Abstract
Binge alcohol consumption in adolescents is increasing, and studies in animal models show that adolescence is a period of high vulnerability to brain insults. The purpose of the present study was to determine the deleterious effects of binge alcohol on hippocampal neurogenesis in adolescent nonhuman primates. Heavy binge alcohol consumption over 11 mo dramatically and persistently decreased hippocampal proliferation and neurogenesis. Combinatorial analysis revealed distinct, actively dividing hippocampal neural progenitor cell types in the subgranular zone of the dentate gyrus that were in transition from stem-like radial glia-like cells (type 1) to immature transiently amplifying neuroblasts (type 2a, type 2b, and type 3), suggesting the evolutionary conservation of milestones of neuronal development in macaque monkeys. Alcohol significantly decreased the number of actively dividing type 1, 2a, and 2b cell types without significantly altering the early neuronal type 3 cells, suggesting that alcohol interferes with the division and migration of hippocampal preneuronal progenitors. Furthermore, the lasting alcohol-induced reduction in hippocampal neurogenesis paralleled an increase in neural degeneration mediated by nonapoptotic pathways. Altogether, these results demonstrate that the hippocampal neurogenic niche during adolescence is highly vulnerable to alcohol and that alcohol decreases neuronal turnover in adolescent nonhuman primate hippocampus by altering the ongoing process of neuronal development. This lasting effect, observed 2 mo after alcohol discontinuation, may underlie the deficits in hippocampus-associated cognitive tasks that are observed in alcoholics.
Keywords: self-administration, binge drinking, hippocampal stem cells, neuronal development, cell death
The teenage or adolescent developmental interval connects childhood with adulthood. Adolescence has been associated with a lack of behavioral maturity and increased experimental curiosity. These behavioral observations are paralleled by a transition state in many aspects of body and brain development. Therefore, it is concerning that binge alcohol consumption in teenagers is on the rise, with a significant percentage (>60%) of individuals vulnerable to developing alcohol use disorders (1, 2). Studies in animal models confirm that adolescence is a period of high vulnerability, with various developmental, behavioral, neuroendocrine, and pharmacological factors, as well as alcohol-induced neuroplastic and neurodegenerative outcomes, which drive the adolescent's propensity to excessively or compulsively drink alcohol in adulthood (3–8).
The hippocampal memory system may be at particular risk for alcoholic insult. One of the neuromodulatory effects of binge alcohol intake is decreased adult hippocampal neurogenesis (9). Many aspects of the development of adult hippocampal neurons and the integration of their synapses into the preexisting neurocircuitry of hippocampal trisynaptic pathways are still under investigation (10, 11). What is known are the developmental stages of these newly born adult hippocampal progenitors and their pathway to attain a neuronal phenotype in the adult brain (12, 13). However, detailed investigation of the different aspects of hippocampal neurogenesis has yet to be completed in the adolescent brain.
There is robust evidence for alcohol-induced reductions in postnatal hippocampal neurogenesis in rodent models (7), but the underlying mechanisms are still under investigation. Importantly, recent research in young, adolescent, and adult rodents supports the hypothesis that postnatal hippocampal neurogenesis may be associated with alcohol abuse and alcohol dependence (7). Although direct support for this proposition is not available from human studies, several indirect observations in adolescent alcoholics support the hypothesis. For example, deficits in behavior dependent on the hippocampus, including spatial and working memory, have been observed in adolescent alcoholics (14–16). Such deficits in memory in alcoholics suggest morphological and neuroplastic alterations in hippocampal formation after alcohol exposure (17–19). Postnatal hippocampal neurogenesis contributes significantly to the morphology and neuroplasticity of hippocampal formation (20–22) and declines with age (23), suggesting greater susceptibility of the adolescent brain to alcoholism (17). Although a direct correlation between the duration of alcohol exposure and altered hippocampal morphology and anatomy is unavailable, levels of intoxication (e.g., binge drinking vs. chronic drinking) may be more useful in predicting the neuromodulatory effects of alcohol. Therefore, investigating how chronic binge alcohol consumption affects hippocampal neurogenesis and hippocampal volume in adolescent nonhuman primate models may elucidate the neurobiological mechanisms contributing to the pathology of alcohol addiction in adolescent human alcoholics (24).
Studies examining alcohol-induced neurobiological alterations in nonhuman primate models have several advantages over rodent models. For example, nonhuman primates are genetically more similar to humans than rodents, exhibit wide cognitive capabilities, readily consume alcohol to the point of intoxication, and are similar to humans in many of the physiological, neuroanatomical, and behavioral systems potentially affected by alcohol (25). Furthermore, hippocampal neurogenesis has been extensively demonstrated in nonhuman primates (26–30) and humans (23, 31–33), suggesting evolutionary conservation of the phenomenon. Lastly, obtaining postmortem tissue from adolescent human subjects with a clear history of alcohol abuse is difficult, if not impossible, confounding the interpretation of human studies with variables such as the amount of alcohol consumed, the duration of exposure, and concurrent use of other illicit drugs.
Given the benefits of nonhuman models in establishing a causal role of alcohol exposure in producing neuroplastic alterations in adolescent alcohol users, the present study examined the impact of ongoing alcohol exposure on postnatal hippocampal neurogenesis using an adolescent nonhuman primate model. An established monkey model of adolescent alcohol binge drinking was used (34, 35) in combination with published and other unique protocols of histological techniques to determine the cellular mechanisms underlying alcohol-induced alterations in hippocampal neurogenesis.
Results
Alcohol Induction, Maintenance, and Blood Alcohol Levels.
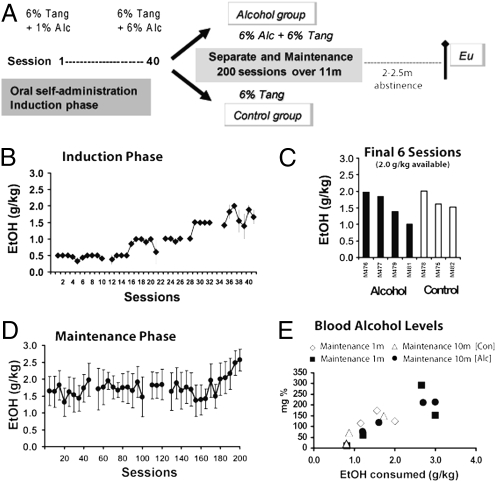
The experimental timeline of alcohol induction and consumption and perfusion is shown in Fig. 1A. All seven animals underwent the alcohol induction process, readily consuming all of the alcohol available when the session limit was ≤1.5 g/kg (Fig. 1B). When the available ethanol was ≥2.0 g/kg/session, animals appeared to titrate their dose, consistent with previous reports from this laboratory (34, 35). The average daily intake (Fig. 1C) from the last six sessions (in which 2.0 g/kg was available) was used to rank the animals for group assignment. The alcohol group was thereafter permitted to consume up to 3.0 g/kg alcohol in Tang during 1-h daily sessions and consumed an average of 1.74 g/kg of alcohol per session during the 11-mo maintenance phase (Fig. 1D). Vehicle solutions of equivalent volume were made available to the control group. Blood samples were obtained on two occasions (1 and 10 mo after assignment to treatment groups) for determination of blood alcohol levels (BALs; Fig. 1E). Samples were collected 30 min after consumption in a 30-min alcohol session in which all animals (including the controls) had the opportunity to consume 3.0 g/kg alcohol in the standard solution. Blood alcohol levels increased as a function of ethanol consumed (34, 35).
Fig. 1.
(A) Time line of alcohol induction and maintenance. Sessions are indicated in days (Alc, alcohol; Eu, euthanize; m, month). (B) Mean ethanol (EtOH) intake during the induction phase (n = 7, ±SEM). (C) Individual animal ethanol intake from the alcohol group for the final six sessions of induction during which 2.0 g/kg was made available. Individuals are grouped by subsequent treatment assignment. (D) Mean (n = 4, ±SEM) daily EtOH consumption more than 200 sessions of EtOH maintenance conducted over ≈11 mo. Data are expressed as sequential five-session averages, and a gap in the series indicates when one individual was briefly discontinued from EtOH access for health reasons unrelated to EtOH drinking. (E) Blood alcohol levels from alcohol and control monkeys either during 1st month or 10th month of maintenance phase after a single session in which 3.0 g/kg was made available.
Alcohol Decreases Hippocampal Neurogenesis in Adolescent Nonhuman Primates.
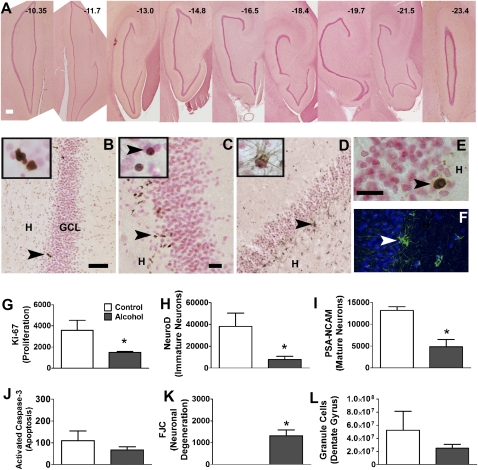
Between 2 and 2.5 mo after the final alcohol (or vehicle) session, monkeys were immobilized with 10 mg/kg ketamine and 5 mg/kg xylazine and euthanized with 10 mg/kg pentobarbital followed by transcardial perfusion with ice-cold PBS. Brains were hemisectioned, transported on dry ice, and frozen at −80 °C. Hippocampal sections from control and alcohol groups [bregma −10.35 to −21.60 mm representing every 30th section (36); Fig. 2A] were processed and examined for changes in cell proliferation [Ki-67-immunoreactivity (IR); Fig. 2B], immature neurons [neurogenic differentiation factor 1(NeuroD1)-IR; Fig. 2C], and immature postmitotic neurons [polysialic acid-neural cell adhesion molecule (PSA-NCAM)-IR; Fig. 2D]. Hippocampal subfields were combined for quantitative analysis; therefore, regional comparisons along the anterior–posterior axis of the hippocampal formation were not conducted. Continued alcohol exposure significantly decreased cell proliferation in the subgranular zone (SGZ) (P = 0.046, Fig. 2G). NeuroD1-IR cells were quantified to assess changes in young immature neurons. Alcohol-exposed animals showed a significant decrease in NeuroD1-IR cells compared with controls (P = 0.035, Fig. 2H). PSA-NCAM-IR cells were quantified to assess changes in older immature neurons. Alcohol-exposed animals also exhibited a significant decrease in PSA-NCAM-IR cells compared with controls (P = 0.01, Fig. 2I). Alcohol reduced NeuroD1-IR cells to a greater extent than Ki-67-IR cells (% decrease: Ki-67, 58.4 ± 2.7; NeuroD1, 79.5 ± 7.7; P = 0.04). Cell death was analyzed by probing for two markers: activated caspase-3 (AC-3) to measure apoptotic cell death (AC-3-IR; Fig. 2E) and Fluoro-Jade C (FJC-IR; Fig. 2F) to measure neural degeneration. Neural degeneration was not observed in controls. Alcohol exposure increased neural degeneration in the granule cell layer compared with controls (P = 0.009, Fig. 2K), and apoptosis was unchanged in alcohol-exposed animals compared with controls (P > 0.05, Fig. 2J). The total number of hippocampal granule cells remained unchanged by chronic binge alcohol consumption (Fig. 2L).
Fig. 2.
Qualitative representative images from the SGZ and quantitative analysis of endogenous markers. (A) Representative sections of the hippocampus containing the dentate gyrus that were used for quantitative analysis from one control animal. Bregma values according to Paxinos et al. (36) are indicated for each section. (B–F) Representative images of Ki-67-IR cells (B), NeuroD1-IR cells (C), PSA-NCAM-IR cells (D) from one control animal, AC-3-IR cells (E), and FJC-IR cells (F) in the granule cell layer from one alcohol animal. Insets in B show Ki-67-IR clusters containing more than one cell; in C, NeuroD1-IR cells that look more mature than the cells in the main panel that look immature and in clusters; and in D, PSA-NCAM-IR cells that always had staining bordering around the granule cell neurons (seen as pink background cells). Arrowhead in B points to a Ki-67-IR cluster of cells. Arrowheads in C point to immature NeuroD1-IR cells in the main panel and mature NeuroD1-IR cell in the Inset. Arrowhead in D, PSA-NCAM-IR cell; in E, AC-3-IR cell; and in F, fluorescently labeled FJC-IR cell in FITC (green) and background nuclear staining with DAPI. Orientation of the dentate gyrus is indicated by hilus (H) and granule cell layer (GCL). [Scale bar, (A) 200 μm; (B) 50 μm (applies also to D); (C) 20 μm; and (E) 50 μm (applies also to Insets B–D and F).] (G–L) Quantitative analysis of immunoreactive cells. Data are expressed as mean ± SEM (n = 3–4 per group). *, P < 0.05, compared with controls (Student's t test).
Decreased Hippocampal Neurogenesis During Adolescence Is Attributable to Distinct Effects on Types of Actively Dividing Hippocampal Progenitors During Their Initial Phases of Neuronal Development.
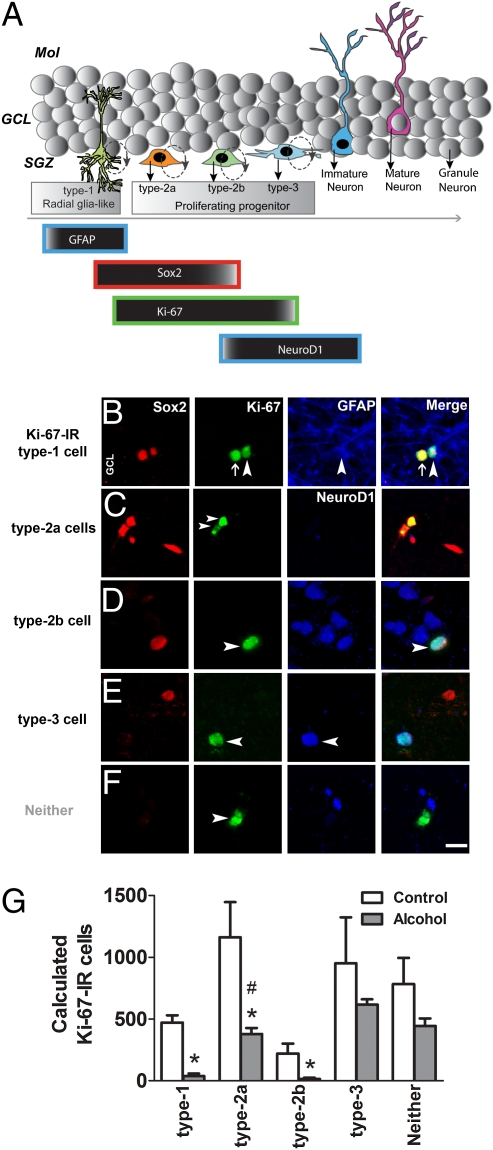
Using endogenous markers of radial glia-like precursors, proliferating progenitors, and differentiating progenitors, the types of actively dividing SGZ progenitors through initial phases of neuronal development were identified and quantified. For example, Ki-67 was used to mark actively dividing type 1, type 2a, type 2b, and type 3 progenitors. Glial fibrillary acidic protein (GFAP) was used as a stem-like marker to distinguish between type 1 and type 2a progenitors (12), in which type 1 cells express GFAP and type 2 do not. Sex-determining region Y-box 2 (Sox2) was used as a marker to distinguish between preneuronal type 2 and neuronal type 3 progenitors (12), in which type 2 cells express Sox2 and type 3 cells do not. NeuroD1 was used to distinguish type 2a and type 2b progenitors (12), in which type 2b express NeuroD1 and type 2a do not. Therefore, using a unique combination of endogenous markers—GFAP, Sox2, Ki-67, and NeuroD1—and the current neurogenesis model for the initial phases of neuronal development of dividing progenitors (12), progenitors were distinctly labeled as type 1, type 2a, type 2b, and type 3. Specifically, actively dividing type 1 progenitors were identified as GFAP+/Sox2+/Ki-67+ cells. Actively dividing type 2a progenitors were identified as Sox2+/Ki-67+/NeuroD1− cells. Actively dividing type 2b progenitors were identified as Sox2+/Ki-67+/NeuroD1+ cells. Actively dividing type 3 progenitors were identified as Sox2−/Ki-67+/NeuroD1+ cells (Fig. 3A). A significant number of Ki-67-IR cells were types 1, 2, and 3 progenitors (74.7% in controls vs. 70.2% in the alcohol group; P > 0.05, unpaired t test; Fig. 3 B–D, F, and G). Our analysis indicated that 14% of Ki-67-IR cells were type 1 cell types, which is greater than the percentage of actively dividing type 1 cells in mouse brain [6% of 2-h 5-bromo-2-deoxyuridine (BrdU)-IR cells according to ref. 37]. In the rat brain, a greater percentage of Sox-2-IR cells are Ki-67-IR (24%, ref. 38), suggesting that the rat brain may have a higher proportion of actively dividing type 1 cells compared with mouse brain. Our present results demonstrate that nonhuman primate brain harbors more type 1 cells than mouse brain. Alcohol altered the ratio of type 1, type 2a, and type 2b progenitors (Fig. 3G), in which alcohol decreased the number of actively dividing type 1, type 2a, and type 2b progenitors compared with the control group (P < 0.01, unpaired t tests). Additionally, separate analysis by two-way analysis of variance (ANOVA) confirmed a significant effect of alcohol on neuronal development, in which alcohol significantly reduced only type 2a progenitors (F4,25 = 21.6, P < 0.01, Bonferroni's post hoc test; Fig. 3G). Alcohol did not significantly alter type 3 progenitors, and alcohol exposure did not significantly change Ki-67-IR cells that were none of these cell types (Fig. 3G).
Fig. 3.
(A) Schematic of the hippocampal granule cell layer demonstrating the sequence of preneuronal, early neuronal, and postmitotic cell types during postnatal neurogenesis (modified from ref. 13). (B–F) Qualitative images of Ki-67-IR cells that are type 1 (B), type 2a (C), type 2b (D), type 3 (E), and none of these cell types (F). Four panels for each cell type are shown indicating single-labeling of each marker, followed by a merge panel. Orientation of each of these panels is indicated by granule cell layer (GCL). Arrowhead in B points to a type 1 cell. Arrowhead in C points to a type 2a cell. Arrowhead in D points to a type 2b cell. Arrowhead in E points to a type 3, and in F, to a Ki-67-IR cell that was none of these cell types. Arrow in B points to a Ki-67-IR type 2a cell next to type 1 cell. [Scale bar in F merge, 10 μm (applies also to B–F).] (G) Quantitative data of calculated cell types that were type 1, type 2a, type 2b, type 3, or none of these cell types. Two separate analyses were performed to analyze significant differences between alcohol and control animals. First, unpaired t test revealed significant differences between control and alcohol animals with only type 1, type 2a, and type 2b cell types. Type 3 cells and cells not classified as any of the cell types (neither) were not significantly different from alcohol animals. Second, two-way ANOVA revealed a significant effect of alcohol on only type 2a cells. Data are expressed as mean ± SEM (n = 3–4 per group). *, P < 0.05, compared with controls by unpaired t test; #, P < 0.01, compared with controls by two-way ANOVA followed by Bonferroni's post hoc analyses.
Discussion
The present study used an Old World monkey model of heavy, binge drinking that produced high BALs equivalent to human BALs during intoxication (and above the legal limit for driving a car), showing that chronic alcohol consumption during adolescence decreases hippocampal neurogenesis. Prior studies using rodent models have demonstrated alcohol-induced decreases in hippocampal neuroplasticity during adulthood (7), but very few studies have explored the neurobiological and behavioral effects of alcohol in adolescents (6, 39–42). Given the short adolescent window in rats, adolescent nonhuman primate models of acute or chronic alcohol drinking are particularly useful in modeling distinct patterns of alcohol use in humans that range from casual drinking to alcohol dependence (25, 34). Our study is unique in that all monkeys received initial alcohol exposure to balance the groups on initial alcohol preference. Thereafter, one group was maintained on alcohol for an extended period of 11 mo. Brain measures for neurogenic and neurotoxic markers were analyzed after 8–10 wk of abstinence. It is possible with this model that the magnitude of differences between controls and alcohol-maintained monkeys was less than might be observed with completely alcohol-naive controls or if animals were killed after a shorter discontinuation interval. This possibility makes it all the more impressive that changes were observed after 2 mo of abstinence from chronic alcohol drinking. This result shows that mechanisms of lasting damage may be triggered relatively early within the context of a multiyear or multidecade pattern of human alcoholism.
The present data also suggest that alcohol-induced changes in neurogenesis may precede and possibly cause the neurodegeneration and hippocampal deficits that have been associated with later adult alcoholism. Chronic binge alcohol consumption in this study produced no change in hippocampal apoptosis but increased neural degeneration, an effect that was evident months after cessation of alcohol consumption. These results show that chronic alcohol consumption decreased neuronal turnover in the hippocampus, thereby producing persistent effects on the hippocampal neurogenic niche.
Alcohol consumption in this model clearly decreased Ki-67-IR proliferating cells. Mechanistically, several pathways may be involved in alcohol's effect on proliferation in the adolescent hippocampus. For example, alcohol could alter how proliferating progenitors cycle through the cell cycle. Notably, the cycling cells in the postnatal hippocampus are not homogeneous, and the process of postnatal neurogenesis is an uncoordinated cluster of developmental stages that progress in parallel, including cycling cells that are radial glia-like (type 1), preneuronal (type 2a), intermediate (type 2b), and early neuronal (type 3) (12) (Fig. 3A). Therefore, alcohol could alter the cell cycle of a distinct type of dividing progenitor or the specific cell cycle phase of all types of dividing progenitors. Although studies in rats show that single or acute binge alcohol exposure during adolescence decreases S phase cells specifically without altering the proliferating pool of progenitors (6), one may speculate that altering cell cycle events may precede the decrease in the proliferating progenitor pool seen with chronic binge exposure (present results). Although a time course of alcohol exposure with simultaneous quantification of S phase cells and the progenitor pool is necessary to support this hypothesis, the present and previous results (6, 40) suggest that this may be a valid mechanism.
In addition to demonstrating that the proliferating progenitor pool in the adolescent nonhuman primate hippocampus is decreased by chronic alcohol exposure, the specific cycling cell types that were affected by alcohol were identified. For example, radial glia-like (type 1), preneuronal (type 2a), intermediate (type 2b), and early neuronal (type 3) cycling cell types in the nonhuman primate hippocampal SGZ were distinctly labeled on the basis of previous combinatorial labeling in adult rodent studies (12, 38). Ki-67-IR was used as a key marker for actively dividing type 1, type 2, and type 3 proliferating progenitors for two reasons. First, in rodents, Ki-67 is expressed in type 1, type 2, and type 3 cells (38, 43). Second, the short half-life of Ki-67 and maximal colabeling with 2-h BrdU-IR cells (44, 45) indicate that almost all Ki-67 cells are nonquiescent, actively dividing progenitors. Therefore, by incorporating previously published and our unique combinations of the endogenous cell cycle marker Ki-67 with GFAP, Sox2, and NeuroD1, the present results in nonhuman primates demonstrate that most of the Ki-67-IR cells (>70%) were a collection of types 1, 2, and 3 cells. Chronic alcohol exposure specifically decreased the number of proliferating cells that are radial glia-like type 1, preneuronal type 2a, and intermediate type 2b, without significantly altering early neuronal type 3 cells. Alcohol did not significantly alter the number of Ki-67-IR cells that were not type 1, type 2, or type 3. The phenotype of Ki-67-IR cells that were none of these cell types could be glial or endothelial hippocampal progenitors because, unlike in the rodent, the proliferative hippocampal zone in nonhuman primates generates a substantial number of cells that are endothelial, oligodendrocytic, and astrocytic (26). Altogether, the present data demonstrate the unique finding that chronic binge alcohol consumption decreases hippocampal neurogenesis by initially altering the precursor cell pool, followed by altering the transition of developmental stages of proliferating progenitors from glial to neuronal phenotypes. Whether the altered types 1 and 2 cells of proliferating progenitors harbor specific receptors or neurochemicals that are exclusive to alcohol's neuropharmacology is yet to be determined and will be a highly productive pursuit for future studies.
The quantitative analyses demonstrate a greater decrease in differentiation and immature neurons than proliferating progenitors. The data suggest that the abnormal decrease in the percentage of actively dividing progenitors that were preneuronal cell types (types 1 and 2) resulted in a greater reduction in maturation and survival of postmitotic cells. This effect of alcohol is not exclusive to nonhuman primates and could be generalized to all mammalian hippocampal progenitors to conclude that hindered proliferation may preferentially contribute to neuronal loss in alcoholics (6, 17).
Reduced hippocampal neurogenesis after chronic binge alcohol consumption did not produce significant hippocampal degeneration. A few factors, such as time of sacrifice after the last alcohol consumption, the model of alcohol consumption, sex differences, and age, may have contributed to the lack of a significant effect (7). For example, the persistent decreases in hippocampal neurogenesis seen months after the last alcohol exposure may bring about significant hippocampal degeneration at a much later time point during abstinence. Despite the lack of immediate significant effects on granule neurons, lower levels of neurogenesis may subsequently promote hippocampal neuronal loss through multiple mechanisms.
Studies in rodent models have suggested that alcohol reduces proliferation and neurogenesis in the adolescent and adult hippocampus through increased cell death (6, 46–48). Active programmed cell death (apoptosis) and neural degeneration were analyzed in the present study. Chronic binge alcohol consumption continued to increase neural degeneration months after abstinence but did not increase apoptosis. These data indicate that changes in apoptosis are short-lived, alcohol's effects on degeneration were lasting, and cells may die through other cell death pathways. For example, long-term exposure to moderate or high alcohol levels may produce cell death via necrosis (46), which could underlie the permanent effects of alcohol on hippocampal volume and morphology. One of the several possible mechanisms for alcohol-induced neurotoxicity may be attributable to alcohol's modulation of hypothalamic–pituitary responsiveness by nitric oxide-generating systems (49). For example, alcohol may regulate nitric oxide synthase, a rate-limiting enzyme for nitric oxide to increase local nitric oxide levels, thus producing neurotoxicity (50, 51). Although a few articles support these hypotheses, a consensus mechanism for alcohol's neurotoxicity has yet to be demonstrated. Nevertheless the current and prior evidence suggests that acute alcohol consumption may first initiate programmed cell death, an effect that is then followed by passive nonprogrammed degeneration of neurons with increasing alcohol exposure (6, 48).
The cellular alterations produced by chronic binge alcohol consumption in a nonhuman primate model may underlie some of the behavioral effects of alcohol drinking in humans. Alcohol abuse in humans produces cognitive deficits on tasks that may be at least partially dependent on hippocampal circuitry. These include increased evidence of impulsivity as well as deficits in spatial learning, short-term memory, and executive function (52). The present results suggest that alcohol-induced reductions in hippocampal neurogenesis during adolescence may lead to hippocampal degeneration as one of several factors that may increase the vulnerability to alcohol use disorders (53).
Materials and Methods
Subjects.
Seven adolescent male rhesus monkeys (Macaca mulatta, Chinese origin; ≈4–5 years of age; mean weight, 7.7 kg at the beginning of the study) were included in the study. Animals were pair-housed and fed in their home cage after completion of the daily behavioral and ethanol sessions. Chow (Lab Diet 5038, PMI Nutrition International) was supplemented with fruit or vegetables 7 d/wk, and water was available in the home cage. Animals were immobilized with ketamine (5–10 mg/kg, i.m.) no less than semiannually for routine care and health monitoring. All monkeys were subjected to behavioral testing (i.e., visiospatial and working memory tasks) during alcohol exposure (manuscript submitted). National Institutes of Health guidelines for laboratory animal care (54) were followed, and protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Ethanol Induction/Group Assignment.
Oral ethanol self-administration (SI Materials and Methods) was induced using a procedure in which the concentration (%) or amount (g/kg) of ethanol in a palatable solution was gradually increased over a series of daily limited-access sessions that identify stable individual preferences for ethanol (Fig. 1A) (34). All seven monkeys were initially given the opportunity to consume alcohol. Alcohol sessions were thereafter discontinued in the control animals, and maintenance of drinking was continued in the alcohol group throughout the study. Blood alcohol levels (SI Materials and Methods) were analyzed with an Analox AM1.
Tissue Preparation.
Blocks of fixed brain tissue (SI Materials and Methods) were cryoprotected in 30% sucrose, after which they were sectioned coronally on a freezing microtome into 40-μm sections. Hippocampal sections were serially collected in six wells and stored in PBS containing sodium azide (0.1%) for subsequent use. Every 30th section through the hippocampus was used for quantitative and qualitative analyses (26).
Antibodies and Immunohistochemistry.
The following primary antibodies were used for immunohistochemistry (IHC): Ki-67, GFAP, NeuroD1, Sox2, PSA-NCAM, and AC-3 (SI Materials and Methods). For single labeling and quantification the DAB calorimetric method was used. The fluorescent labeling (SI Materials and Methods) pattern mimicked the DAB staining pattern for all antibodies tested. Care was taken to avoid possible cross-reactivity between goat anti-Sox2 and goat anti-NeuroD1 staining. For example, staining for Ki-67 separated NeuroD1 and Sox2, such that goat serum was added during Ki-67 staining to prevent any cross-reactivity for the second anti-goat antibody. Omission or dilution of the primary antibodies resulted in a lack of specific staining, thus serving as a negative control for antibody experiments.
Fluoro-Jade C Staining.
Fluoro-Jade C staining (SI Materials and Methods) was performed as previously described (48).
Data Analysis.
Alcohol self-administration data and Ki-67, NeuroD1, PSA-NCAM, AC-3, and FJC data comparing alcohol self-administering and control groups were analyzed by Student's t test. Phenotypic analyses were performed using two-way analysis of variance (ANOVA) using GraphPad Prism version 5 and SPSS software. All analyses were followed by Bonferroni's or Tukey's post hoc tests. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Christopher C. Lay and Stefani N. Von Huben for their expert technical assistance and Drs. Floyd Bloom and George Koob for critical reading of the manuscript. We appreciate the editorial assistance of Michael Arends. This work was supported by National Institutes of Health Grants AA013972, AA06420, and AA016807 (to M.A.T.) from the National Institute on Alcohol Abuse and Alcoholism and Grant DA022473 (to C.D.M.) from the National Institute on Drug Abuse. This is Publication 20325 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912810107/-/DCSupplemental.
References
- 1.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Vol I. Bethesda, MD: National Institute on Drug Abuse; 2009. Monitoring the Future National Survey Results on Drug Use, 1975–2008. Secondary School Students, NIH Publication No. 09-7402. [Google Scholar]
- 2.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 3.Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 5.Spear LP. Alcohol's effects on adolescents. Alcohol Res Health. 2002;26:287–291. [PMC free article] [PubMed] [Google Scholar]
- 6.Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2009;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 10.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Steiner B, et al. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 13.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- 15.Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- 16.Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- 17.De Bellis MD, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 18.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 21.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 23.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue F, et al. Biological properties of neural progenitor cells isolated from the hippocampus of adult cynomolgus monkeys. Chin Med J (Engl) 2006;119:110–116. [PubMed] [Google Scholar]
- 28.Coe CL, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 29.Gould E, et al. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis MA, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci USA. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 33.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katner SN, et al. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28:873–883. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katner SN, et al. Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug Alcohol Depend. 2007;91:236–243. doi: 10.1016/j.drugalcdep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Huang X, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic; 2000. [Google Scholar]
- 37.Filippov V, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 38.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- 41.Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- 42.Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 43.Kronenberg G, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 44.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 45.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- 47.Herrera DG, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson HN, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo DO, Rivier C. Interaction between alcohol and nitric oxide on ACTH release in the rat. Alcohol Clin Exp Res. 2003;27:989–996. doi: 10.1097/01.ALC.0000071737.84882.C4. [DOI] [PubMed] [Google Scholar]
- 50.Lancaster FE. Alcohol and the brain: What's NO got to do with it? Metab Brain Dis. 1995;10:125–133. doi: 10.1007/BF01991860. [DOI] [PubMed] [Google Scholar]
- 51.Davis RL, Syapin PJ. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res Brain Res Rev. 2005;49:494–504. doi: 10.1016/j.brainresrev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Uekermann J, Daum I, Schlebusch P, Wiebel B, Trenckmann U. Depression and cognitive functioning in alcoholism. Addiction. 2003;98:1521–1529. doi: 10.1046/j.1360-0443.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- 53.Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci. 2007;257:261–270. doi: 10.1007/s00406-007-0730-6. [DOI] [PubMed] [Google Scholar]
- 54.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.