Abstract
TCR-mediated recognition of β-linked self-glycolipids bound to CD1d is poorly understood. Here, we have characterized the TCR repertoire of a CD1d-restricted type II NKT cell subset reactive to sulfatide involved in the regulation of autoimmunity and antitumor immunity. The sulfatide/CD1d-tetramer+ cells isolated from naïve mice show an oligoclonal TCR repertoire with predominant usage of the Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments. The CDR3 regions of both the α- and β-chains are encoded by either germline or nongermline gene segments of limited lengths containing several conserved residues. Presence of dominant clonotypes with limited TCR gene usage for both TCR α- and β-chains in type II NKT cells reflects specific antigen recognition not found in the type I NKT cells but similar to the MHC-restricted T cells. Although potential CD1d-binding tyrosine residues in the CDR2β region are conserved between most type I and type II NKT TCRs, CDR 1α and 3α regions differ significantly between the two subsets. Collectively, the TCR repertoire of sulfatide-reactive type II NKT cells exhibits features of both antigen-specific conventional T cells and innate-like cells, and these findings provide important clues to the recognition of β-linked glycolipids by CD1d-restricted T cells in general.
Keywords: autoimmunity, CD1, liver, sulfatide, thymic selection
Antigen recognition of peptides presented by the classical polymorphic major histocompatibility complex (MHC) class Ia or class II molecules to TCRs has been extensively analyzed both structurally and functionally. Generally, the complementarity-determining region (CDR) loops CDR1 and CDR2 interact with the MHC portion of the MHC–antigen complex, whereas it is the CDR3 region that contacts the peptide antigen, with some exceptions (1). Lipid antigens are recognized by TCRs when presented by the β2-microglobulin-associated MHC-like molecule CD1. CD1 molecules are monomorphic and can be classified into three groups: group 1 comprises CD1a, CD1b, and CD1c, group 2 CD1d, and group 3 CD1e (2). CD1d is highly conserved among the species and is involved in presenting lipids, glycolipids, and lipoproteins of self or foreign origin to natural killer T (NKT) cells (reviewed in refs. 3–5).
CD1d-restricted NKT cells can be categorized into two groups: type I and type II. Type I NKT cells use a TCR α-chain encoded by the Vα14-Jα18 and a β-chain predominantly using Vβ8.2, Vβ7, or Vβ2 gene segments in mice (3–5). These cells are strongly reactive with the marine sponge-derived glycolipid α-galactosyl ceramide (αGalCer) and can recognize bacterial-derived lipids or a self-glycolipid, isoglobotrihexosyl ceramide (iGb3) (6–8). Recently, crystallographic and alanine mutagenesis analyses have examined type I NKT TCR interactions with the αGalCer/CD1d complex (9–13). Unlike the TCR interaction with peptide/MHC complexes, crucial residues for this interaction are mainly located in the CDR2β loop contacting the CD1d molecule, in the CDR3α loop contacting both glycolipid antigen and the CD1d molecule, and in the CDR1α loop contacting solely the α-linked galactose moiety of the antigen (1, 9–13).
Type II NKT cells are thought to use diverse TCRs (3, 14, 15). A detailed analysis of the TCR repertoire of type II NKT cell subsets has not been carried out. Earlier studies of non-Vα14, CD1d-reactive T cell hybrids in MHC class II-deficient mice revealed a predominant Vβ8 and Vα3.2/Vα8 gene segment usage with diverse CDR3 regions (16). We have identified a major subset of type II NKT cells reactive to the self-glycolipid 3′-sulfated galactosyl ceramide, sulfatide (17, 18). The crystal structure of a sulfatide/murine CD1d complex shows how the β-linked galactose head group of sulfatide projects out and away from the binding pocket of CD1d, resulting in a conformational change associated with greater exposure of CD1d residues (19) compared with the conformation of an α-linked galactose in the αGalCer/CD1d complex. It is noteworthy that β-linked glycolipids are more prevalent among mammals and type II NKT cells are also abundant in humans (20–23). How β-linked self-glycolipids bound to the CD1d molecules are recognized by TCRs remains unknown. This study examines in detail the TCR V gene repertoire of a major type II NKT cell subset reactive to a β-linked glycolipid, sulfatide, involved in a novel immunoregulatory pathway controlling autoimmunity and antitumor immunity (24, 25).
Here, we show that sulfatide-reactive type II NKT cells isolated from naïve, unprimed animals have an oligoclonal TCR repertoire with predominant usage of Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments. CDR3 regions of both the TCR α- and β-chains are encoded by germline or nongermline sequences and have defined lengths containing several conserved residues. Thus, features of both innate-like cells and antigen-specific conventional T cells are present in the type II NKT cells. This study has implications for how TCRs discriminate between β-linked and α-linked glycolipids, and for recognition of self-glycolipids by TCRs in general.
Results
Sulfatide/CD1d-Tetramer+ Cells Use Predominantly TCR Vβ8.1/Vβ3.1 and Jβ2.7 Gene Segments with Dominant Clonotypes of Restricted CDR3 Lengths.
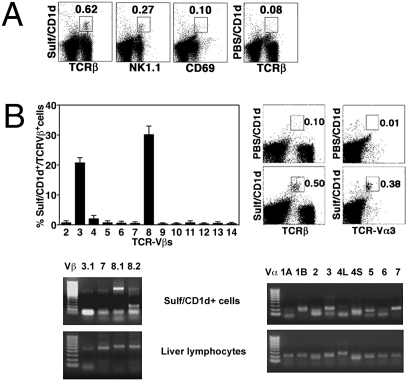
To determine the TCR Vβ repertoire of sulfatide-reactive type II NKT cells, liver mononuclear cells (MNCs) were stained with sulfatide/CD1d-tetramers and a broad spectrum of anti-TCR Vβ chain antibodies (Fig. 1B). Jα18−/− mice were used to exclude contamination from type I NKT cells. Because of the very low frequency of type II NKT cells in spleen and thymus, the analysis of tetramer+ cells was restricted to the liver. As noted earlier in ref. 17, ~35% of sulfatide/CD1d-tetramer+ cells are NK1.1+, and most of them do not express the early activation marker CD69 (Fig. 1A). As shown in Fig. 1B, tetramer+ cells predominantly stained with anti-Vβ8 (33%) and anti-Vβ3 (24%) mAbs. Absence of staining with anti-Vβ8.3 mAb alone suggested tetramer+ cells to be Vβ8.1+ or/and Vβ8.2+.
Fig. 1.
Preferential use of TCR Vβ8.1/Vβ3.1 and Vα3/Vα1 gene segments by sulfatide-reactive type II NKT cells. (A) Flow cytometric profiles of liver MNCs from Jα18−/− mice following staining with PE-labeled sulfatide/CD1d-tetramer (Sulf/CD1d) or unloaded tetramer (PBS/CD1d) and FITC-labeled anti-TCRβ, anti-NK1.1, or anti-CD69. (B Upper Left) Bar graphs depicting the percentage of staining of sulfatide/CD1d-tetramer+ cells with different anti-Vβ mAbs. Percentage was calculated in relation to total tetramer+ cells after subtracting background with unloaded tetramer. (B Upper Right) Tricolor flow cytometric analysis of liver MNCs following staining with PE-labeled unloaded (PBS) or sulfatide-loaded tetramers, PE-Cy5-labeled anti-TCRβ, and FITC-labeled anti-TCR Vα3. Numbers within boxes indicate the percentage of positive cells in total lymphocytes. (B Lower) Gel images showing RT-PCR products of indicated Vβ (Left) or Vα (Right) chains expressed by sorted tetramer+ cells or unsorted liver lymphocytes. A 50- or 100-bp DNA ladder was used. Data are representative of two to four individual experiments.
Sulfatide/CD1d-tetramer+ cells were sorted from liver MNCs pooled from 10 mice (>90% purity) and examined for antigen reactivity and TCR V gene analysis. Sorted cells proliferated and secreted IFN-γ upon in vitro culture with dendritic cells pulsed with sulfatide, but not with αGalCer or mono-GM1 (an irrelevant glycolipid) or without stimulation (Fig. S1). However, modest response of tetramer-sorted cells to sulfatide may suggest their highly unstable nature or their inherent ability to respond poorly. Next, the RT-PCR analysis of the sorted tetramer+ cells showed predominant expression of Vβ8.1, but not Vβ8.2, and minor expression of Vβ3.1/Vβ7 gene segments (Fig. 1B). As control, sorted sulfatide/CD1d-tetramer-negative cells from Jα18−/− mice, as well as total liver MNCs and splenocytes from WT mice, showed broad expression of the different Vβ genes (Fig. 1B). For comparison, RT-PCR was also performed on NK1.1+ TCRβ+ cells from Jα18−/− and CD1d−/− mice not selected for sulfatide reactivity. This population included CD1d-independent NKT and other NK1.1+ T cells. As expected these NK1.1+ T cells are poly-reactive and accordingly express a broad spectrum of Vβ chains, including equivalent levels of Vβ8.1, Vβ8.2, and Vβ8.3. Collectively, FACS and RT-PCR data showed the predominant expression of the TCR Vβ8.1/Vβ3.1 chains by sulfatide/CD1d-tetramer+ cells. The difference between the RT-PCR and FACS data for the Vβ3 may be related to the suboptimal efficiency of the PCR amplification.
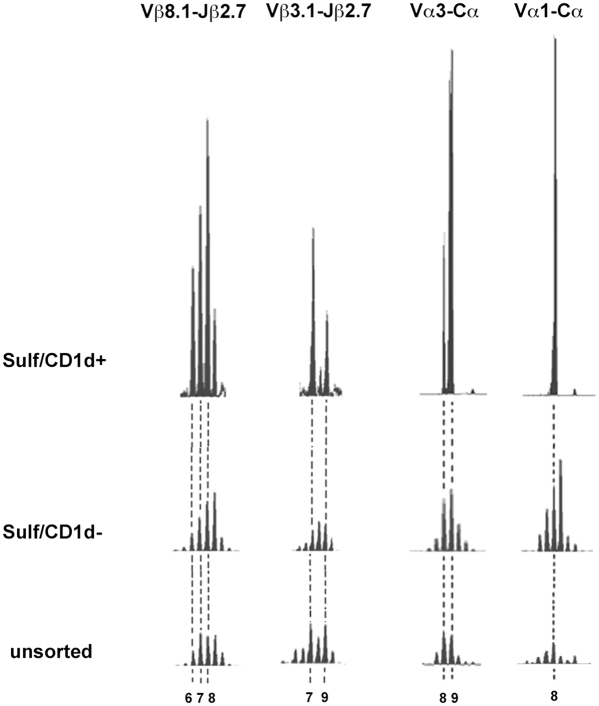
Next, spectratyping analysis was carried out to further examine the CDR3β regions of sulfatide/CD1d-tetramer+ cells. Initial Cβ run-off reactions showed significant expansions for only Vβ8.1, Vβ3.1, and Vβ7 genes, but not for Vβ8.2. As control, typical Gaussian distributions of CDR3 lengths were seen for all Vβ-Cβ products from sorted tetramer-negative cells and from total splenocytes. Following Jβ run-off reactions with 12 different Jβ primers, significant expansions were found only for Vβ8.1-Jβ2.7 and Vβ3.1-Jβ2.7 products, as shown in Fig. 2. No significant expansions were detected for any other Jβ gene segment. Predominant CDR3 lengths of 6, 7, and 8 aa were found for the Vβ8.1-Jβ2.7 and of 7 and 9 aa for the Vβ3.1-Jβ2.7 products, revealing few dominant clonotypes of restricted CDR3β lengths. As control, tetramer-negative cells and total splenocytes showed no specific expansions for any Jβ run-off product examined, including Vβ8.1, Vβ3.1, and Vβ7 (Fig. 2).
Fig. 2.
Spectratyping analysis of TCR Vβ-Jβ and Vα-Cα products reveals a few dominant clonotypes of limited CDR3 lengths in sulfatide-reactive type II NKT cells. RNA was extracted from sorted tetramer+ cells (Sulf/CD1d+), tetramer-negative cells (Sulf/CD1d-), and unsorted liver lymphocytes and splenocytes for Vα and Vβ, respectively. (Left) Vβ-Cβ PCR products subjected to Jβ run-off extension reactions, and significant spectratyping profiles of Vβ8.1-Jβ2.7 and Vβ3.1-Jβ2.7. (Right) Vα-Cα PCR products were subjected to Cα run-off extension reactions, and significant spectratyping profiles of Vα3-Cα and Vα1-Cα are shown. CDR3 lengths are depicted below. For comparison of profiles heights are shown in relation to a reference peak (10 aa for Vβ8.1, 8 aa for Vβ3.1, 12 aa for Vα3, and 11 aa for Vα1).
To further analyze the respective CDR3β regions in sulfatide/CD1d-tetramer+ cells, Vβ-Cβ PCR products were cloned and sequenced. Of 63 Vβ8.1+ clone sequences, 50.8% were redundant—i.e., the nucleotide sequences were shared by more than one clone (Table 1)—whereas another 12.7% were redundant at the amino acid level. Most sequences (41.3%) used Jβ2.7, whereas the remaining sequences showed diverse Jβ gene segment usage (Table 1). Interestingly, none of the Vβ3.1+ clone sequences were redundant, and 18.2% used the Jβ2.7 gene segment (Table 1). Consistent with spectratyping results, Vβ8.1-Jβ2.7 sequences showed predominant CDR3 lengths of 6, 7, and 8 aa, and Vβ3.1-Jβ2.7 sequences were of 7 and 9 aa (Table 1). Collectively, sequencing and spectratyping analyses showed predominant usage of Vβ8.1/Vβ3.1 and Jβ2.7 gene segments with restricted CDR3 lengths by sulfatide/CD1d-tetramer+ cells.
Table 1.
TCR Vβ-Dβ-Jβ gene usage by sulfatide-reactive type II NKT cells
| Vβ aa seq. | N-Dβ-N | Jβ aa seq. | Jβ | Redun-dancy | CDR3 length | |
| Vβ 8.1 clone # | ||||||
| 1, 6, 8, 102, 120 | CAS | SLGGAR | EQYFGPG | Jβ2.7 | 5 | 8 |
| 76, 78, 85, 96, 112 | CAS | YD | SYEQYFGPG | Jβ2.7 | 5 | 6 |
| 44, 114 | CAS | SDGTGG | YEQYFGPG | Jβ2.7 | 2 | 9 |
| 101, 118 | CAS | SDG | SYEQYFGPG | Jβ2.7 | 2 | 7 |
| 3, 45 | CAS | SVTGG | YEQYFGPG | Jβ2.7 | 2 | 8 |
| 19 | CAS | SVTGG | YEQYFGPG | Jβ2.7 | 1 | 8 |
| 22 | CAS | SED | SYEQYFGPG | Jβ2.7 | 1 | 7 |
| 60 | CAS | RDRGH | EQYFGPG | Jβ2.7 | 1 | 7 |
| 62 | CAS | SDAGT | EQYFGPG | Jβ2.7 | 1 | 7 |
| 66 | CAS | TPGQ | EKQYFGPG | Jβ2.7 | 1 | 7 |
| 89 | CAS | SGTGGL | SYEQYFGPG | Jβ2.7 | 1 | 10 |
| 99 | CAS | SDRWGV | YEQYFGPG | Jβ2.7 | 1 | 9 |
| 105 | CAS | SDRWGV | YEQYFGPG | Jβ2.7 | 1 | 9 |
| 113 | CAS | SRLGG | YEQYFGPG | Jβ2.7 | 1 | 8 |
| 115 | CAS | SGDWGGG | EQYFGPG | Jβ2.7 | 1 | 9 |
| 12, 79, 83 | CAS | ENRGY | TEVFFGKG | Jβ1.1 | 3 | 8 |
| 58, 107 | CAS | SDVQK | NTEVFFGKG | Jβ1.1 | 2 | 9 |
| 61 | CAS | SDAQGA | NTEVFFGKG | Jβ1.1 | 1 | 10 |
| 77 | CAS | SES | NTEVFFGKG | Jβ1.1 | 1 | 7 |
| 95 | CAS | RPDSA | NTEVFFGKG | Jβ1.1 | 1 | 9 |
| 17, 51 | CAS | RTGG | YAEQFFGPG | Jβ2.1 | 2 | 8 |
| 9 | CAS | SDMAK | NYAEQFFGPG | Jβ2.1 | 1 | 10 |
| 10 | CAS | SEGTGG | YAEQFFGPG | Jβ2.1 | 1 | 10 |
| 41 | CAS | RDWGV | YAEQFFGPG | Jβ2.1 | 1 | 9 |
| 91, 117 | CAS | SARA | GNTLYFGEG | Jβ1.3 | 2 | 8 |
| 124 | CAS | SARA | GNTLYFGEG | Jβ1.3 | 1 | 8 |
| 7 | CAS | SEGG | SGNTLYFGEG | Jβ1.3 | 1 | 9 |
| 84, 92, 132 | CAS | RMGGD | SNERLFFGHG | Jβ1.4 | 3 | 10 |
| 106 | CAS | SDVWA | SNERLFFGHG | Jβ1.4 | 1 | 10 |
| 81, 126 | CAS | RPGQG | YNSPLYFAAG | Jβ1.6 | 2 | 10 |
| 74 | CAS | SDRTGG | SYNSPLYFAAG | Jβ1.6 | 1 | 12 |
| 75 | CAS | SDAS | SYNSPLYFAAG | Jβ1.6 | 1 | 10 |
| 5, 86 | CAS | TDWGGAV | SAETLYFGSG | Jβ2.3 | 2 | 1 |
| 63 | CAS | TGRD | SAETLYFGSG | Jβ2.3 | 1 | 9 |
| 88 | CAS | SVSWGS | SQNTLYFGAG | Jβ2.4 | 1 | 11 |
| 90 | CAS | SDGP | SQNTLYFGAG | Jβ2.4 | 1 | 9 |
| 100 | CAS | SDSAG | QNTLYFGAG | Jβ2.4 | 1 | 9 |
| 122 | CAS | SDSPGTGG | NTLYFGAG | Jβ2.4 | 1 | 11 |
| 123 | CAS | KTGK | DTQYFGPG | Jβ2.5 | 1 | 7 |
| 128 | CAS | SDNR | NQDTQYFGPG | Jβ2.5 | 1 | 9 |
| 111 | CAS | SGTGG | NTGQLYFGEG | Jβ2.2 | 1 | 10 |
| 131 | CAS | SDARGSD | TGQLYFGEG | Jβ2.2 | 1 | 11 |
| 130 | CAS | PQGA | NSDYTFGSG | Jβ1.2 | 1 | 8 |
| Vβ 3.1 clone # | ||||||
| 15 | CAS | SLNA | NTEVFFGKG | Jβ1.1 | 1 | 8 |
| 18 | CAS | SLYY | TEVFFGKG | Jβ1.1 | 1 | 7 |
| 25 | CAS | SPGTG | NTEVFFGKG | Jβ1.1 | 1 | 9 |
| 1 | CAS | SLWG | NYAEQFFGPG | Jβ2.1 | 1 | 9 |
| 10 | CAS | SLMGAD | YAEQFFGPG | Jβ2.1 | 1 | 10 |
| 13 | CAS | SRWGG | YAEQFFGPG | Jβ2.1 | 1 | 9 |
| 2 | CAS | SLNWGG | YEQYFGPG | Jβ2.7 | 1 | 9 |
| 19 | CAS | SLIFD | EQYFGPG | Jβ2.7 | 1 | 7 |
| 16 | CAS | SLHS | QDTQYFGPG | Jβ2.5 | 1 | 8 |
| 21 | CAS | SLG | NQDTQYFGPG | Jβ2.5 | 1 | 8 |
| 6 | CAS | RRTGR | NTGQLYFGEG | Jβ2.2 | 1 | 10 |
RNA extracted from sorted tetramer+ cells was subjected to RT-PCR, and Vβ-Cβ PCR products were cloned and sequenced. Sixty-three sequences were obtained from 133 Vβ8.1 clones and 11 sequences from 25 Vβ3.1 clones. Redundancy signifies identical nucleotide sequences found among the respective clones.
Predominant Use of TCR Vα3/Vα1 and Jα7/Jα9 Gene Segments and Limited CDR3 Lengths by Sulfatide/CD1d-Tetramer+ Cells.
To examine the TCR Vα repertoire, sulfatide/CD1d-tetramer+ cells were stained with anti-Vα3 antibody and analyzed by flow cytometry. Fig. 1B shows that a majority (∼70%) of the tetramer+ cells are Vα3+. Because Abs against most Vα chains are not available, sorted tetramer+ cells were subjected to RT-PCR analysis using all Vα primers (Vα1A-Vα20) and Cα primer. As shown in Fig. 1B, tetramer+ cells expressed predominantly Vα3, Vα1, and Vα7 and showed minimal expression of the Vα2, Vα5, Vα10, Vα14, and Vα18 gene segments (Fig. 1B and Fig. S2). Notably, sequencing analysis yielded no functional sequence for Vα7 or Vα14. As control, tetramer-negative cells and total liver lymphocytes showed a broad expression of all Vα gene segments (Fig. 1B and Fig. S2).
To further investigate the TCR Vα gene usage, spectratyping analysis was carried out. As depicted in Fig. 2, significant expansions were found for the Vα3-Cα and Vα1-Cα products: two dominant Vα3 clonotypes with CDR3 lengths of 8 and 9 aa and one single predominant Vα1 clonotype with a CDR3 length of 8 aa were detected. In contrast, control populations (tetramer-negative cells and total liver lymphocytes) showed Gaussian distributions for these gene segments (Fig. 2).
Sequencing analysis (Table 2) confirmed predominant usage of Vα3 and Vα1 gene segments. Table 2 shows that most tetramer+ cells use the Jα7 (≥50% of Vα3 and Vα1) or Jα9 (>46% of Vα1 and >7% of Vα3) gene segments. Another 25% of the Vα3 sequences used the Jα12 gene segment. The sequences were highly redundant (>97%), with all Vα1 and > 96% of Vα3 nucleotide sequences being shared by different clones. In accord with spectratyping analysis (Fig. 2), sequencing analysis also detected predominant CDR3α lengths of 8 and 9 aa (Table 2; for Vα3, 53.6% CDR3 length of 8 aa, 35.7% of 9 aa; for Vα1, 84.6% CDR3 length of 8 aa). Collectively these results show a highly oligoclonal TCR Vα repertoire with predominant usage of Vα1/Vα3 and Jα7/Jα9 gene segments and limited CDR3α lengths for sulfatide/CD1d-tetramer+ cells.
Table 2.
TCR Vα-Jα gene use by sulfatide-reactive type II NKT cells
| Vα aa seq. | N-Jα | Jα aa seq. | Jα | Redun-dancy | CDR3 length | |
| Vα 3 clone # | ||||||
| 3, 7, 23, 34 | YFCAV | SA | YSNNRLTLGKG | Jα7 | 4 | 8 |
| 15, 22, 28, 35 | YFCAV | SMG | YSNNRLTLGKG | Jα7 | 4 | 9 |
| 6, 10, 14 | YFCAV | V | YSNNRLTLGKG | Jα7 | 3 | 7 |
| 21, 30, 33 | YFCAA | SMP | YSNNRLTLGKG | Jα7 | 3 | 9 |
| 2, 8, 9, 13, 17, 20 | YFCAV | GA | TGGYKVVFGSG | Jα12 | 6 | 8 |
| 19 | YFCAV | SIR | TGGYKVVFGSG | Jα12 | 1 | 9 |
| 12, 18, 27 | YFCAV | NP | GGNYKPTFGKG | Jα6 | 3 | 8 |
| 36, 47 | YFCAV | SAGG | MGYKLTFGTG | Jα9 | 2 | 9 |
| 1, 11 | YFCAV | SAP | GYNKLTFGKG | Jα11 | 2 | 8 |
| Vα 1 clone # | ||||||
| 3, 6, 13, 21, 25 | YLCAV | RE | YSNNRLTLGKG | Jα7 | 5 | 8 |
| 14, 19 | YLCAV | KA | SNNRLTLGKG | Jα7 | 2 | 7 |
| 1, 4, 5, 16, 17, 22 | YLCAV | SK | NMGYKLTFGTG | Jα9 | 6 | 8 |
RNA extracted from sorted tetramer+ cells was subjected to RT-PCR, and Vα-Cα PCR products were cloned and sequenced. 28/50 (Vα3), 13/25 (Vα1), 3/50 (Vα2), 0/25 (Vα7), and 0/25 (Vα14) were functional sequences. Redundancy signifies identical nucleotide sequences found among the respective clones.
CDR3β and CDR3α Regions of Sulfatide-Reactive Type II NKT Cells Contain Conserved Motifs and Are Encoded by Both Germline and Nongermline Sequences.
To examine whether CDR3β and CDR3α regions are encoded by germline or N-additions, DNA sequences were compared with germline matrices (IMGT). We found that CDR3 regions of Vβ8.1, Vβ3.1, and Vα3 are encoded by both germline and nongermline: the proportion of germline sequences was 12.7% for Vβ8.1, 27.3% for Vβ3.1, and 14.3% for Vα3. However, all Vα1 CDR3 regions were encoded by N-additions. CDR3 regions of the most prevalent Vβ8.1-Jβ2.7 and Vα3-Jα7 chains encoded by germline or by N-additions are shown in Fig. S3.
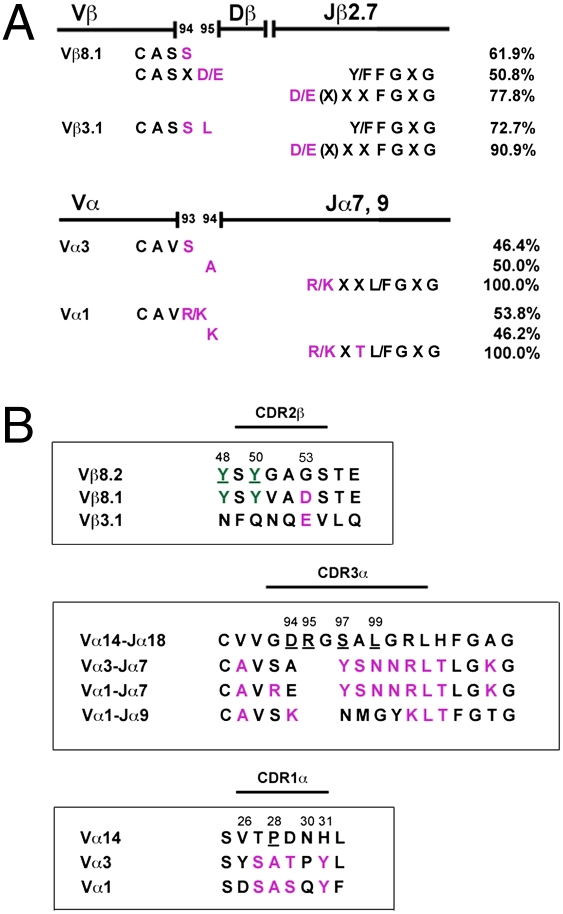
CDR3 regions were examined for conserved amino acid residues. A basic amino acid residue, either lysine or arginine, was found at the end of all CDR3α regions, four residues upstream of the GXG motif (Fig. 3A and Table 2). Additionally, all Vα1 sequences had another basic amino acid residue at position 93 or 94. Most Vα3 sequences displayed the neutral residues serine and alanine at these positions. In vast majority of CDR3β regions, an acidic amino acid (aspartic or glutamic acid) was present at the end, 4–5 residues upstream of the GXG motif (Fig. 3A and Table 1). Furthermore, a serine residue was predominantly (>66%) found at position 94 (Fig. 3A and Table 1). Whereas most Vβ8.1 sequences (50.8%) coded for another acidic amino acid at position 95, most Vβ3.1 sequences (72.7%) encoded the neutral residue leucine (Fig. 3A and Table 1).
Fig. 3.
Analysis of CDR region amino acid sequences. (A) Conserved CDR3 amino acid residues (purple) and their frequency (%) among the TCR Vβ8.1, Vβ3.1, Vα3, or Vα1 chains. (B) Comparison of the CDR2β, CDR3α, and CDR1α regions between the type I and type II NKT cells. The type I and type II NKT TCR amino acid sequences are depicted in top and bottom rows, respectively. Conserved residues between type I and II are shown in green, whereas those among type II TCRs are depicted in purple. The underlined residues are crucial in binding to the αGalCer/CD1d complex (10).
These conserved CDR3 residues on TCR α- and β-chain of tetramer+ cells originate either from germline or from rearranged N-additions. Thus, S93 in Vα3 sequences is always, and L95 in Vβ3.1 sequences in >87%, encoded by V gene germline alone. In contrast, all R/K 93/94 residues among Vα1 sequences are encoded by N-additions. The residues D95 in Vβ8.1 and A94 in Vα3 sequences are encoded either by germline (69% and 43%, respectively) or by N-additions (31% and 57%, respectively).
Whereas CDR2β Residues Are Conserved, CDR3α and CDR1α Regions Are Highly Diverse Between Type I and Type II NKT Cells.
Because recent studies suggested the importance of CDR1α, CDR3α, and CDR2β residues of the invariant TCR in the recognition of glycolipid bound to CD1d (1, 9, 10, 12), we compared these regions between sulfatide-reactive type II NKT cells and type I NKT cells (Fig. 3B). In the CDR2β regions, the crucial tyrosine residues, at position 48 and 50, were found to be conserved among most Vβ8+ type I and type II NKT cells (Fig. 3B). In contrast, these residues were found in neither Vβ3.1 chains, used by type II NKT cells (Fig. 3B), nor Vβ2 chains, used by type I NKT cells. On the other hand, there appears to be a bias between the two NKT cell subsets in the CDR2β region: At position 53, Vβ8.1 and Vβ3.1 chains carry a negatively charged residue, aspartic acid or glutamic acid (Fig. 3B), whereas Vβ8.2, Vβ8.3, and Vβ2 chains hold a neutral glycine residue. The CDR1α and CDR3α regions showed significant differences between type I and type II NKT cells (Fig. 3B). Interestingly, among type II NKT cell TCRs, a number of residues were conserved regardless of Vα3 or Vα1: at positions 27–29, SAT and SAS motives for most Vα3 and Vα1, respectively, were found, and at position 31, a tyrosine residue was found.
Sulfatide/CD1d-Tetramer+ Cells Are Increased in Mice Deficient in Sulfoglycolipids.
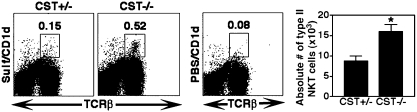
To determine whether absence of the self-glycolipid influences frequency of type II NKT cells, liver MNCs from mice deficient in ceramide galactosyl sulfotransferase (CST−/− mice) were analyzed. CST−/− mice lack all sulfoglycolipids including sulfatide (26), because the CST enzyme is involved in the synthesis through addition of a sulfate group to galactosyl ceramide. FACS analysis showed a significant increase in the number of sulfatide/CD1d-tetramer+ cells in livers of CST−/− mice in comparison with their littermates (Fig. 4). These data suggest that other CD1d-binding self-glycolipids might be sufficient for the thymic selection of sulfatide-reactive type II NKT cells.
Fig. 4.
Increased numbers of sulfatide-reactive type II NKT cells are present in sulfoglycolipid-deficient mice. (Left and Center) Flow cytometric profiles of liver MNCs from CST−/− or CST+/− mice following staining with sulfatide-loaded or unloaded (PBS) tetramers and anti-TCRβ. Numbers within boxes indicate the percentage of positive cells in total lymphocytes. Data are representative of three individual experiments. (Right) Bar graphs summarizing absolute numbers of sulfatide/CD1d-tetramer+ cells in CST−/− vs. CST+/− mice (n = 3 per group). *, P < 0.05.
Discussion
This study characterizes the TCR V gene repertoire of a major CD1d-restricted type II NKT cell subset recognizing a β-linked self-glycolipid, sulfatide. Sulfatide-reactive NKT cells are oligoclonal and use predominantly the TCR Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments with few dominant clonotypes of restricted CDR3 lengths and conserved amino acid residues. CDR3 regions of both TCR α- and β-chains are encoded by germline sequences or germline sequences with N-additions. Collectively, our data emphasize this type II NKT cell subset displays both innate-like features and characteristics of antigen-specific conventional MHC-restricted T cells.
The oligoclonal TCR V gene usage by sulfatide/CD1d-tetramer+ cells isolated from naïve or unprimed animals is quite significant. Consistently, only a low expression of CD69 is found on most of the tetramer+ cells (Fig. 1A), similar to other Vα3.2/Vβ9+ type II NKT cells (27, 28). It is remarkable that in the absence of any external immunization with the antigen, spectratyping (Fig. 2) and sequencing analysis (Tables 1 and 2) indicate the presence of dominant clonotypes of limited CDR3 lengths in this NKT subset. Because tetramers are loaded with bovine brain-derived sulfatide consisting of several molecular species only differing in the CD1d-binding domain (19), the TCR V-J gene usage is likely to be overlapping among TCRs reactive to individual sulfatides. Two findings are consistent: (i) cells stained with tetramers loaded with an immunodominant synthetic cis-tetracosenoyl sulfatide also are predominantly Vβ8+, Vα3+, and Vβ3+ (Fig. S4); and (ii) antigen fine specificity of a sulfatide-reactive hybridoma, Hy19.3, showed that it is only strongly reactive to lyso-sulfatide (17, 18, 29) and utilizes the TCR Vα1-Jα26 and Vβ16-Jβ2.1 gene segments with similar CDR1α, CDR3α, and CDR2β sequences to the TCRs of sulfatide/CD1d-tetramer+ cells (Fig. S5). Further analysis of individual T cell hybridomas is required to clarify this issue.
Only some but not all type II NKT cells recognize sulfatide and accordingly should be reactive to other lipid antigens and should use distinct TCR repertoires. Consistent with this idea, a number of CD1d-reactive T cell hybrids isolated from MHC class II-deficient mice (15, 16, 28) are Vα3+, Vα8+, and Vα4+ and do not show any reactivity to sulfatide (17, 18). Accordingly, none of them expressed the TCR Vα-Jα and Vβ-Jβ gene segments predominantly used by sulfatide/CD1d-tetramer+ cells. These data suggest that the TCR repertoire of this type II NKT cell subset also has key features similar to that of the conventional MHC-restricted self-protein-reactive T cells and can distinguish different lipids.
Several observations suggest the antigen specificity as well as selection of certain conserved amino acid residues among the TCRs of sulfatide-reactive type II NKT cells. (i) Distinct TCR clones with different DNA sequences in the CDR3β region encode for the same conserved amino acids [Vβ8.1 clones 3, 45 vs. 19, Vβ8.1 clone 99 vs. 105, and Vβ8.1 clones 91, 117 vs. 124 (Table 1)]. (ii) Conserved amino acid residues in the CDR3 regions can be generated by germline DNA sequences or by N-additions—for example, D95 in Vβ8.1 and A94 in Vα3 are encoded by N-additions in 31% and 57%, respectively. (iii) Conserved basic amino acids at position 93 or 94 of Vα1 are all encoded by N-additions. (iv) A predominant usage of the Jβ2.7 gene segment, restricted lengths, and several conserved residues suggest a key role of the CDR3β region in recognition of sulfatide, similar to that in conventional MHC-restricted T cells. Conserved residues selected in different CDR regions of the TCRs are likely to play an important role in binding to sulfatide/CD1d complex.
Sulfatide-reactive type II NKT cells also display several features of innate-like lymphocytes. These cells express NK cell markers and secrete cytokines rapidly upon stimulation with their self-lipid ligand (17, 25). The TCR α- and β-chains of type II NKT are encoded by germline gene segments, and some of the conserved CDR3 region residues (e.g., S93 in Vα3) are only generated by the germline sequences. Despite the sharing of some of these features, there are important differences among type II and type I NKT cells or MR-1-restricted mucosal-associated invariant Vα19-Jα33+ T (MAIT) cells (3, 30, 31). For example, the latter subsets have an activated/memory phenotype, and their TCRs are comprised of an invariant α-chain. Accordingly, CDR3α regions of type I NKT and MAIT cells are 75–88% encoded by germline gene segments, whereas only ∼14% are in the case of type II NKT cells. In contrast, 13–27% of CDR3β regions among type II NKT cells, but none among type I NKT or MAIT cells, are germline-encoded. Furthermore, CDR3β regions in type II NKT cells show a predominant usage of the Jβ2.7 gene segment, restricted lengths, and conserved residues not found in type I NKT or MAIT cells (3–5, 30–32). The TCR repertoire of type II NKT cells is highly oligoclonal with respect to both the TCR α- and the β-chains, whereas it is primarily restricted to the α-chain for type I NKT and MAIT cells.
Recent structural studies have suggested the involvement of crucial residues within the CDR2β, CDR1α, and CDR3α loops of the semi-invariant TCR in recognition of the αGalCer/CD1d complex (9–12). It is notable that two conserved residues (48Y and 50Y) in the CDR2β loop of the type I NKT TCR that contact the CD1d molecule (9–12) are also present in the Vβ8.1 TCRs of type II NKT cells (Fig. 3B). Consistent with the idea that other CDR2β region residues in the Vβ2 and Vβ7 type I NKT TCRs (11, 13) can also bind to CD1d, these tyrosine residues are not present in the Vβ3.1 TCRs of the type II NKT cells. It is noteworthy that all Vβ8.1 and Vβ3.1 TCRs of type II NKT cells carry a negatively charged residue at position 53 in the CDR2β region, whereas a neutral glycine residue is present in most TCRs of type I NKT cells (Fig. 3B). It will be interesting to examine whether residue 53 may play a key role in recognition of the sulfatide/CD1d complex. In contrast to the CDR2β region, CDR3α as well as CDR1α regions between the type I vs. type II subsets are quite different in almost every residue. However, CDR1α regions among TCRs of the type II NKT cells are very similar and comprise abundant hydroxyl groups. Because this region of the invariant TCR contacts solely the galactose moiety (9–11), it is possible that these hydroxyl groups bind to the hydrophilic sulfated galactose. A conserved CDR3β region among TCRs of type II NKT cells is likely to be involved in the recognition of the sulfatide/CD1d complex, consistent with the recent studies of invariant TCRs (11, 13). The β-linkage of galactose and the presence of a negatively charged sulfate group in sulfatide indicates that the sulfatide/CD1d complex is likely to have a different conformation from αGalCer/CD1d for TCR recognition (19) and may reflect differences in recognition of the β-linked vs. the α-linked glycolipids.
Several studies on experimental models of autoimmune diseases, parasitic diseases, and antitumor immunity suggest that type I and type II NKT cells play opposite or cross-regulating roles (24, 25, 33, 34). Thus, sulfatide-reactive type II NKT cells suppress antitumor immunity in several tumor models, whereas type I NKT cells promote immune responses against cancer (24, 35). Furthermore, sulfatide-mediated activation of type II NKT cells protects animals from experimental autoimmunity including EAE and Con A-induced hepatitis, models for multiple sclerosis and liver injury, respectively (17, 25). Earlier data (17) showed that sulfatide-reactive type II NKT cells infiltrate into the CNS during EAE. Because tissues are enriched in different sulfatides, tissue-infiltrating type II NKT cells may display distinct TCR repertoires during disease.
Whether and how the TCR repertoire of self-glycolipid-reactive CD1d-restricted T cells is influenced by the presence or absence of the respective glycolipid is not yet clear. Some of the complicating factors in these studies are related to the presence of minute quantities of glycolipids, alternate synthesis pathways, and ability to detect low levels of lipids that can potentially influence thymic selection. Nevertheless, except for the earlier report related to the complete absence of type I NKT cells in iGb3-deficient mice (7), other studies have demonstrated normal levels of NKT cells in the self-glycolipid–deficient animals (36, 37). We have consistently found an increased number of sulfatide-reactive type II NKT cells in mice lacking sulfatide: CST−/− mice deficient in all sulfolipids and CGT−/− mice devoid of all galactolipids (17). These data suggest that self-ligands other than sulfatide and iGb3 can potentially select type II and type I NKT cells, respectively, in the thymus similar to that for conventional MHC-restricted T cells (38). Furthermore, sulfatides may be involved in the negative selection of sulfatide-reactive type II NKT cells.
Studies presented here describe the nature of the TCR repertoire of a major self-glycolipid-reactive type II NKT cell subset involved in the regulation of autoimmune diseases and in suppression of antitumor responses. The findings provide a molecular tool for tracking these cells under physiological conditions in vivo and have implications for the recognition of β-linked glycolipids by T cells in general. In this regard, a detailed knowledge of glycolipid–CD1d–TCR interactions conserved from mice to humans may help in the development of HLA-independent immunotherapeutics for T cell-mediated inflammatory diseases.
Material and Methods
Animals.
C57BL/6 mice were purchased from The Jackson Laboratory. CD1d−/− and Jα18−/− BL/6 mice were originally generated by Van Kaer and Taniguchi (6), respectively. C57BL/6-CST−/− mice were acquired from K. Honke (Osaka University, Japan). All mice were bred and maintained in specific pathogen-free conditions. Treatment of animals was in compliance with federal and institutional guidelines and approved by the Torrey Pines Institute for Molecular Studies Animal Care and Use Committee.
Lipids and Tetramers.
Purified bovine myelin-derived sulfatide (>90% pure) was purchased from Matreya, mono-GM1 was from Sigma, and synthetic α-GalCer was provided by Y. Koezuka (Kirin Brewery Co.). All lipids were dissolved in vehicle (0.5% Tween-20 and 0.9% NaCl solution) and diluted in PBS. PE-labeled murine CD1d tetramers were generated using a baculovirus expression system as described in ref. 17.
Cell Isolation, Sorting, and Flow Cytometry.
MNCs were isolated from murine livers, using Percoll gradient as described in ref. 25. For cell sorting, liver MNCs were collected from a total of 40 Jα18−/− mice (10 per sort). After blocking, cells were stained with sulfatide/mCD-tetramer-PE and anti-TCRβ-FITC, resuspended in basic sorting buffer [PBS containing 1 mM EDTA, 25 mM Hepes (pH 7.0), and 1% FCS, filtered], and sorted on a BD FACSAria instrument (at The Scripps Research Institute). For FACS analysis, after blocking with anti-mouse FcR-γ (BD Pharmingen), MNCs were stained with CD1d-tetramers or indicated mAbs (BD Pharmingen or eBioscience) and analyzed on a FACSCalibur instrument using CellQuest software (version 4.0.2; BD).
Coculture Assay.
CD11c+ DCs were isolated from spleens of Jα18−/− mice, using CD11c MicroBeads (Miltenyi Biotec) as described in ref. 25. Splenocytes from CD1d−/− mice served as filler cells. Sorted sulfatide/CD1d-tetramer+ cells (16,000 cells per well) were cultured with 20,000 DCs per well and filler cells (400,000 cells per well), and incubated with sulfatide (20 μg/mL), αGalCer (10 ng/mL), or monoGM-1 (20 μg/mL), or without antigen. IFN-γ levels in supernatants by ELISA (17, 39) and [3H]thymidine incorporation was assessed after 48 and 72 h, respectively (17, 39).
RT-PCR and DNA Sequencing.
RNA was extracted using the RNeasy Mini Kit (Qiagen) from two different sorts from Jα18−/− mice and from unsorted hepatic MNCs or splenocytes from BL/6 mice. Following reverse transcription, equal amounts of cDNA were subjected to PCR using TCR Vβ-Cβ and Vα-Cα primers (40, 41). Purified PCR products (Qiagen) were ligated into pCR 2.1-TOPO TA cloning vector (Invitrogen). Purified plasmid DNA was sequenced (Retrogen), and sequences were analyzed using DNA Strider 1.2 software. The CDR3 region DNA sequences were compared with germline matrices from the IMGT databank, and amino acid sequences were deduced from the respective alleles in the IMGT databank.
Spectratyping Analysis.
TCR CDR3 length spectratyping analysis was performed as described in ref. 42. For TCR CDR3α analysis, cDNA was amplified accordingly with respective Vα primers and Cαa primer (41). Respective Vβ-Cβ and Vα-Cα PCR products were subjected to run-off extension reactions with fluoresceinated Cβ5 (5′-FAMCTTGGGTGGAGTCACATTTCTC-3′) or Cαb primers (5′-FAM-ACACAGCAGGTTCTGGGTTC-3′) and different fluoresceinated Jβ primers (40). PCR products were separated on the basis of their length on automated ABI PRISM 3100 genetic analyzer, and CDR3 lengths were calculated.
Statistics.
Data are expressed as mean ± SD for each group. Statistical differences between groups were evaluated by unpaired, two-tailed Student's t test using GraphPad Prism 4.03 software. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. Randle Ware and Marc Hertz for critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01-CA100660 (to V.K.), the Juvenile Diabetes Research Foundation (V.K.), the Multiple Sclerosis National Research Institute (V.K.), the Diabetes National Research Group (V.K.), and Deutsche Forschungsgemeinschaft Grant AR 645/1-1 (to P.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000576107/-/DCSupplemental.
References
- 1.Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28:304–314. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 5.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kawano T, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 8.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 9.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 10.Wun KS, et al. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med. 2008;205:939–949. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellicci DG, et al. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott-Browne JP, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 13.Mallevaey T, et al. T cell receptor CDR2β and CDR3β loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- 15.Cardell S, et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SH, et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193:893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahng A, et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy KC, et al. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 19.Zajonc DM, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Exley MA, et al. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 21.Fuss IJ, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaulov A, et al. Peripheral blood progenitor cell product contains Th1-biased noninvariant CD1d-reactive natural killer T cells: Implications for posttransplant survival. Exp Hematol. 2008;36:464–472. doi: 10.1016/j.exphem.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang DH, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosino E, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: A new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 25.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honke K, et al. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci USA. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenström M, et al. Surface receptors identify mouse NK1.1+ T cell subsets distinguished by function and T cell receptor type. Eur J Immunol. 2004;34:56–65. doi: 10.1002/eji.200323963. [DOI] [PubMed] [Google Scholar]
- 28.Duarte N, et al. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol. 2004;173:3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 29.Blomqvist M, et al. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol. 2009;39:1726–1735. doi: 10.1002/eji.200839001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilloy F, et al. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda JL, et al. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor β repertoire and small clone size. Proc Natl Acad Sci USA. 2001;98:12636–12641. doi: 10.1073/pnas.221445298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–192. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallevaey T, et al. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75:2171–2180. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renukaradhya GJ, et al. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porubsky S, et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speak AO, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshizawa I, Bronson R, Dorf ME, Abromson-Leeman S. T-cell responses to myelin basic protein in normal and MBP-deficient mice. J Neuroimmunol. 1998;84:131–138. doi: 10.1016/s0165-5728(97)00205-1. [DOI] [PubMed] [Google Scholar]
- 39.Jahng AW, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currier JR, Robinson MA. Spectratype/immunoscope analysis of the expressed TCR repertoire. Curr Protoc Immunol. May 2001;10.28 doi: 10.1002/0471142735.im1028s38. 10.1002/0471142735.im1028s38. [DOI] [PubMed] [Google Scholar]
- 41.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: Implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madakamutil LT, Maricic I, Sercarz EE, Kumar V. Immunodominance in the TCR repertoire of a [corrected] TCR peptide-specific CD4+ Treg population that controls experimental autoimmune encephalomyelitis. J Immunol. 2008;180:4577–4585. doi: 10.4049/jimmunol.180.7.4577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.