Abstract
In the human brain, cognitive-control processes are generally considered distinct from the unconscious mechanisms elicited by subliminal priming. Here, we show that cognitive control engaged in situations of response conflict interacts with the negative (inhibitory) phase of subliminal priming. Thus, cognitive control may surprisingly share common processes with nonconscious brain mechanisms. In contrast, our findings reveal that subliminal inhibition does not, however, interact with control adaptation—the supposed modulation of current control settings by previous experience of conflict. Therefore, although influential models have grouped immediate cognitive control and control adaptation together as products of the same conflict detection and control network, their relationship to subliminal inhibition separates them. Overall, these results suggest that the important distinction lies not between cognitive or top-down processes on the one hand and nonconscious priming mechanisms on the other hand but between responsive (poststimulus) mechanisms that deal with sensorimotor activation after it has occurred and preparatory (prestimulus) mechanisms that are modulated before stimulus arrival.
Keywords: automatic, executive, negative compatibility, voluntary, flanker task
A hallmark of cognitive control is the ability, under conditions of conflict and uncertainty, to select a required response among competing alternatives. Such control has been extensively investigated with tasks that contain situations of potential response conflict, such as the influential Eriksen flanker task (1). In this task, a target stimulus is flanked by stimuli that are either associated with the same response (congruent) or with another response (incongruent). Responses are normally slower with incongruent flankers because of coactivation of conflicting responses to flankers and target. Such conflict is assumed to be minimized by a control process that is sensitive to task demands and attempts to ensure that the correct response is made (1, 2). Moreover, this control process is thought to be adaptive so that, after a conflict situation, the system is better prepared for the next instance of conflict (3–8).
A paradigmatic example of unconscious mechanisms, however, is subliminal priming (9). In this case, stimuli are masked so that they are not consciously perceived, but such masked primes nevertheless affect subsequent responses. Investigations have also revealed an important oscillation in the priming effect (10). Initially, the prime unconsciously activates the response associated with it so that responses are sped up for subsequent targets that require the same (compatible) response and slowed down if prime and target are associated with different (incompatible) responses. This is referred to as a positive compatibility effect (PCE). However, if the delay between prime and target is extended, the priming effect reverses, producing a negative compatibility effect (NCE): primes now facilitate incompatible responses and slow down compatible responses (11, 12).
There is now a wealth of evidence that this reversal is caused by a nonconscious inhibitory mechanism that suppresses the initial, subthreshold motor activation evoked by the prime (11, 13–19). The rationale for such inhibition in everyday life is that priming of the motor system that does not lead to an immediate action needs to be suppressed so that other actions can be made. In the laboratory setting, a delay between priming and response cues of ~200 ms is long enough to observe such inhibition being deployed. Oscillations of motor priming such as this must arise entirely automatically when the participants cannot consciously report the primes.
Here, we ask whether goal-directed cognitive control is entirely distinct from such oscillation of motor priming, although the latter is automatic, unconscious, and blind to the goal-directed response. To probe their relationship, we combine a masked-priming paradigm with the flanker interference task. If they are distinct, the flanker effect and the priming compatibility effect are predicted to be additive. In other words, both would be present in the data, and their effects would simply add up in their influence on the final response time (Fig. 1 prediction box). However, if the inhibitory mechanisms involved in masked priming and flanker paradigms are overlapping, the effects would be expected to interact, such that when the unconscious mechanism swings into the inhibitory phase, it becomes especially difficult to inhibit flankers that do not coincide with the direction of unconscious inhibition.
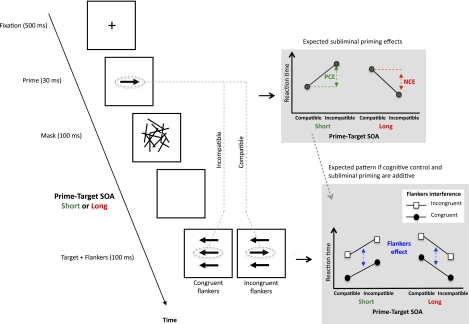
Fig. 1.
Schematic illustration of the task, which combines subliminal priming and flanker interference. If the stimulus onset asynchrony (SOA) between prime and target (response) cue is short (70 ms), a PCE in response times is found (green). If the SOA is longer (180 ms), an NCE is found (red). Compatibility refers to whether the direction of prime arrow and target (response) arrow is the same (compatible) or different (incompatible). Targets are flanked by arrows. If the flankers are congruent with the target, then response times are shorter than if flankers are incongruent (the flanker interference effect; blue plot). The question is whether these effects interact. The stimuli represent the ones used in most experiments, but they are schematic. The sequence illustrated is that for the long SOA condition. In the short SOA condition, there was no blank screen between mask and target, and the mask (always 100 ms in duration) was still on the screen when the target-flanker set appeared (Fig. S1). For this reason, in both conditions, the target-flanker set was actually presented at a random position in a virtual annulus around the mask locus (Fig. S1).
Results and Discussion
Flanker Interference Interacts with Automatic Inhibition.
We combined the flanker paradigm with two versions of the masked-prime paradigm (Fig. 1 and Fig. S1). One of these used a short stimulus onset asynchrony (SOA) of 70 ms between prime and target (the response cue), which previous studies have shown to produce a positive priming effect (PCE). The other paradigm used a longer SOA of 180 ms, by which time priming has reversed to produce the NCE (11, 19). In both tasks, we could examine the effects of flankers being congruent or incongruent with the direction of the target. Thus, we used a 2 × 2 study design that allowed us to examine the effects of compatible/incompatible primes and the effects of congruent/incongruent flankers. Importantly, in all experiments, we ascertained that the prime stimuli were not consciously perceived (all forced choice identification rates were between 46.5% and 51.9%; comparison with 50%: all had t < 1.3 and P > 0.2).
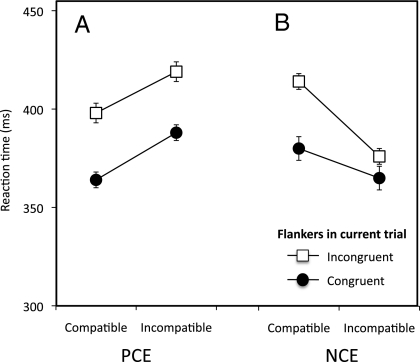
In the PCE block, the incongruent flankers and incompatible subliminal primes both prolonged responses, as expected, and their effects were simply additive (Fig. 2A): the flanker effect is simply the vertical displacement between the two lines [F(1, 9) = 9.3; P = 0.014; η2p = 0.5], whereas the priming effect is the slope of the lines [F(1, 9) = 23.7; P = 0.001; η2P = 0.72]. Additivity is indicated by the lines being parallel [interaction is not significant; F(1, 9) < 1].
Fig. 2.
For positive priming (PCE), the priming effect (slope of lines) was additive with the effect of flanker interference (distance between lines). For the NCE, which measures subliminal inhibition, there was an interaction with flanker interference. For consistency throughout, we refer to primes as compatible or incompatible and flankers as congruent or incongruent. Error bars are the SE of the compatibility effect.
However, in the NCE block where the priming effect is negative, we found a clear interaction with flanker interference (Fig. 2B): F(1, 9) = 27.8; P = 0.001; η2p = 0.75; main effect of flankers conflict: F(1, 9) = 9.52; P = 0.013; main effect of priming: F(1, 9) = 51.1; P < 0.001. In other words, the key finding was that the effect of the flankers depends on the effect of the subliminal primes. Incongruent flankers had little or no additional costs compared with congruent ones when primes were incompatible with target cues. However, when primes were compatible with targets, the presence of incongruent flankers increased response times. Because this interaction might be considered surprising, we replicated it two times with different sets of stimuli, and we confirmed that, relative to neutral flankers, it is the incongruent, not congruent, flankers that are the source of the interaction (Figs. S2 and S3).
In turn, these findings suggest that the inhibitory process involved in controlling flanker interference is not independent from automatic inhibition in subliminal priming. When the unconscious mechanism swings into the inhibitory phase, it becomes relatively easy to inhibit flankers that coincide with the direction of unconscious inhibition. For example, if a left prime is followed by a right target flanked by leftward arrows, there is no significant cost of these incongruent flankers. Because the flankers are in the direction of the prime, it seems that the system already has a head start in inhibiting them. However, if the flankers do not coincide with the direction of unconscious inhibition (for example, when a right prime is followed by a right target with leftward flankers, as in Fig. 1), then it is much more difficult to inhibit these flankers [evidence that there is a hindering effect as well as a helping effect comes from a supplementary study involving neutral primes; the flankers effect for compatible primes was increased with respect to the neutral ones, t(11) = 2.8 and P < 0.017 (one-tailed), and also, the flankers effect for incompatible primes was decreased with respect to that of neutral primes, t(11) = 2.5 and P < 0.028 (one-tailed)]. Importantly, the effect that the prime has on the difficulty of dealing with the flankers is over and above the additive effect that would be expected if their mechanisms were separate (compare Fig. 2A, showing an additive effect, to Fig. 2B, showing the interaction). Instead, the attempt to inhibit flanker interference seems to critically depend on the direction of subliminal inhibition that has been evoked. Thus, we conclude that conflict control and subliminal inhibition share essential neural machinery.
The interaction we measure must stem from the process of controlling flanker interference after it arises (i.e., poststimulus) rather than changing the amount of motor activation caused by the flankers in the first place (e.g., through perceptual priming of the flanker stimuli or facilitating motor activation by the flankers). This is because, for flanker activation, it should not matter whether the flankers are congruent or incongruent with the target—all flankers are expected to activate their associated action plans, and thus, all would be subject to modulation by priming (processing of incongruent flankers might be more suppressed after incompatible primes but so would processing of congruent flankers after compatible primes). Thus, the NCE would be enhanced with both congruent and incongruent flankers, and we would not observe the behavioral interaction that we measure.
The fact that perceptual or motor priming of the flankers themselves would not manifest as a behavioral interaction means, of course, that it may be present as an additional hidden effect in either the PCE or NCE conditions of Fig. 2. Fig. S3, however, provides some evidence against this: if the measured priming effects are enhanced by priming flankers as well as targets, then the measured priming effect should be larger with either congruent or incongruent flankers than with neutral flankers, which could not be primed. We found no evidence for this (Fig. S3).
The most important point, however, is that priming the flankers (at either perceptual or motor levels) could not explain the interaction that we repeatedly measure between the NCE and flanker effect. This requires an interaction between subliminal inhibition and a top-down process that is engaged only when the flankers conflict with the target (or whose effects are much more apparent in these circumstances than for congruent flankers).
Other explanations are ruled out by the fact that the behavioral interaction occurred only for the inhibitory phase of subliminal priming, not for the positive phase (interaction of SOA × flanker conflict × prime compatibility: F(1, 7) = 35.8; P = 0.001; η2p = 0.84). If flanker interference interacted with the net outcome of priming in a final motor map, we would expect an interaction symmetrically with positive and inhibitory phases. Because this does not occur, the interaction must be specific to the inhibitory mechanism of subliminal priming. Note that the dissociation between positive and negative priming phases also rules out an explanation in terms of the object updating or mask-induced priming interpretations of the NCE (20, 21), because, in these, the NCE is caused by positive priming from the mask just as the PCE is cause by positive priming from the prime; therefore, we should not expect PCE and NCE to interact differently with the flankers. Additionally, there is a wealth of previous evidence in favor of an inhibitory interpretation of the NCE (13–15, 17).
Prestimulus Control Does Not Interact with Automatic Inhibition.
Having established that cognitive control interacts with nonconscious inhibition, we ask whether all aspects of cognitive control similarly interact or whether automatic inhibition can be used as a means to distinguish different types of cognitive control. A key aspect of the conflict-control process is its supposed ability to adapt so that after a conflict situation, the system is better prepared for the next instance of conflict (3–6).
According to this influential idea, when conflict occurs, it triggers—perhaps automatically—a boost to the control mechanism that has been set up to deal with it, thus modulating the strength of control before the next trial. The main evidence for this is that the degree of measured flanker interference (the congruency effect) depends on whether conflict occurred in the previous trial or not (the previous-trial or Gratton effect) (22). However, this evidence and the supposed mechanism of adaptive control remain controversial (7, 8, 23).
A strong prediction arises from the idea that the same cognitive-control mechanism that resolves conflict as it occurs is also boosted to prepare the system for the next trial. If subliminal inhibition interacts with control on the current trial, then it should also interact with the boosting effect produced by the previous trial. Given suggestions that the feedback part of the system causing the boost could be automatic rather than a conscious process (3, 24, 25), it may seem especially likely that this mechanism, like flanker interference itself, would interact with subliminal inhibition. If it does not, the previous trial effect must arise from a distinct process.
To have enough power in analyzing sequential effects, we performed an experiment with double the number of trials and without neutral trials. All expected basic effects were clearly present: the PCE, NCE, flanker interference, and previous trial effect [for the long SOA condition, the influence of flankers was, on average, 19 ms after an incongruent trial and 49 ms after a congruent trial; t(19) = 5.4; P < 0.001] (Fig. S4).
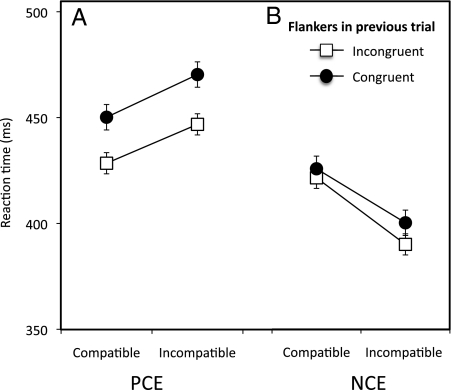
As above, we found an interaction between automatic inhibition and the flanker effect on the current trial, and again, this interaction was absent for positive priming [three-way interaction of priming, flanker effect, and SOA: F(1, 9) = 8.4; P = 0.018; η2p = 0.48]. However, surprisingly, there was no sign of an interaction between automatic inhibition and the previous trial effect (Fig. 3) [the NCE on incongruent trials was 39 ms for previous congruent trials and 42 ms for previous incongruent trials; t(9) < 1]. It is also essential to note that the pattern of results was identical for trials in which the target did not repeat and for trials in which the target did repeat (Fig. S4B), showing that it cannot be explained by simple stimulus-repetition effects (7).
Fig. 3.
The congruency of flankers in the previous trial did not interact with subliminal priming, either for the PCE or NCE. Error bars are the SE of the compatibility effect.
Thus, whereas the process of controlling flanker conflict interacts with automatic inhibition on the same trial, the effect of previous flanker conflict does not interact. This dissociation is not consistent with the idea that previous trial conflict modulates the same cognitive-control mechanism that deals with flanker interference on the current trial. However, we must be cautious with null results—the absence of an interaction of previous conflict with automatic inhibition. We, therefore, further investigated the previous trial effect using a psychophysical approach (four individuals with many trials each). In every participant, current flanker conflict interacted with automatic inhibition, but previous conflict, although producing the standard previous trial effect, did not affect automatic inhibition (Fig. S5). Thus, we conclude that the modulation process that occurs before stimulus onset (causing the previous trial effect) must be distinct from the control mechanism that deals with subliminal primes and conflicting flankers after they occur.
Discussion
Automatic and unconscious sensorimotor mechanisms have traditionally been considered separate from cognitive control (2). Here, we have found that a popular and highly influential measure of cognitive control—flanker interference—interacts with the negative phase of subliminal priming. The interaction is very robust, emerging clearly in all five experiments, and suggests that cognitive conflict control employs a common process with subliminal inhibition. Specifically, the control process dealing with flanker-evoked response conflict seems to have a head start when subliminal inhibition has been evoked in the required direction and be hindered when subliminal inhibition has been evoked in the opposite direction (over and above a simple additive effect).
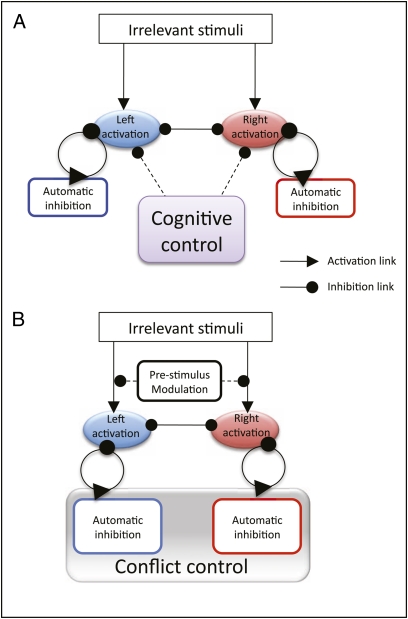
Thus, cognitive control may share essential neural machinery with, or be built on, subliminal inhibition (Fig. 4). This proposal is not as radical as it may sound. It is consistent with and builds on evidence that priming can influence, and is influenced by, top-down executive control processes (attention and intention) (26–30), and that subliminal processes may involve brain areas traditionally considered to mediate voluntary control (14).
Fig. 4.
(A) Representation of traditional separation between subliminal inhibition and cognitive-control mechanisms. (B) Illustration of our hypothesis in which subliminal inhibition is a component part of cognitive control and the important distinction lies between reactive mechanisms and prestimulus processes, not between cognitive and subliminal systems. Placing the influence of prestimulus control on the incoming sensorimotor connections is one way in which it could have a general influence before the direction of the stimuli is known and without globally increasing response latency.
We also found that automatic inhibition did not interact with the previous trial effect, which has previously been taken as evidence for the adaptive nature of cognitive control (3–6). However, if controlling flankers and adapting for the next trial occur through the same control mechanism, then both these effects (or neither) should interact with automatic inhibition. Our data, therefore, drive a distinction between the type of mechanism that responds to flanker interference on a given trial (poststimulus) and the type of mechanism indexed by the previous trial effect—a separate prestimulus process.
The control mechanism that interacts with subliminal inhibition seems to be inhibition of the motor plans evoked by the flankers after conflict has arisen (poststimulus or stimulus-evoked). This interpretation differs from models in which flanker interference is controlled by an attention-like mechanism regulating how much the flankers get processed in the first place (3, 31). Such attentional modulation may occur in addition to poststimulus inhibition, but it is unlikely to be the source of our interaction for two reasons. First, if it modulates the processing of all flankers, then any interaction with priming would be expected to create symmetrical effects for both congruent and incongruent flankers, which, critically, would not result in the behavioral interaction that we measured. Second, subliminal inhibition has been shown to occur at a motor locus, not a perceptual one (14, 19, 32, 33).
Then, the nature of the prestimulus process has several candidates. We can rule out the possibility that it is stimulus-response priming from repeating the same stimuli from one trial to the next (7), because we found the same previous trial effect when the target was repeated and when it was not repeated (Fig. S4). It is possible that more complex stimulus-repetition effects make a contribution (flanker to target and target to flanker) (8), because we used only two different stimuli; however, these cannot easily account for the same-sized previous trial effect when the target was repeated and when it was not, and moreover, in a study with multiple stimulus types, Mayr and Awh (8) found that, although the previous trial effect can be modulated by target to flanker repetitions, it is not accounted for by them. Thus, the previous trial effect truly seems to index a cognitive-control process and may be related to the proactive inhibition investigated in other kinds of paradigms (34–36). However, like an adjustment of cautiousness (a modulation of baseline activity or response criterion), this would predict an overall slowing in the speed of responding after conflict as well as a reduction in the influence of distracting stimuli such as flankers. Such slowing did not occur in our data (if anything, the reverse occurred) (Fig. 3).
Alternatively, prestimulus control could be caused by narrowing of spatial attention around the expected target location. However, in our case, the targets occurred anywhere in an annulus around the prime and mask location so that their location could not be predicted. Still, it may be that prestimulus control acts in a way akin to spatial attention in that it modulates the degree to which flankers cause motor activation in the first place rather than attempting to control that activation after it has happened (Fig. 4). Thus, the distinction between poststimulus control, which interacts with subliminal inhibition, and prestimulus control, which does not, may be most parsimoniously attributed to mechanisms that inhibit motor activation in the former case and mechanisms that modulate perceptual processing in the latter case (37). Note that this is an important departure from models in which a single control mechanism is put in place to deal with interfering stimuli (whether on the motor or perceptual side), and prestimulus control operates through that same mechanism (3, 5).
We can then ask how the prestimulus control process is modulated by previous conflict and whether conscious awareness is necessary. Mayr and Awh (8) discuss four alternative ways in which modulation could occur: explicit feedback from conflict detection (3, 5), passive carryover of control settings (38), learned associations between stimulus arrangement and control state (39), or a deliberate prestimulus regulatory process. In their basic form, the first three of these all envisage that the previous trial effect comes from changing the state or settings (by different means) of the same control process that deals with incongruent flankers when they occur. Thus, they predict that subliminal inhibition should have interacted with both (or neither) the flanker effect and the previous trial effect.
However, on one hand, these ideas could be adapted to a model in which prestimulus control and poststimulus control are separate, but the former is still modulated within a trial and affects the next trial. On the other hand, previous data seem most consistent with deliberate prestimulus control. Mayr and Awh (8) found that the previous trial effect was most prominent only in the first 200 or so trials of testing, after which, it declines. Although this decline may not be present in all data [it is not present in ours—previous trial effects for first and last 200 trials were 20 ms and 17 ms; t(19) = 1.45; P = not significant), its occurrence in any data is difficult to explain in terms of the first three explanations listed above, which would expect it to stay the same throughout (feedback loop or passive carryover) or grow (instance-based learning). More persuasively perhaps, Kunde (25) reported that control adaptation occurs only with conscious awareness of conflict and not for conflict induced by subliminal primes (40, 41).
In sum, our data indicate that prestimulus control is distinct from poststimulus (stimulus-evoked) control, with only the latter being related to subliminal inhibition. Previously, there has been an intuitive tendency to group subliminal and reflexive mechanisms together on the one hand and cognitive, goal-directed processes together on the other hand. However, our results suggest that this is the wrong conceptual distinction. We suggest a more neuronally meaningful segregation should be drawn on other lines between prestimulus control and poststimulus inhibitory mechanisms (Fig. 4B). Note that prestimulus control must be general, because the direction of the target and flankers is not known before they appear, but both poststimulus control and automatic inhibition share the essential feature that they are evoked to inhibit a specific (directional) response. Thus, it is not too speculative to suppose that neural machinery can be shared between subliminal inhibition and voluntary, top-down control (42).
Methods
Participants.
A total of 34 individuals from Cardiff University (21 females; age: 18–35 y) participated in the experiments reported here for payment or course credit. All were right-handed, had normal or corrected-to-normal vision, and were naïve to the purpose of the experiments.
Apparatus (All Experiments).
Stimulus presentation was performed by a personal computer-controlled Cambridge Research Systems (CRS) Visage connected to a 21-in Sony GDM-F520 Trinitron monitor placed at a viewing distance of 70 cm. Stimulus presentation was synchronized with the screen refresh rate of 100 Hz, and timings were controlled and measured by the CRS clock and thus, were not subject to the errors produced by normal PC operating systems. Manual responses were collected using a CRS-CB6 button box.
Stimuli and Procedure.
In the main experiments reported above, participants had to make speeded responses with a left- or right-hand key press to the orientation of arrows targets (1.8° × 0.5°), which occurred in random order and were located at 3.5° from fixation in a random direction from fixation. These targets were flanked by two other arrows, appearing at 1° at the top and the bottom (Fig. 1 and Fig. S1). The direction of the two could be congruent or incongruent with the target. At the beginning of the trial, a fixation cross was visible at the center of the screen. After a brief blank (100 ms), primes were briefly presented (30 ms) that were identical to either one of the possible targets and appeared within 0.5° of fixation (i.e., in the same vicinity as the target but not in an identical location on any trial). The prime was followed by a mask of 3° × 3° and constructed of 45 randomly orientated lines, excluding any orientation closer than ±5° to the vertical or the horizontal (0° / 90°). A new mask was constructed on each trial but appeared always in the same place, centered on fixation. We used two timing conditions: one with mask-target SOA of 40 ms and the other with a long SOA of 150 ms. These are known to produce PCE and NCE, respectively. In the first main experiment, 400 trials were presented in each condition with brief breaks every 50 trials. In the second main experiment, there was double that number. Participants carried out the two conditions in counterbalanced order with a pause of about 20 s between each condition. Figs. S1–S5 show details of the supplementary experiments.
Supplementary Material
Acknowledgments
We thank Jamie Ballard for assistance with some data collection and Chris Chambers, Frederick Verbruggen, Aline Bompas, and Jen Mcbride for comments. The Wellcome Trust and the Wales Institute of Cognitive Neuroscience (WICN) supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001925107/-/DCSupplemental.
References
- 1.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 2.Shiffrin RM, Schneider W. Controlled and automatic human information-processing. 11. Perceptual learning, automatic attending, and a general theory. Psychol Rev. 1977;84:127–190. [Google Scholar]
- 3.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 4.Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: It's not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- 5.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 6.Verbruggen F, Notebaert W, Liefooghe B, Vandierendonck A. Stimulus- and response-conflict-induced cognitive control in the flanker task. Psychon Bull Rev. 2006;13:328–333. doi: 10.3758/bf03193852. [DOI] [PubMed] [Google Scholar]
- 7.Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- 8.Mayr U, Awh E. The elusive link between conflict and conflict adaptation. Psychol Res. 2009;73:794–802. doi: 10.1007/s00426-008-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann O, Klotz W. Motor-responses to nonreportable, masked stimuli—where is the limit of direct parameter specification. In: Umiltà C, Moscovitch M, editors. Attention and Performance XV: Conscious and Nonconscious Information Processing. Cambridge, MA: MIT Press; 1994. pp. 123–150. [Google Scholar]
- 10.Eimer M, Schlaghecken F. Effects of masked stimuli on motor activation: Behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform. 1998;24:1737–1747. doi: 10.1037//0096-1523.24.6.1737. [DOI] [PubMed] [Google Scholar]
- 11.Eimer M, Schlaghecken F. Response facilitation and inhibition in subliminal priming. Biol Psychol. 2003;64:7–26. doi: 10.1016/s0301-0511(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 12.Sumner P. Negative and positive masked-priming—implications for motor inhibition. Advances in Cognitive Psychology. 2007;3:317–326. doi: 10.2478/v10053-008-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlaghecken F, Eimer M. Active masks and active inhibition: A comment on Lleras and Enns (2004) and on Verleger, Jaskowski, Aydemir, van der Lubbe, and Groen (2004) J Exp Psychol Gen. 2006;135:484–494. doi: 10.1037/0096-3445.135.3.484. [DOI] [PubMed] [Google Scholar]
- 14.Sumner P, et al. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner P. Mask-induced priming and the negative compatibility effect. Exp Psychol. 2008;55:133–141. doi: 10.1027/1618-3169.55.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Sumner P, Brandwood T. Oscillations in motor priming: Positive rebound follows the inhibitory phase in the masked prime paradigm. J Mot Behav. 2008;40:484–489. doi: 10.3200/JMBR.40.6.484-490. [DOI] [PubMed] [Google Scholar]
- 17.Jaśkowski P. Negative compatibility effect: The object-updating hypothesis revisited. Exp Brain Res. 2009;193:157–160. doi: 10.1007/s00221-008-1700-6. [DOI] [PubMed] [Google Scholar]
- 18.Jaśkowski P. The negative compatibility effect with nonmasking flankers: A case for mask-triggered inhibition hypothesis. Conscious Cogn. 2008;17:765–777. doi: 10.1016/j.concog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Boy F, Sumner P. Tight coupling between positive and reversed priming in the masked prime paradigm. J Exp Psychol Hum Percept Perform. 2010;36(4) doi: 10.1037/a0017173. doi:10.1037/a0017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verleger R, Jaśkowski P, Aydemir A, van der Lubbe RH, Groen M. Qualitative differences between conscious and nonconscious processing? On inverse priming induced by masked arrows. J Exp Psychol Gen. 2004;133:494–515. doi: 10.1037/0096-3445.133.4.494. [DOI] [PubMed] [Google Scholar]
- 21.Lleras A, Enns JT. Negative compatibility or object updating? A cautionary tale of mask-dependent priming. J Exp Psychol Gen. 2004;133:475–493. doi: 10.1037/0096-3445.133.4.475. [DOI] [PubMed] [Google Scholar]
- 22.Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 23.Hommel B, Proctor RW, Vu KP. A feature-integration account of sequential effects in the Simon task. Psychol Res. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- 24.Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci. 2008;12:374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Kunde W. Sequential modulations of stimulus-response correspondence effects depend on awareness of response conflict. Psychon Bull Rev. 2003;10:198–205. doi: 10.3758/bf03196485. [DOI] [PubMed] [Google Scholar]
- 26.Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends on temporal attention. Psychol Sci. 2002;13:416–424. doi: 10.1111/1467-9280.00474. [DOI] [PubMed] [Google Scholar]
- 27.Kunde W, Kiesel A, Hoffmann J. Conscious control over the content of unconscious cognition. Cognition. 2003;88:223–242. doi: 10.1016/s0010-0277(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 28.Sumner P, Tsai P-C, Yu K, Nachev P. Attentional modulation of sensorimotor processes in the absence of perceptual awareness. Proc Natl Acad Sci USA. 2006;103:10520–10525. doi: 10.1073/pnas.0601974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau HC, Passingham RE. Unconscious activation of the cognitive control system in the human prefrontal cortex. J Neurosci. 2007;27:5805–5811. doi: 10.1523/JNEUROSCI.4335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbruggen F, Logan GD. Automaticity of cognitive control: Goal priming in response-inhibition paradigms. J Exp Psychol Learn Mem Cogn. 2009;35:1381–1388. doi: 10.1037/a0016645. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JD, Servan-Schreiber D, McClelland JL. A parallel distributed processing approach to automaticity. Am J Psychol. 1992;105:239–269. [PubMed] [Google Scholar]
- 32.Schlaghecken F, Bowman H, Eimer M. Dissociating local and global levels of perceptuo-motor control in masked priming. J Exp Psychol Hum Percept Perform. 2006;32:618–632. doi: 10.1037/0096-1523.32.3.618. [DOI] [PubMed] [Google Scholar]
- 33.Schlaghecken F, Klapp ST, Maylor EA. Either or neither, but not both: Locating the effects of masked primes. Proc Biol Sci. 2009;276:515–521. doi: 10.1098/rspb.2008.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulinguez P, Ballanger B, Granjon L, Benraiss A. The paradoxical effect of warning on reaction time: Demonstrating proactive response inhibition with event-related potentials. Clin Neurophysiol. 2009;120:730–737. doi: 10.1016/j.clinph.2009.02.167. [DOI] [PubMed] [Google Scholar]
- 35.Jaffard M, et al. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 36.Lo CC, Boucher L, Paré M, Schall JD, Wang XJ. Proactive inhibitory control and attractor dynamics in countermanding action: A spiking neural circuit model. J Neurosci. 2009;29:9059–9071. doi: 10.1523/JNEUROSCI.6164-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert SJ, Shallice T. Task switching: A PDP model. Cognit Psychol. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- 39.Logan GD. Toward an instance theory of automatization. Psychol Rev. 1988;95:492–527. [Google Scholar]
- 40.Dehaene S, et al. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: The role of the anterior cingulate. Proc Natl Acad Sci USA. 2003;100:13722–13727. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayr U. Conflict, consciousness, and control. Trends Cogn Sci. 2004;8:145–148. doi: 10.1016/j.tics.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Sumner P, Husain M. At the edge of consciousness: Automatic motor activation and voluntary control. Neuroscientist. 2008;14:474–486. doi: 10.1177/1073858408314435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.