Abstract
To better understand intrinsic brain connections in major depression, we used a neuroimaging technique that measures resting state functional connectivity using functional MRI (fMRI). Three different brain networks—the cognitive control network, default mode network, and affective network—were investigated. Compared with controls, in depressed subjects each of these three networks had increased connectivity to the same bilateral dorsal medial prefrontal cortex region, an area that we term the dorsal nexus. The dorsal nexus demonstrated dramatically increased depression-associated fMRI connectivity with large portions of each of the three networks. The discovery that these regions are linked together through the dorsal nexus provides a potential mechanism to explain how symptoms of major depression thought to arise in distinct networks—decreased ability to focus on cognitive tasks, rumination, excessive self-focus, increased vigilance, and emotional, visceral, and autonomic dysregulation—could occur concurrently and behave synergistically. It suggests that the newly identified dorsal nexus plays a critical role in depressive symptomatology, in effect “hot wiring” networks together; it further suggests that reducing increased connectivity of the dorsal nexus presents a potential therapeutic target.
Keywords: blood oxygen level-dependent signal, resting state
Functional brain imaging has provided an important window into human brain function by identifying brain systems involved in a variety of tasks and stimulus processing activities. Most depression-related functional neuroimaging differences have been identified in task-related studies. Previous functional imaging studies have tended to focus on particular regions of interest or, more recently, on regions within particular networks. One important system, the cognitive control network (CCN), serves attention-demanding cognitive tasks and exhibits activity increases in frontal and parietal regions associated with top-down modulation of attention and working memory tasks (1). The CCN is involved in decision making and conflict resolution and is known to be impaired in depression (2). A number of previous studies have found depression-related abnormalities in the CCN, mostly involving decreased task-related activity (3–5). Other studies have identified increased task-related “effective” connectivity of the dorsolateral prefrontal cortex (DLPFC) and dorsal cingulate in depression (6, 7).
Regions in a separate system, the default mode network (DMN), are defined functionally by their coordinated behavior and commonly have the greatest activity at rest and decreased activity during the performance of goal-directed tasks (8, 9). The DMN is important in self-referential activities, including evaluating salience of internal and external cues, remembering the past, and planning the future (9–11). We recently identified depression-related overactivity in tasks involving explicit emotional regulation in regions that fell within the DMN (12), the second network we examined in the present study. Among these DMN regions are medial BA8 and medial BA9 regions, which are involved in self-inspection (13) and emotional regulation (14) and demonstrate increased activity in depression (15).
A third system, the affective network (AN), comprises the connections of the affective division of the anterior cingulate cortex (ACC: subgenual and pregenual cingulate) (16). The AN is involved in emotional processing (16–21) and, through its connections with other regions (18), is important in fear, vigilance, and autonomic and visceral regulation. Although the AN overlaps the DMN as broadly defined (11), it has strong local connections to other regions involved in emotion processing, including the hypothalamus, amygdala, entorhinal cortex, nucleus accumbens, and other limbic structures (18), and is particularly involved in the relationship of emotion/mood to visceral function. As such, it has been a focus of numerous metabolic and task-based studies of mood disorders (17, 19–21). An extensive literature describes alterations in AN activity in major depression (reviewed in refs. 17 and 22).
Along with delineating these networks during a task or stimulus, it is also possible to image these networks without a task, using spontaneous brain activity studied with functional MRI (fMRI). When a person is resting quietly in the scanner, the “resting state” demonstrates slow (<0.1 Hz) spontaneous fluctuations in the blood oxygen level–dependent (BOLD) signal in regional brain activity. These spontaneous fluctuations are coherent (i.e., correlate in magnitude) within specific networks (23), a phenomenon observed in most cortical and subcortical systems, including the DMN (see refs. 10 and 11 for recent reviews). Networks identified as having similar behavior during task performance typically have correlated spontaneous fluctuations even in the absence of tasks and even when component regions are widely separated anatomically.
Because these networks can be determined for each individual based on the strength of correlation to an a priori seed location, group statistical differences in networks can be evaluated on an image-wide basis. In the present study, we used fMRI to investigate altered resting-state functional connectivity in major depression. To determine the localization and specificity of depression-related connectivity differences, we examined three different networks: the CCN, the DMN, and the AN.
Results
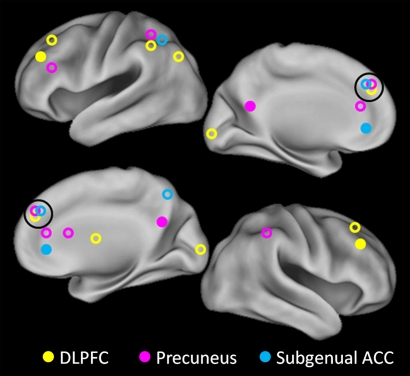
Using the three a priori seed regions shown in Fig. 1, we determined significant differences in mean correlation coefficients between depressed and control subjects for each network. The locations of regions with significant differences were color-coded by seed region origin (Fig. 1), and a correlation map for each network was created.
Fig. 1.
Seed regions and targets. Each of the three solid circles corresponds to a seed region in the DLPFC, part of the CCN (yellow); precuneus, part of the DMN (pink); and subgenual and pregenual cingulate cortex, the affective division of the ACC (16) (turquoise). The correspondingly colored open circles represent regions with significantly increased connectivity with the respective seed regions.
A region of increased connectivity in depression was identified in all three maps (z = 30) (Fig. 2) in the bilateral dorsomedial prefrontal cortex (DMPFC). To explore the specificity of this overlapping connectivity more formally, we created a conjunction map of regions with altered connectivity in depression using the connectivity differences in all three networks (Fig. 3). Depressed subjects exhibited significantly increased functional connectivity in this bilateral DMPFC region, which we term the “dorsal nexus,” indicating the overlapping connections with all three networks. We then conducted functional connectivity analyses focusing on this dorsal nexus region, and found extensive prefrontal, anterior, and posterior cingulate cortex and precuneus regions with dramatically increased connectivity to the dorsal nexus in depressed subjects compared with controls (Fig. 4 and Table 1).
Fig. 2.
Comparison of connectivity maps for depressed and control subjects across three networks. Connectivity of the CCN for control participants (A), depressed participants (B), and between-group differences (C); connectivity of DMN for controls (D), depressed participants (E), and between-group differences (F); and connectivity of the AN for controls (G), depressed participants (H), and between-group differences (I). Networks were identified by seed regions placed in the DLPFC (A–C), precuneus (D–F), and subgenual ACC (G–I). In each network, the dorsal nexus is seen at z = 30. The color bar indicates that images were thresholded at z = 2.58, P < 0.01.
Fig. 3.
A 3D image of the dorsal nexus. (A) Left (−24, 35, 28). (B) Right (18, 34, 29). The nexus was created from a conjunction analysis of the overlap of the areas of significantly increased connectivity among the three networks arising from seeds placed in the DLPFC, precuneus, and subgenual ACC. The individual connectivity maps for these seed regions are shown in Fig. 2. The nexus is comprised primarily of medial BA9, a portion of the ACC (BA32), and a small portion of medial BA8.
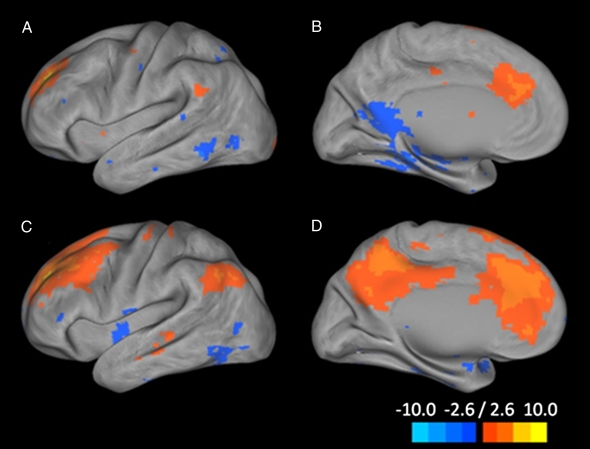
Fig. 4.
Connectivity map from the dorsal nexus to all of the voxels in the brain. Pictured are lateral and medial surface connectivity of the left hemisphere for both control (A and B) and depressed (C and D) participants. Dramatically higher connectivity for depressed participants is evident.
Table 1.
Regions with significantly different connectivity of the dorsal nexus to other brain regions in depressed versus control participants
| Regions | (x y z) | Voxels | P value |
| Dorsal frontal gyrus | |||
| Medial BA9 | +01_+48_+18 | 22 | <10−3 |
| Left medial BA8 | −12_+37_+42 | 73 | <10−3 |
| Right medial BA8 | +07_+38_+36 | 75 | <10−4 |
| Left medial BA8 | −21_+15_+46 | 229 | <10−4 |
| Right medial BA8 | +21_+15_+44 | 178 | <10−4 |
| Left lateral BA9 | −35_+19_+32 | 269 | <10−5 |
| ACC | |||
| Left pregenual ACC BA32 | −09_+29_+19 | 52 | <10−7 |
| Right pregenual ACC BA24 | +09_+26_+19 | 23 | <10−4 |
| Right subgenual ACC BA24 | +05_+30_−02 | 167 | <10−5 |
| Posterior cingulate cortex/precuneus | |||
| Left posterior cingulate | −05_−41_+38 | 167 | <10−5 |
| Left posterior cingulate | −08_−50_+15 | 245 | <10−6 |
| Right posterior cingulate | +09_−56_+19 | 233 | <10−7 |
| Superior precuneus | +01_−57_+38 | 167 | <10−4 |
| Hippocampus/parahippocampus | |||
| Right hippocampus | +29_−34_−03 | 28 | <10−4 |
| Left parahippocampus | −21_−32_−10 | 102 | <10−6 |
| Left visual cortex BA19 | −04_−72_+32 | 76 | <10−3 |
Listed are the Talairach coordinates, voxel number (size), and significance level for each region.
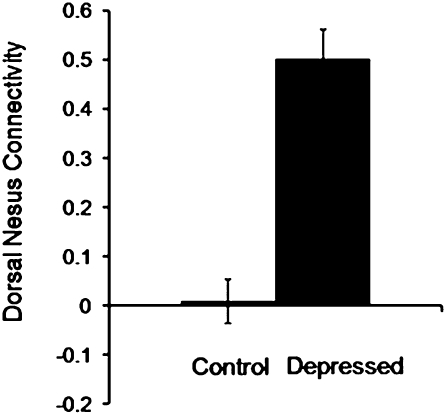
We compared depressed and control subjects in dorsal nexus connectivity to the original three network seeds using ANCOVA, with age, sex, and education level as covariates. There were significant between-group differences (Fig. 5) [F(1, 29) = 57.9; P < 0.0001], but age (P = 0.70), sex (P = 0.97), and education (P = 0.47) did not contribute to the model. In addition, we correlated dorsal nexus connectivity values with Hamilton Depression Rating Scale (HAMD) scores and found a significant relationship (r = 0.85 ± 0.13; P < 0.0001).
Fig. 5.
Comparison of depressed and control participants for mean resting-state connectivity between the dorsal nexus and the combined three seed regions in the DLPFC, precuneus, and subgenual ACC. Images were thresholded at z = 2.58, P < 0.01, as shown in Figure 4.
Discussion
The key finding in this study is the identification in depression of a region in the DMPFC, which we term the dorsal nexus, with increased connectivity to three important networks: the CCN, DMN, and AN. Despite starting with widely separated seeds in these three networks, the increased connectivity converged specifically on the dorsal nexus (Fig. 2). In depression, the dorsal nexus had extremely high connectivity with large regions, including the DLPFC [BA9 (z = 32)], DMPFC [BA8 (z =38–44)], ventral medial prefrontal cortex [BA10 (z = 16)], additional regions in the ACC (dorsal, pregenual, and subgenual), posterior cingulate, and precuneus (Fig. 4 and Table 1). Each of these regions has been implicated separately in depression- and emotion-related functions (2–7, 15–17, 19–22, 24–27), but here we introduce the idea that the joining together of these abnormalities through abnormally high and convergent connectivity gives rise to at least a portion of the complex of dysfunctions seen in depression.
As in the present study, several other recent studies have found increased resting-state functional connectivity in the CCN (24), in contrast with the majority of earlier studies that found decreased task-based activity. The divergence in the CCN between increased resting-state activity and decreased task-based activity might be explained by the presence of higher and more volatile activity in these regions at rest, which in turn could lead to smaller increases during tasks. In the second network that we investigated, the DMN, we found depression-related DMN resting-state connectivity increases similar to those reported by several studies (24, 25). Decreased resting state connectivity also has been reported, however (26). Thus, for the DMN, the majority of studies have found congruence of increased activity both in the resting state and during task performance. A third set of regions that we found to have increased resting state functional connectivity with the dorsal nexus lie within the AN, and also have been studied as part of the medial network (18). Using independent-components analysis, Greicius et al. (27) found that unmedicated depressed patients had increased resting-state connectivity of the DMN with the subgenual ACC, part of the AN.
Our results extend the literature describing abnormally increased resting-state activity in portions of the CCN, DMN, and AN, but now we describe how these findings might fit together. Recent mapping studies in normal brain structural anatomy support high correlation of structural and functional connection patterns within regional nodes, but individual variability in connections between nodes (28), which can have pathological implications (29). Our findings suggest that depression involves systematic alterations in connections among nodes, with increased connection through the dorsal nexus. How this alteration in connections occurs is not clear, but one hypothesis is that it might involve early developmental changes that are further elaborated by experience. For example, the DMN is known to follow a developmental trajectory in humans in which interhemispheric coherence within the DMN appears strong by age 6 years but anterior-posterior coherence between parietal regions and medial prefrontal cortex is weak (30). This suggests an important experiential component in sculpting the DMN. As such, this sculpting also may be affected by early life stressors and trauma that have been shown to predispose to the development of depression (31) through, for example, changes in neurotrophic factors or other factors that could affect neuroplasticity and DMN connectivity (32). Aberrant regulation of neuronal plasticity might result in maladaptive changes in neural networks that underlie the development of major depressive disorder (MDD).
In other studies, the location of the dorsal nexus has been implicated in attentional mechanisms, including attentional expertise in meditation (33). We speculate that through increased resting-state DMN connectivity with the dorsal nexus, an attentional shift with increased self-focus might interfere with task performance in the CCN. Similarly, in the AN, the subgenual and pregenual cingulate have connections to amygdala, hypothalamus, and brainstem nuclei involved in visceral monitoring, including appetite, libido, sleep, and vigilance, all of which are compromised in depression. With increased AN connectivity to the dorsal nexus, these symptoms also may acquire increased connectivity to other DMN regions and cognitive control regions. Thus, the dysregulation of autonomic functioning in the AN and heightened vigilance/heightened self-awareness of the DMN might become the internally focused task that the cognitive network performs (e.g., ruminating) rather than attending to the task at hand. Further, in the present study, the degree of increased dorsal nexus connectivity correlated with the severity of depression, suggesting that the more severe the depression, the greater the hot-wiring of these systems together. In summary, we hypothesize that the marked increase in functional connectivity of the dorsal nexus might underlie some aspects of emotional dysregulation, and that functional mapping in longitudinal and treatment studies will be important in future studies of major depression.
Methods
Participants.
Participants were screened using criteria described previously (34, 35), resulting in 18 individuals with major depression (11 males, 7 females; mean age, 35.9 ± 9.3 yr; mean years of education, 14.6 ± 2.1 yr) and 17 demographically similar controls (5 males, 12 females; mean age, 30.9 ± 6.5 yr; mean years of education, 15.8 ± 1.1 yr). There were no significant group differences in age [t(32) = –1.8; P = 0.08] or sex (χ2 = 3.5; df = 1; P = 0.06); however, participants differed in education [t(32) = 2.2; P = 0.04]. Whereas the 1-year difference between groups in education is unlikely to be meaningful, because it was statistically significant, we used it as a covariate in analyses to demonstrate that it had no effect on the analyses (Hypothesis Testing). Depressed participants met the criteria for a current episode of unipolar recurrent major depression based on DSM-IV criteria (36). All participants were right-handed, free of psychotropic medication for a minimum of 4 wk, underwent a 17-item HAMD evaluation, and were excluded for acute physical illness, history of trauma resulting in loss of consciousness, and lifetime psychiatric disorder other than depression. Depressed participants were included with a 17-item HAMD score ≥18 (mean, 20.2 ± 2.9) were included. Control participants had a HAMD score ≤7 (mean, 0.1 ± 0.3). Nine of the depressed participants had recurrent MDD, with a mean of 2.6 ± 0.7 previous episodes; the other eight were experiencing a first episode. All participants provided written informed consent in accordance with Washington University's Human Subjects Committee criteria, and they were paid $25.00 per hour for their participation.
Structural MRI.
Structural imaging sessions began with acquisition of a scout scan with three orthogonal slices, followed by coarse 3D sagittal T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) to automatically compute fMRI slice tilts and offsets that optimize whole-brain coverage parallel to the anterior-posterior commissure plane. This computation (“preregistration”) standardizes the fMRI coverage across subjects and provides highly reproducible slice positioning in longitudinal studies. High-resolution structural images were acquired using a 3D sagittal T1-weighted MP-RAGE acquisition optimized for contrast-to-noise ratio as described previously (12).
fMRI Scanning Methods.
All scanning was performed on a 3.0-T Siemens Allegra system at the MR Research Facility of the Mallinckrodt Institute of Radiology, Washington University Medical School. The functional images were collected in runs using a gradient spin-echo sequence (TE = 27 ms; TR = 384 ms; field of view = 256 mm; flip angle = 90°) sensitive to blood oxygenation level–dependent (BOLD) contrast (T2* weighting). A total of 36 contiguous 4.0-mm-thick slices were acquired parallel to the anterior-posterior commissure plane (4.0-mm approximately isotropic voxels), providing complete brain coverage. Two fMRI runs included 210 volumes acquired continuously at a TR of 2.5 s (∼7.5 min each).
The MRI data were reconstructed into images and then normalized across runs by scaling whole-brain signal intensity to a fixed value, removing the linear slope on a voxel-by-voxel basis to counteract the effects of drift, correcting for head motion using a six-parameter rigid-body rotation and translation correction that mutually registers all frames in all runs for each subject, and transforming the data to a common atlas space. Then the images were blurred with a 6-mm FWHM Gaussian filter.
Functional Connectivity Analysis of Resting-State Activity.
Standard methods were used for all analyses (23, 37). In preparation for functional connectivity analysis, the BOLD volumetric time series was passed through several additional preprocessing steps: (i) spatial smoothing; (ii) temporal filtering, retaining frequencies in the 0.009- to 0.08-Hz band; and (iii) removal by regression of several sources of variance. To compute functional connectivity maps corresponding to a selected seed region of interest (ROI), the regional time course was correlated against all other voxels within the brain. Correlation maps were produced by extracting the BOLD time course from a seed region, then computing the correlation coefficient between that time course and the time course from all other brain voxels. The principal techniques used are measurement of interregional (ROI–ROI) covariance and correlation, and computation of whole brain, voxelwise intrinsic functional connectivity maps. Thus, each fMRI run provides a series of BOLD signal measurements at each voxel within the field of view. Our main results (i.e., regional functional connectivity) were obtained by computing Pearson correlation coefficients (r) for region pairs. Statistical tests on regional functional connectivity results were computed after application of Fisher's r-to-z transform {z = 0.5 Ln [(1 + r)/(1 − r)]}, which yields variates that are approximately normally distributed.
Seed Region Selection.
For our first analysis, we chose three seeds (Fig. 1) in three different networks—the bilateral DLPFC in the CCN, the precuneus in the DMN, and the subgenual ACC in the affective ACC network—from our previously published task-based ROIs found to be abnormal in depression. The DLPFC a priori seed region (±36, 27, 29) was identified as less active in depressed participants during task performance (35) in an emotion-interference, “conflict” matching task. The a priori precuneus seed (±7, −60, 21) was chosen based on the literature (12, 38). The a priori subgenual ACC seed (±10, 35, −2) was identified as abnormally overactive in depression in response to fearful and neutral faces in the conflict task (35). This seed is similar to other subgenual ROIs reported in the literature with depression- related differences (19, 20, 27), although slightly less ventral than some subgenual regions (17, 27). The seeds were applied to the resting-state connectivity data and group difference significance maps were created using a random-effects analysis on the individual Fisher z-transformed correlation maps as described above. The resulting unpaired t values were converted to z scores and thresholded using P < 0.01 (z = 2.58). Target ROIs were determined from the resulting whole-brain correlation maps. Between-group t tests were calculated for the seed-target correlations (Fig. 2).
Hypothesis Testing of Functional Correlations During Resting-State Activations.
Significance maps were created for the population by combining results across subjects using a random-effects analysis on the Fisher z-transformed correlation maps. The resulting t values were converted to equally probable z scores. Random-effects analyses were carried out in the usual fashion, comparing Fisher z-transformed correlation values across groups. Because education level was statistically different between groups, we conducted ANCOVA analyses, covarying for education when testing differences in connectivity between groups in each of the networks. In all three analyses, the group differences remained significant at P < 0.001, and education level was not significant in a model that included group and education (all P > 0.2).
Identification of the Dorsal Nexus.
Using a z-value threshold at a significance level of P < 0.01 (23), each of the three network seed regions demonstrated the same bilateral region in the DMPFC with significantly increased connectivity in depressed participants compared with control participants (Fig. 2). Therefore, a single region, the dorsal nexus (Fig. 3), was created by a conjunction analysis of the three significance maps generated for the starting seeds. The nexus is composed primarily of medial BA9, a portion of the ACC (BA32), and a small portion of medial BA8. This conjunction region exhibited independent statistically significant increases in connectivity with each of the three separate networks. The dorsal nexus connectivity with other brain regions was determined (Fig. 4).
Acknowledgments
We thank Tony Durbin for his assistance with subject recruitment and fMRI scanning. This work was supported by National Institute of Mental Health Grants R01 MH64821 and K24 MHO79510 (to Y.I.S.).
Footnotes
Conflict of interest statement: Y.I.S. has served on the advisory board and speakers’ bureau of Eli Lilly, Inc. M.A.M. serves as a consultant for Avid Radiopharmaceuticals. Y.I.S. is independent of any commercial provider, had full access to all of the data in this study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. No author named on the title page of this study has any financial interest in the results of the study or any other conflict of interest relevant to the subject matter of this manuscript.
This article is a PNAS Direct Submission.
References
- 1.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MA, et al. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald PB, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Siegle G, Thompson W, Carter C, Steinhauser S, Thase M. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- 7.Schlösser RG, et al. Fronto-cingulate effective connectivity in major depression: A study with fMRI and dynamic causal modeling. Neuroimage. 2008;43:645–655. doi: 10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Shulman G, et al. Common blood flow changes across visual tasks II: Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 9.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Lemogne C, et al. In search of the depressive self: Extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 17.Johansen-Berg H, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 19.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy SH, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 21.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 22.Price J, Drevets W. Neurocircuitry of mood disorders. Neuropsychopharm Rev. 2009;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Grimm S, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 26.Anand A, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Greicius MD, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fair DA, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Uys JD, et al. Developmental trauma is associated with behavioral hyperarousal, altered HPA axis activity, and decreased hippocampal neurotrophin expression in the adult rat. Ann NY Acad Sci. 2006;1071:542–546. doi: 10.1196/annals.1364.060. [DOI] [PubMed] [Google Scholar]
- 33.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 35.Fales C, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Psychiatric Association . DSM-IV-R: Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 37.Sheline Y, et al. Amyloid plaques disrupt resting-state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2009;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]