Ingenuity pathway analysis software (10) also revealed a well-connected cytoskeletal network of pY proteins known to regulate the pseudopodium and focal adhesion dynamics including paxillin, cortactin, talin, shc, GIT1/2, p130Cas, FAK, and Src family kinases (9, 17–26) (Fig. S1D). As expected, signaling pathways involved in regulation of cell cycle and calcium signaling were highly represented in the cell body fraction, which contains the nuclear compartment. Thus, the combined methods of pseudopodium purification, pY protein enrichment, and MudPIT provided a representative sample of the pseudopodium pY proteome and its spatial organization in migrating cells.
Bioinformatic Analyses Indicate That PEAK1 Contains Multiple Motifs, Domains and Consensus Phosphorylation Sites That Couple It to the Cytoskeleton.
Twelve unique phosphoproteins were found to be enriched in the pseudopodium (Table S1). Although these proteins have not been previously studied, their function can be inferred from bioinformatic software, which identify known protein interaction domains and consensus kinase phosphorylation motifs that are based on amino acid sequence homologies (27). Of these 12 proteins, PEAK1 was chosen for detailed analysis. PEAK1’s complete domain structure, its known and predicted phosphorylation sites, and its predicted signaling pathway are shown schematically in Fig. 1A. Previous work has shown that PEAK1 is ubiquitously expressed in tissues with the highest level of expression in brain and kidney (28) and is highly conserved from zebrafish to human. The complete gene structure and cloning strategy for obtaining full-length PEAK1 is shown in Fig. S2.
Fig. 1.
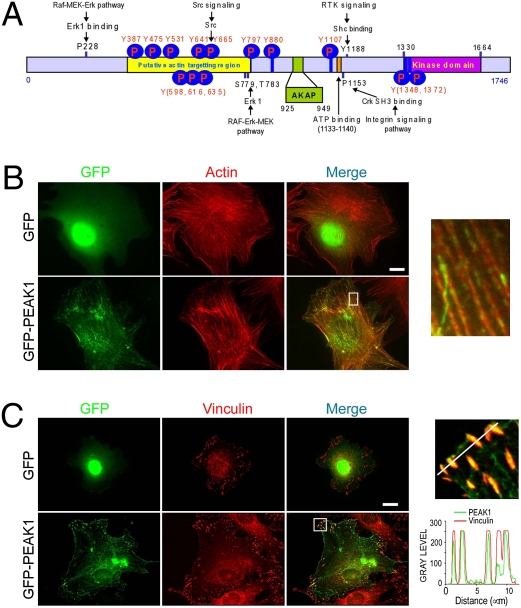
PEAK1 localizes to the actin cytoskeleton and focal adhesions. (A) Schematic showing predicted PEAK1 protein domains, motifs, and known pY sites. (B and C) Immunofluorescence images showing GFP-PEAK1 colocalization with the actin cytoskeleton and vinculin-positive focal adhesions (red) in NIH 3T3 fibroblast cells. White boxes (Inset) show the respective zoomed images. The fluorescence intensity of GFP-PEAK1 and vinculin along the indicated line were scanned by using MetaMorph imaging software, and their colocalization was determined by using the Pearson correlation coefficient (r = 0.91) method. (Scale bars: 15 μm.)
Using the bioinformatic software Scansite (27), PEAK1 is predicted to be phosphorylated by Abl (Y797), Src (Y665), and ERK (S779, T783) kinases (27) (Fig. 1A). All of these enzymes are known to regulate the cytoskeleton and contribute to cancer progression (18, 29–31). Although we have not yet mapped the sites of phosphorylation on PEAK1, at least 13 phosphotyrosine sites have been identified in previous phosphoproteomic studies including Y665 (32) (see also the phosphosites database for the complete list of PEAK1 phosphosites: www.phosphosite.org). There are also several important protein interaction sites present in PEAK1, including an SH2 binding motif for Src kinase (Y665), a proline rich motif for binding to the Crk SH3 domain (P1153), a Shc binding site (Y1188), and an ERK binding motif (P228). The kinase domain spans aa 1330–1664, and the ATP binding site spans aa 1133–1140. Overall, our bioinformatic analyses predict that PEAK1 is a phosphotyrosine protein and an atypical tyrosine kinase that associates with the actin cytoskeleton.
PEAK1 Shows in Vitro Kinase Activity.
All active kinases are predicted to contain three motifs, VAIK, HRD, and DFG, within the kinase domain (33). Each of these motifs contain one highly conserved residue (VAIK: K, HRD: D, DFG: D) that is predicted to be important for full catalytic activity. Sequence analysis revealed that PEAK1 contains all of the three motifs YAVK, HCD, and NFS. The YAVK and HCD motif are highly conserved on the critical K and D residues, but the D residue in the NFS motif has been replaced by N, which classifies it as an atypical kinase (33). However, it is not yet known whether this amino acid substitution can affect kinase catalytic activity or whether this site may be mutated in human cancers to confer full catalytic activity (33). Nonetheless, to investigate whether PEAK1 displays kinase activity, we first determined whether PEAK1 can autophosphorylate in vitro because many kinases phosphorylate themselves (34, 35). For these experiments, GFP-PEAK1 was purified from HEK 293T cells as described in Experimental Procedures and its purity was confirmed by silver staining (Fig. S3A). Autophosphorylation was determined by using an in vitro kinase assay (35) and antiphosphotyrosine Western blotting. PEAK1 displayed significant ability to autophosphorylate on tyrosine residues (Fig. S3A). To determine whether PEAK1 can phosphorylate an exogenous substrate, we performed an in-gel kinase assay by using myelin basic protein (MBP) as a generic substrate and γ-[32P]ATP (36). Under these conditions PEAK1 was able to weakly, but consistently, phosphorylate MBP (Fig. S3B). Finally, to rule out the possibility that a coprecipitating kinase or cofactor from mammalian cell extracts may account for the observed kinase activity of PEAK1 in these experiments, we purified PEAK1’s kinase domain (aa 1289–1746) from Escherichia coli by using a Smt3 tag and size exclusion chromatography (Fig. S3C) and then tested it for tyrosine kinase activity toward MBP in vitro. MBP was phosphorylated by the purified kinase domain under these conditions (Fig. S3D). Together, these results demonstrate that PEAK1 has tyrosine kinase activity.
PEAK1 Localizes to the Actin Cytoskeleton and Focal Adhesions.
PEAK1 subcellular localization was determined using fluorescence microscopy in NIH 3T3 cells transfected with full-length PEAK1 fused with GFP (GFP-PEAK1). PEAK1 strongly colocalizes with the F-actin cytoskeleton and vinculin-positive focal adhesions, whereas control cells expressing GFP showed only diffuse cytoplasmic staining (Fig. 1 B and C). In the majority of cells (>80%), PEAK1 displayed punctate staining along actin cables and cortical actin structures. To map which region of PEAK1 is responsible for its F-actin localization, we expressed a series of truncated forms of PEAK1 fused to GFP at the N terminus in NIH 3T3 cells (Fig. S4A). Using this mutagenesis approach, the actin targeting region was mapped to residues 338–727 (Fig. S4B). Interestingly, this region of PEAK1 also displays the majority of known and predicted tyrosine phosphorylation sites, suggesting that upstream kinases and phosphatases may regulate cytoskeletal localization of PEAK1 (Fig. 1A). In support of this notion, serum starvation reduced PEAK1 localization to F-actin, whereas stimulation of cells with serum or PDGF-BB induced strong PEAK1 colocalization with F-actin, and this response required residues 338–727 (Fig. S4C). In addition, using immunofluorescence staining and a monoclonal antibody to PEAK1, we assessed whether endogenous PEAK1 protein localized to these cytoskeletal structures. Endogenous PEAK1 was observed to localize to actin and focal adhesions (Fig. S4D), whereas cells depleted of PEAK1 by siRNA did not show PEAK1 immunostaining (data not shown). Although the focal adhesion targeting domain in PEAK1 has not yet been ellucidated, our preliminary data suggest that this process will be complex and multifaceted, requiring phosphorylation of PEAK1 and binding of multiple proteins that target PEAK1 to these structures. Nevertheless, these findings indicate that PEAK1 associates with the actin cytoskeleton and localizes to cell-matrix focal adhesions.
PEAK1 Expression in Cells Alters Phosphorylation of Cytoskeleton-Associated Proteins.
Activation of integrin and growth factor receptors promotes tyrosine phosphorylation and the molecular scaffolding of cytoskeleton effector proteins including Src/p130Cas/Crk/Paxillin/Erk (9, 17, 18). Interestingly, exogenous expression of PEAK1 in cells increased the phosphorylation of paxillin (Y31), p130Cas (Y249), and the Erk activation sites (T185/Y187) in response to cell adhesion to fibronectin (Fig. 2A). Paxillin Y31 phosphorylation was shown to regulate the assembly and formation of cell-matrix adhesions, and p130Cas Y249 phosphorylation by Src provides a docking site for the Crk cytoskeleton adaptor protein (37), whereas Erk phosphorylation on T185/Y187 is necessary for its kinase activity. Importantly, the phosphorylation of these proteins at these specific amino acid residues are known to play important roles in modulation of the cytoskeleton and cell migration (17, 18, 30, 38–40). In contrast, depletion of endogenous PEAK1 using a specific siRNA (siPEAK1) decreased the phosphorylation of paxillin (Y31) and Erk under these conditions (Fig. 2B). No obvious change was observed in the phosphorylation of p130Cas (Y249), indicating that PEAK1 can enhance phosphorylation, but is not necessary for phosphorylation at this specific site. Furthermore, we found that p130Cas and Crk coimmunoprecipitated with PEAK1. Under these conditions, Crk coprecipitated only with full-length PEAK1 or the C-terminal truncated forms of PEAK1 (C1 and C2) that contain the predicted Crk-SH3 binding motif (P-X-L-P-X-K) (41) and not the N-terminal truncations that lack this domain (Fig. 2C). On the other hand, p130Cas coprecipitated with wild-type and all mutant forms of PEAK1, suggesting that it may interact with PEAK1 at multiple sites or may associate with other proteins that interact at multiple sites with PEAK1. Taken together, these data indicate that the modulation of PEAK1 protein levels altered the phosphorylation/activation of known cytoskeleton-associated proteins.
Fig. 2.
PEAK1 regulates cytoskeletal and focal adhesion proteins and undergoes Src kinase-dependent tyrosine phosphorylation in response to cell adhesion or EGF stimulation. (A) Protein lysates of 293T cells expressing GFP-PEAK1 (PEAK1) in suspension (Sus) or attached (Atta) to fibronectin (FN) were immunoblotted with indicated total protein and pY site-specific antibodies. (B) Lysates from PEAK1-specific siRNA (siPEAK1) or scrambled control siRNA (siCtrl)-treated cells in suspension or attached to FN for 30 min were immunoblotted as in A. (C) GFP, GFP-PEAK1, and GFP-PEAK1 truncated forms (N1–N3, C1, and C2; Fig. S4A) were immunoprecipitated then immunoblotted for associated Crk and Cas proteins. Whole-cell lysates were also immunoblotted for the indicated proteins. (D and E) pY Western blots of GFP-PEAK1 immunoprecipitated from cells stimulated or not stimulated with EGF for 10 min (D) or stimulated with EGF and in the absence or presence of the Src kinase inhibitor PP2 (E). (F and G) pY Western blots of GFP-PEAK1 immunoprecipitated from cells in suspension (Sus) or reattached to 5 μg/mL of poly-L-lysine (PLL), FN, or laminin (LN), respectively, (F) or cells reattached to FN in the absence or presence of PP2 (G). Total PEAK1 levels in E and F were detected by Ponceau staining. (H) pY immunoprecipitation of total lysates from wild-type (+/+) or Src/Fyn/Yes (SYF) knockout (−/−) MEFs and Western blots for PEAK1 after EGF stimulation for 10 min.
Src Kinase Activity Is Necessary for PEAK1 Tyrosine Phosphorylation Induced by Integrin and Growth Factor Receptor Activation.
We next tested whether PEAK1 is phosphorylated in response to growth factor stimulation or integrin engagement and the role of Src kinase in this response. PEAK1 was maximally tyrosine phosphorylated in HEK 293T cells exposed to EGF for 10 min, and this response was inhibited by the Src kinase inhibitor, PP2 (Fig. 2 D and E). Similar findings were obtained in Src/Yes/Fyn-deficient MEF cells exposed to EGF (Fig. 2H). Cell adhesion to fibronectin also induced PEAK1 tyrosine phosphorylation, and this was inhibited by PP2 (Fig. 2 F and G). Together, these findings indicate that cell adhesion and growth factor stimulation induce PEAK1 tyrosine phosphorylation in a Src-dependent manner.
PEAK1 Expression Modulates Cell Spreading and Migration.
PEAK1 localization to the cytoskeleton and its ability to modulate known focal adhesion proteins suggest that it regulates spreading and migration. To investigate this possibility, Cos-7 or HEK 293T cells were depleted of PEAK1 using siRNA and tested for their ability to spread and migrate in vitro on fibronectin. Knockdown of PEAK1 in Cos-7 cells (Fig. S5C) delayed the initial stages of cell spreading (<90 min) as indicated by a decrease in cell area (Fig. 3A). However, by 180 min there was no difference in the size of control and PEAK1-depleted cells, indicating that other spreading signals can compensate for the loss of PEAK1 (Fig. 3A). On the other hand, exogenous expression of PEAK1 increased cell spreading at an early stage (<30 min) (Fig. 3B). Also, cells depleted of PEAK1 showed significantly reduced chemotaxis compared to control cells (Fig. 3C). Importantly, PEAK1 depletion did not alter cell attachment to fibronectin (Fig. S6A Left). Conversely, Cos-7 cells expressing exogenous PEAK1 showed increased cell chemotaxis toward LPA (Fig. 3D), and this did not alter cell attachment to fibronectin Fig. S6A Center). We further tested whether PEAK1 is necessary for haptotactic migration toward a fibronectin gradient. For these experiments, we used a specific shRNA to stably deplete PEAK1 from XPA-1 pancreatic cancer cells (Fig. S5D). These cells showed significantly reduced migration toward fibronectin compared with the control cells stably expressing a nontargeting shRNA (Fig. 3E). Again, depletion of PEAK1 in XPA-1 cells did not significantly change adhesion to the ECM (Fig. S6A Right). Additionally, we analyzed the migration tracks of these cells over 24 h using time-lapse video microscopy in combination with Metamorph software. This test revealed that PEAK1 promotes a more persistent type of migration of cancer cells (Fig. 3 F and G). Finally, because Src can regulate PEAK1 phosphorylation (Fig. 2 E, G, and H), we wanted to determine whether PEAK1 played a role in Src-induced cell migration. In this case, exogenous expression of Src-enhanced detectable Src activity (Fig. S5E), leading to an increase in the velocity of cell migration that depended on endogenous PEAK1 protein (Fig. 3H). Taken together, these data show that PEAK1 is sufficient and necessary for proper cell spreading and migration in response to fibronectin and growth factors as well as Src kinase activity. Although the mechanism through which PEAK1 modulates cell spreading and migration is not yet understood, PEAK1 expression in cells was observed to significantly increase focal adhesion length (Fig. S4E), suggesting that it may play a role in regulating adhesion dynamics.
Fig. 3.
PEAK1 regulates cell spreading and directional cell migration. Cos-7 cells treated with siPEAK1 or siCtrl (A) or HEK 293T cells expressing exogenous GFP-PEAK1 or GFP (B) only were allowed to attach and spread on 5 μg/mL fibronectin for the indicated times. Cell areas were measured by using MetaMorph. Bars indicate mean ± SD in all figures unless indicated otherwise. *P < 0.01; **P < 0.001. Cos-7 cell chemotaxis toward LPA after treatment with siPEAK1 or siCtrl (C) or overexpression of GFP-PEAK1 or GFP (D). (E) Cell migration toward FN of XPA-1 pancreatic cancer cells treated with shRNA specific for PEAK1 (shPEAK1) or a scrambled control shRNA (shCtrl). (F and G) Cells (5 × 104) as in E were plated onto FN-coated six-well plates, and >70 cells were tracked in each population by using phase-contrast microscopy and MetaMorph software during the subsequent 24 h. The resulting endpoint displacement (F) and cell migration persistance (G) were plotted. (H) After cell migration tracking as in F and G, cell velocity was quantified for shCtrl and shPEAK1 cell populations that were transiently transfected with empty vector or Src overexpression vector. ***P < 0.0001.
PEAK1 Promotes Anchorage-Independent Cancer Cell Growth in Vitro and Tumor Progression in Mice.
The deregulation of kinases and cytoskeletal proteins often contribute to cancer progression (42). Therefore, we wanted to determine whether PEAK1 plays a role in cancer progression. The ability of cells to grow in soft agar in the absence of integrin adhesions to the ECM is a hallmark of cancer (43–45). To determine whether PEAK1 modulates cancer cell growth, human MDA-MB-435 cancer cells stably expressing exogenous PEAK1 were cultured in soft agar and the number and size of colonies measured after 14 d. Exogenous expression of PEAK1 increased the number and size of soft agar colonies and was associated with increased Erk kinase activity (Fig. 4 A and C). In contrast, depletion of exogenous PEAK1 protein with an shRNA construct inhibited this response (Fig. 4 B and C and Fig. S5 A and B). These findings indicate that PEAK1 provides a growth advantage to tumor cells independent of integrin adhesion signals.
Fig. 4.
PEAK1 promotes oncogenic growth of cancer cells in vitro and in vivo and is up-regulated in human colon cancer. Comparison of soft agar colony size and number in MDA-435 cells expressing GFP-PEAK1 or GFP only (A) or depleted of GFP-PEAK1 by shRNA (B) (see also Fig. S5). The area of the colonies is shown as mean ± SEM. (C) Cells from A and B were cultured under suspension conditions for 30 min followed by lysis and Western blot analysis of P-Erk and total Erk proteins. (D) Whole-animal fluorescent images of MDA-435-GFP (GFP) and MDA-435-PEAK1 (PEAK1) cancer cells allowed to grow s.c. in nude mice for 9 wk. (E) Tumor data from mice shown in D: Upper, average tumor weight after tumor excision; Lower, average tumor area as measured by whole-body fluorescence imaging throughout the course of the experiment. (F) Average tumor size of XPA-1 human pancreatic cancer cells stably expressing control scrambled or PEAK1 shRNA and growing orthotopically in the pancreas of nude mice for 4 and 5 wk. The data are shown as mean ± SEM for E and F. (G) Representative in situ hybridization images of PEAK1 expression levels (red stain) in normal colon tissue (NC), primary colon cancer tissue (CC), and a liver metastasis (LM) taken from the same patient.
Having established that PEAK1 is important for anchorage-independent tumor cell growth, we wanted to determine whether PEAK1 could contribute to tumor formation in vivo. MDA-MB-435 cells stably expressing GFP (MDA-435-GFP) or GFP-PEAK1 (MDA-435-PEAK1) were injected s.c. into the flanks of nude mice. Tumor progression was monitored weekly by using whole-body fluorescence imaging as described (46). MDA-435-PEAK1 showed a significant advantage in tumor formation as indicated by the increase in tumor size over the 9-wk period compared with MDA-435-GFP (Fig. 4D). At the end of the 9 wk, the animals were sacrificed; the tumors were removed and weighed. Consistent with our in vitro data, cells overexpressing PEAK1 formed tumors that showed increased area and weight compared with GFP control tumors (Fig. 4 D and E). We also used shRNA to stably knockdown endogenous PEAK1 expression in human XPA-1 pancreatic cancer cells and determined their ability to form tumors when transplanted orthotopically into the pancreas of nude mice. In this case, PEAK1-depleted tumor cells showed a reduction in tumor growth as indicated by reduced tumor size compared with control cells (Fig. 4F). These findings indicate that PEAK1 is necessary for tumor growth in vitro and in vivo.
PEAK1 Expression Is Up-Regulated in Human Colon Cancer and Liver Metastases.
The observation that modulation of PEAK1 protein levels in cancer cells alters tumor growth prompted us to examine whether expression of PEAK1 mRNA is altered in human cancer. In situ hybridization was performed to analyze expression of PEAK1 mRNA in healthy colon tissues and the corresponding colon tumors and liver metastases by using human colon cancer tissue array that included samples from 22 patients. Of these, 18 patients (81.8%; P < 0.001) showed elevated PEAK1 staining in the primary tumor compared with the normal colon tissue (Fig. 4G and Fig. S6B). Twelve of these patients (54.5%) showed positive staining in their corresponding liver metastases (P < 0.05) (Fig. 4G and Fig. S6B). In contrast, only 5 patients (22.7%) showed positive PEAK1 staining in normal colon tissues. Taken together these data indicate that PEAK1 is a unique cytoskeleton-associated kinase and a member of the Src/p130Cas/Crk/paxillin/Erk signaling pathways that regulate cell migration and promote cancer progression.
Discussion
Our ability to affinity purify pY proteins from isolated pseudopodia proved to be a robust system to identify proteins involved in cell migration. Also, the solubilization of pseudopodial proteins in SDS buffer significantly improved our yield of pY proteins, which can be tightly associated with the insoluble cytoskeleton (15). This approach allowed us to identify many low abundant pY proteins involved in cell migration and led to the discovery of PEAK1. Collectively, our findings demonstrate that PEAK1 is a unique nonreceptor tyrosine kinase that operates within the Src-p130Cas-Crk-Paxillin signaling pathway to regulate cell spreading, migration, and cancer progression.
Modulation of PEAK1 affected the phosphorylation level of several known cytoskeleton regulatory proteins including paxillin, p130Cas, and Erk, and it was found to associate with the Crk adaptor protein. PEAK1 has a proline-rich sequence (P-X-L-P-X-K) that conforms to the predicted binding site for the N-terminal SH3 domain of Crk (41). Our finding that Crk coprecipitates with the C-terminal region of PEAK1 has important implications for how PEAK1 could regulate the cytoskeleton. Crk is an adaptor protein that regulates cells spreading and migration by coupling critical signaling proteins such as EGFR, PDGFR, and C-Abl to the cytoskeleton and focal adhesions including the scaffolding protein p130Cas (47). p130Cas and its family members are necessary for cell migration and cancer cell invasion and have been associated with cancer progression in patients (48–51). Interestingly, p130Cas coprecipitates with PEAK1 and PEAK1 modulates p130Cas-Y249 phosphorylation (Fig. 2 B and C). Y249 is known to be phosphorylated by Src family kinases and to provide a binding site for the Crk SH2 domain (38, 47, 52–55). The Src/p130Cas/Crk complex has been shown to modulate Rac activity, pseudopodium protrusion, cell migration, and cancer progression (9, 17, 48, 56–59). Together, these findings suggest a possible scenario in which integrins and growth factors activate Src that, in turn, phosphorylates p130Cas Y249, leading to Crk binding through its SH2 domain. PEAK1 is bound to Crk's SH3 domain and, thus, is recruited to the p130Cas/Crk scaffold, where it is phosphorylated by Src at Y665, the Src consensus site (Fig. 1A and Fig. S5E). Consistent with this idea, Src kinase activity is necessary for PEAK1 tyrosine phosphorylation in response to growth factor and integrin receptor activation (Fig. 2 D–H). Under these conditions, PEAK1 could serve several important purposes. First, it may modulate protein–protein interactions by directly phosphorylating components of the p130Cas/Crk/Paxillin scaffold via its tyrosine kinase activity (Fig. S3). Second, given that PEAK1 translocates to focal adhesions and the actin cytoskeleton after growth factor stimulation (Fig. 1 and Fig. S4), it could provide a mechanism to transport the Src/p130Cas/Crk scaffold to these structures. Third, its strong association with the actin cytoskeleton suggests that it may tether the Src/p130Cas/Crk complex to the cytoskeleton. Finally, it may deliver unique effector proteins to focal adhesions and the cytoskeleton that, in turn, modulate cell migration and/or proliferation. It is intriguing that the majority of the known and predicted phosphorylation sites cluster in the actin localization region of PEAK1. It seems plausible then that PEAK1’s association with the cytoskeleton is regulated by phosphorylation of one or more of these sites. In any case, our observations that PEAK1 can interact with and modulate the Src/p130Cas/Crk/paxillin and Erk signaling pathways point to a central role for this unique protein in mediating cell migration and proliferation in normal and cancer cells.
Although we directly study the function of PEAK1, several independent lines of evidence also suggested that PEAK1 and its only family member sgk223 (33% overall homology to PEAK1) play an integral role in regulation of cell motility and tumor progression (60, 61). For example, sgk223 has been reported to be a unique effector of Rnd2 GTPase. It has also been shown to stimulate RhoA activity in HeLa cells and mediated cancer cell invasion in a Src-dependent manner (61). More recently, sgk223 and PEAK1 were both identified as the potential targets that mediate Src invasive activity in advanced colon carcinoma cells in a quantitative phosphoproteomics study (60). Here, we demonstrate that PEAK1 modulates anchorage-independent growth in soft agar and tumor formation in nude mice. Similar to sgk223, Src mediates PEAK1 tyrosine phosphorylation (Fig. 2 D–H). Finally, large shRNA genomic screens have also revealed that PEAK1 is involved in cancer cell proliferation (62). Together, these findings suggest that PEAK1 and sgk223 are cytoskeleton-associated kinases that regulate cell migration and cancer progression in response to Src kinase activity. The fact that Src is a major contributor to human cancers and our findings that PEAK1 levels are amplified in >80% of colon cancer patients underscore the importance of this protein in human cancer and warrants further investigation.
Experimental Procedures
Protein Sample Preparation and Identification.
Pseudopodia and cell bodies were purified from Cos-7 cells as described (9–11). PY proteins were purified by immunoprecipitation and identified by MudPIT and mass spectrometry (see SI Experimental Procedures for details).
SiRNA and shRNA Assay.
The siRNA pool specific for PEAK1 was purchased from Dharmacon, and transfection was performed according to manufacturer's instructions. The shRNA was designed using the software RNAi OligoRetriever (63) and cloned into the lentiviral vector FG12. The depletion of PEAK1 was confirmed by measuring the mRNA level using RT-PCR and Western blot analysis. See Table S2 for the sequence information of all siRNAs and shRNAs.
Tumor Progression Assays.
The soft agar assay was performed as described (64) by using lentivirus infected, and FACS sorted MDA-MB-435 cells that stably express GFP or GFP/PEAK1. An aliquot of these cells were injected s.c. in nude mice for tumor formation. A second in vivo experiment consisted of establishing orthotopic human pancreatic cancer xenografts in nude mice by direct injection of XPA-1-GFP shCtrl and XPA-1-GFP shPEAK-1 into their pancreas (65) (see SI Experimental Procedures for further details).
Supplementary Material
Acknowledgments
We thank Drs. Olivier Pertz, and Hisashi Kato for assistance in lentivirus production, Spencer Wei for imaging assistance, and Tiffany Taylor, Ryan Matson, and Elizabeth Hampton for assisting with the molecular biology and biochemistry work. We also thank Dr. Stephen K. Burley and his research team at New York SGX Research Center for Structural Genomics (J. Michael Sauder, Shawn S. Chang, Kevin Bain, Jacqueline Freeman, and Tarun Gheyi) for the construct design, cloning, expression, purification, and MS analysis of the PEAK1 kinase domain (a.a. 1289–1746) for our in vitro kinase assays. Finally, we thank Drs. Beverley Emerson and Matthias Kaeser (Salk Institute for Biological Studies) for their kind gift of the CMV-MCS lentiviral expression vector. This work was supported by the Susan G. Komen Foundation Grant PDF0503999 (to Y.W.), National Institutes of Health Grants GM068487 (to R.L.K.) and CA097022 (to R.L.K.), and Cell Migration Consortium Grant GM064346 (to R.L.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914776107/-/DCSupplemental.
References
- 1.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Gupton SL, Gertler FB. Filopodia: The fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 5.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: Where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 6.Chodniewicz D, Klemke RL. Guiding cell migration through directed extension and stabilization of pseudopodia. Exp Cell Res. 2004;301:31–37. doi: 10.1016/j.yexcr.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 8.Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 9.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725––736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Profiling signaling polarity in chemotactic cells. Proc Natl Acad Sci USA. 2007;104:8328–8333. doi: 10.1073/pnas.0701103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Methods for pseudopodia purification and proteomic analysis. Sci STKE. 2007;2007:pl4. doi: 10.1126/stke.4002007pl4. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P. The regulation of protein function by multisite phosphorylation—a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 13.Mann M, et al. Analysis of protein phosphorylation using mass spectrometry: Deciphering the phosphoproteome. Trends Biotechnol. 2002;20:261–268. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 14.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 15.Polte TR, Hanks SK. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130(Cas)) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shahrour F, et al. BABELOMICS: A systems biology perspective in the functional annotation of genome-scale experiments. Nucleic Acids Res. 2006;34:W472–W476. doi: 10.1093/nar/gkl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemke RL, et al. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 19.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner CE. Paxillin interactions. J Cell Sci. 2000;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 21.Goksoy E, et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell. 2008;31:124–133. doi: 10.1016/j.molcel.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, et al. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11:624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 25.Weed SA, Parsons JT. Cortactin: Coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 26.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 27.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohara O, et al. Characterization of size-fractionated cDNA libraries generated by the in vitro recombination-assisted method. DNA Res. 2002;9:47–57. doi: 10.1093/dnares/9.2.47. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb M, Rufini A, Barilà D, Klemke RL. Deregulation of proteasome function induces Abl-mediated cell death by uncoupling p130CAS and c-CrkII. J Biol Chem. 2006;281:2430–2440. doi: 10.1074/jbc.M508454200. [DOI] [PubMed] [Google Scholar]
- 30.Klemke RL, et al. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src's hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- 32.Rikova K, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 34.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuazon PT, Chinwah M, Traugh JA. Autophosphorylation and protein kinase activity of p21-activated protein kinase gamma-PAK are differentially affected by magnesium and manganese. Biochemistry. 1998;37:17024–17029. doi: 10.1021/bi982103o. [DOI] [PubMed] [Google Scholar]
- 36.Wooten MW. In-gel kinase assay as a method to identify kinase substrates. Sci STKE. 2002;2002:pl15. doi: 10.1126/stke.2002.153.pl15. [DOI] [PubMed] [Google Scholar]
- 37.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg GS, et al. Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J Biol Chem. 2003;278:46533–46540. doi: 10.1074/jbc.M307526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vindis C, Teli T, Cerretti DP, Turner CE, Huynh-Do U. EphB1-mediated cell migration requires the phosphorylation of paxillin at Tyr-31/Tyr-118. J Biol Chem. 2004;279:27965–27970. doi: 10.1074/jbc.M401295200. [DOI] [PubMed] [Google Scholar]
- 40.Petit V, et al. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumacher C, et al. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J Biol Chem. 1995;270:15341–15347. doi: 10.1074/jbc.270.25.15341. [DOI] [PubMed] [Google Scholar]
- 42.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 43.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: Correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 44.Gallimore PH, McDougall JK, Chen LB. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977;10:669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- 45.Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci USA. 1975;72:4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman RM, Yang M. Whole body imaging with fluorescent proteins. Nat Protoc. 2006;1:1429–1438. doi: 10.1038/nprot.2006.223. [DOI] [PubMed] [Google Scholar]
- 47.Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Brábek J, et al. CAS promotes invasiveness of Src-transformed cells. Oncogene. 2004;23:7406–7415. doi: 10.1038/sj.onc.1207965. [DOI] [PubMed] [Google Scholar]
- 49.Cho SY, Klemke RL. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorssers LC, et al. The prognostic value of BCAR1 in patients with primary breast cancer. Clin Cancer Res. 2004;10:6194–6202. doi: 10.1158/1078-0432.CCR-04-0444. [DOI] [PubMed] [Google Scholar]
- 51.van der Flier S, et al. BCAR1/p130Cas expression in untreated and acquired tamoxifen-resistant human breast carcinomas. Int J Cancer. 2000;89:465–468. doi: 10.1002/1097-0215(20000920)89:5<465::aid-ijc11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: Involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: A role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: Signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 55.Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol Cell Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brábek J, et al. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3:307–315. doi: 10.1158/1541-7786.MCR-05-0015. [DOI] [PubMed] [Google Scholar]
- 57.Pratt SJ, et al. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J Cell Biol. 2005;168:813–824. doi: 10.1083/jcb.200406083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A, Mayer BJ. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biol. 2008;9:50. doi: 10.1186/1471-2121-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith HW, Marra P, Marshall CJ. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol. 2008;182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leroy C, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–2286. doi: 10.1158/0008-5472.CAN-08-2354. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka H, Katoh H, Negishi M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol Chem. 2006;281:10355–10364. doi: 10.1074/jbc.M511314200. [DOI] [PubMed] [Google Scholar]
- 62.Schlabach MR, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319:620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kain KH, Gooch S, Klemke RL. Cytoplasmic c-Abl provides a molecular ‘Rheostat’ controlling carcinoma cell survival and invasion. Oncogene. 2003;22:6071–6080. doi: 10.1038/sj.onc.1206930. [DOI] [PubMed] [Google Scholar]
- 65.Katz MH, et al. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21:7–12. doi: 10.1023/b:clin.0000017160.93812.3b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.