Abstract
In Egypt, efforts to control highly pathogenic H5N1 avian influenza virus in poultry and in humans have failed despite increased biosecurity, quarantine, and vaccination at poultry farms. The ongoing circulation of HP H5N1 avian influenza in Egypt has caused >100 human infections and remains an unresolved threat to veterinary and public health. Here, we describe that the failure of commercially available H5 poultry vaccines in Egypt may be caused in part by the passive transfer of maternal H5N1 antibodies to chicks, inhibiting their immune response to vaccination. We propose that the induction of a protective immune response to H5N1 is suppressed for an extended period in young chickens. This issue, among others, must be resolved and additional steps must be taken before the outbreaks in Egypt can be controlled.
Keywords: maternally transferred antibodies, immune, suppression
H5N1 highly pathogenic avian influenza (HPAI) virus has so far not consistently been transmitted from human to human (1), despite its circulation in multiple Eurasian epicenters for more than a decade (2). However, the pandemic (H1N1) 2009 influenza virus has spread globally in humans after transmission from the zoonotic reservoir (3), and it could potentially acquire the high lethality of the H5N1 HPAI virus for humans via reassortment (4, 5). This possibility increases the potential public health threat of H5N1 HPAI. Since its emergence in domestic geese in Guangdong Province, China (6, 7), the H5N1 HPAI virus has evolved to form at least 10 distinct phylogenetic clades (8) and has spread to wild migratory waterfowl (9) and to multiple mammalian hosts (10–16). In April 2005, a massive die-off of wild waterfowl was caused by H5N1 HPAI viruses at Qinghai Lake in China (17, 18). After that event, one genotype spread westward to Central and Southern Asia, Europe, and Africa (19). The World Organization for Animal Health (OIE) and the World Health Organization (WHO) have now reported H5N1 HPAI infection in >60 countries and >400 human infections in 16 of these countries (5).
In Africa, H5N1 HPAI infection of domestic birds was reported first in Nigeria in early 2006 and subsequently in Egypt, Niger, Cameroon, Burkina Faso, Sudan, Cote d'Ivoire, and Togo (5). Of these countries, Egypt has been most severely affected by continuous outbreaks, resulting in severe losses in the poultry industry, with >100 human cases, and 34 human deaths (5, 20). As of July 2008, Egypt reported outbreaks in nine governorates (Gharbiyah, Minufiyah, Kafr Ash Shaykh, Daqahliyah, Sharqiyah, Minya, Jizah, Suhaj, and Luxor) in commercial and backyard poultry, and poultry in live bird market from 7 February to 14 June 2008. At this time, the national veterinary Service (GOVS) declares H5N1 to be endemic in Egypt; Indonesia is the only other country with endemic H5N1 HPAI (5, 20). To control and attempt to eradicate the H5N1 HPAI viruses, the Egyptian agricultural authorities have used vaccination, attempted to heighten biosecurity, and used quarantine measures on poultry farms (5, 20). However, even three doses of vaccine (inactivated oil–whole-virus emulsion H5N1 vaccines imported from China and Europe) have failed to provide the expected level of protection against the currently circulating clade 2.2.1 H5N1 viruses (21). Despite the attempted implementation of these measures, the current strategies have limitations (22).
Antibodies to the circulating virus strain had been detected in day-old chicks in Egypt (see below). Because passive transfer of maternal antibody through the yolk sac is known to interfere with immunization against both infectious bursal disease virus (23, 24) and Newcastle disease virus (NDV) (25, 26), we hypothesized that maternally transferred antibody was inhibiting vaccine induction of anti-H5N1 immunity. We examined the immunogenicity and protective efficacy of the imported commercial H5 influenza vaccines against the circulating Egyptian H5N1 HPAI isolate (21) in chickens that had and had not been exposed to passive antibody transfer. Here, we demonstrate that the vaccine failure was indeed caused by transfer of maternal immunity through the yolk sac.
Results
Characterization of A/Chicken/Qalubia-Egypt/1/08 (H5N1) Influenza Virus.
Antigenic drift in the circulating clade 2.2.1 H5N1 viruses was one possible explanation for the failure of commercially available H5N1 poultry vaccines in Egypt. To determine whether antigenic drift had occurred in the currently circulating A/Chicken/Qalubia-Egypt/1/08 (Ck/Qal-Egypt/1/08) isolate, we compared the hemagglutination inhibition (HI) titers of sequential isolates, using clade 2.2 H5N1 ferret antisera. The HI titers of the viruses isolated from domestic poultry in Egypt in 2007 and 2008 differed from those of the first viruses isolated in Egypt (in 2006) by a factor of 4 (Table 1), demonstrating only minor antigenic drift. Sequence comparison of the hemagglutinin (HA) of representative H5N1 Egyptian isolates from 2006 onward and comparison of their amino acid sequences gave no clear indicator of antigenic drift (27). The Ck/Qal-Egypt/1/08 virus was uniformly lethal to chickens within 5 d at doses >102.5 EID50, also causing hemorrhages of the legs and face and central nervous system disease signs (data not shown). On the basis of this result, the challenge dose for subsequent studies was 10 LD50 (103.5 EID50).
Table 1.
Cross-reactions between Egyptian H5N1 influenza viruses in hemagglutination inhibition (HI) tests with ferret antisera
| H5N1 viruses | Antisera to | ||||
| WSM-05 | BHG-05 | T15-06 | CEG-06 | TQE-07 | |
| A/WHOOPER SWAN/MONGOLIA/244/2005 (WSM-05) | 80 | 80 | 40 | 160 | 80 |
| A/BAR-HEADED GOOSE/QINGHAI/1A/2005 (BHG-05) | 80 | 80 | 40 | 160 | 80 |
| A/TURKEY/15/2006 (human) (T15-06) | 80 | 80 | 80 | 160 | 80 |
| A/CHICKEN/EGYPT/1C/2006 (CEG-06) | 80 | NA | 80 | 160 | 80 |
| A/TURKEY/QALUBIA-EGYPT/7/2007 (TQE-07) | 20 | 20 | 10 | 80 | 80 |
| A/CHICKEN/QALUBIA-EGYPT/1/2008 | 20 | 10 | 10 | 40 | 10 |
HI assays were performed using 0.5% CRBCs. NA, not available.
Protective Efficacy of Four Commercial H5 Influenza Vaccines in Chickens.
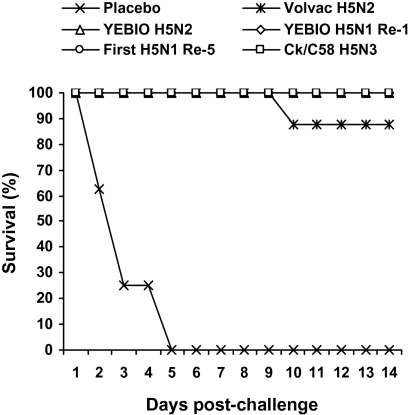
We compared death, clinical signs, and shedding of the challenge virus in groups of eight chickens vaccinated with four commercial inactivated oil emulsion–whole-virus H5 influenza vaccines and then challenged with a lethal dose of Ck/Qal-Egypt/1/08 (H5N1) virus. A placebo vaccine containing virus-free allantoic fluid and a standardized, inactivated oil emulsion–whole-virus H5N3 influenza vaccine containing 1.2 μg of hemagglutinin protein were tested as well (Fig. 1 and Table 2).
Fig. 1.
Survival curves of chickens (n = 8 per group) immunized with two doses of one of the four commercial H5 influenza vaccines, of placebo vaccine containing virus-free allantoic fluid, or of control Ck/C58 H5N3 vaccine (known to be efficacious). After challenge with Ck/Qal-Egypt/1/08 (H5N1) virus, the chickens were observed for 14 d.
Table 2.
Tracheal and cloacal titers of Ck/Qal-Egypt/1/08 (H5N1) virus in chickens immunized with commercial H5 vaccines
| Vaccine | Virus titers* | |||||||
| Trachea | Cloaca | |||||||
| 3 dpc | 5 dpc | 7 dpc | 9 dpc | 3 dpc | 5 dpc | 7 dpc | 9 dpc | |
| Placebo | 3.5 ± 1.1 (3/3) | ndb | nd | nd | 1.1 ± 0.9 (3/3) | nd | nd | nd |
| Volvac H5N2 | 2.4 ± 1.6 (2/8) | 4.3 ± 0.0 (1/8) | 4.5 ± 0.0 (1/8) | 2.5 ± 0.0 (1/8) | <(0/8) | <(0/8) | <(0/8) | 3.3 ± 0.0 (1/8) |
| YEBIO H5N2 | <(0/8)† | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) |
| YEBIO H5N1 Re-1 | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) |
| First H5N1 Re-5 | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) |
| Ck/C58 H5N3‡ | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) | <(0/8) |
dpc, days postchallenge; nd, not determined because of death.
*Log10 EID50/mL, determined in eggs. Data are the mean ± SD from positive samples (≥0.75 log10 EID50/mL). Values in parentheses are number shedding/number tested.
†<, the titer was below the limit of detection (<0.75 log10 EID50/mL).
‡Vaccine containing 1.2 μg of HA protein of A/Chicken/Vietnam/C58/04 (H5N1).
Fig. 1 compares survival after challenge. Three of the four commercial H5N1 vaccines and the standardized Ck/C58 vaccine provided complete protection: there were no deaths, disease signs, or detectable virus shedding from the cloaca or the trachea. One of eight birds in the Volvac H5N2 vaccine group died after showing severe disease signs (severe listlessness and depression with episodic neurological dysfunction); the remaining seven birds survived with no disease signs. Two birds in this group shed virus (≤4.5 log10 EID50), mainly from the trachea (Table 2). All birds given placebo vaccine were dead by day 5 postchallenge.
Serologic Responses to Commercial H5 Vaccines.
The immunogenicity of the vaccines was examined by determining vaccinated chickens’ serum HI antibody titers to the challenge virus, using chicken red blood cells (CRBCs) or horse red blood cells (HRBCs) (Table 3). All birds were seronegative for H5N1 viruses before vaccination. When measured with CRBCs, HI antibody titers were undetectable 4 wk after immunization with three of the vaccines (Table 3); the Re-5 and control Ck/C58 vaccines induced low titers. After boost vaccination, the Re-5 vaccine produced modest antibody levels [geometric mean titer (GMT), 87], but the remaining vaccines induced low antibody levels (HI titer ≤24) (Table 3). After challenge, only the Re-5 vaccine had induced significant HI titers (GMT, 80) (Table 3).
Table 3.
Serologic responses (HI antibody titers) induced by the commercial H5 vaccines against Ck/Qal-Egypt/1/08 (H5N1) virus
| Vaccine | HI GMT* | |||||||
| CRBCs | HRBCs | |||||||
| Prevac | 4 wpv | 3 wpb | 2 wpc | Prevac | 4 wpv | 3 wpb | 2 wpc | |
| Placebo | <10 (0/8) | <10 (0/8) | <10 (0/8) | nd | <10 (0/8) | <10 (0/8) | <10 (0/8) | nd |
| Volvac H5N2 | <10 (0/8) | <10 (0/8) | 20 (4/8) | 40 (3/7) | <10 (0/8) | 10 (4/8) | 54 (7/8) | 98 (7/7) |
| YEBIO H5N2 | <10 (0/8) | <10 (0/8) | 14 (2/8) | 17 (4/8) | <10 (0/8) | 40 (3/8) | 145 (8/8) | 195 (8/8) |
| YEBIO H5N1 Re-1 | <10 (0/8) | <10 (0/8) | 24 (4/8) | 20 (4/8) | <10 (0/8) | 20 (5/8) | 127 (8/8) | 160 (8/8) |
| H5N1 Re-5 | <10 (0/8) | 11 (6/10) | 87 (8/8) | 80 (8/8) | <10 (0/8) | 67 (8/8) | 1076 (8/8) | 987 (8/8) |
| Ck/C58 H5N3 | <10 (0/8) | 10 (1/10) | 14 (6/8) | 12 (8/8) | <10 (0/8) | 27 (7/8) | 175 (8/8) | 160 (8/8) |
GMT, geometric mean titer; wpv, weeks postvaccination; wpb, weeks postboost; wpc, weeks postchallenge; prevac, prevaccination; nd, not determined because of death.
*Four weeks after administration of the indicated H5 vaccines, boost vaccination was given. Lethal challenge with Ck/Qal-Egypt/1/08 (H5N1) occurred 3 wk postboost. HI titers against the challenge virus are the reciprocals of the highest dilutions of sera that inhibited hemagglutination by 4 HA units of virus. Values in parentheses are number of chickens with positive sera /total number.
When tested with HRBCs (Table 3), the HI antibody responses varied according to the level of protection provided by the vaccines. The Re-5 vaccine was the most immunogenic, inducing modest levels of HI antibodies 4 wk postvaccination (GMT, 67) and high levels after boost vaccination (GMT, 1076) and challenge (GMT, 987). Two of the commercial vaccines (Yebio H5N2 and Yebio H5N1 Re-1) and the control vaccine (Ck/C58) induced significant antibody levels (HI titer >40) only after boost vaccination. Only the Volvac H5N2 vaccine failed to induce significant antibody levels in all birds, but seven of the eight birds showed significant antibody titers (GMT, 54) after boost vaccination. The placebo vaccine induced no detectable antibodies to H5N1 virus.
Do Antibodies to H5N1 Interfere with Induction of Immunity?
The above results suggest that most of the commercially available H5N1 vaccines tested should be efficacious in Egyptian chickens. However, these vaccines had been only partially efficacious on Egyptian poultry farms (20, 22). To investigate whether maternal antibodies interfere with induction of protective immunity, we injected 7-d-old, seronegative, specific-pathogen-free (SPF) chickens i.p. with pooled anti-H5 chicken sera (HRBC HI titer, 80). One day later, the chickens were immunized with the YEBIO H5N1 Re-1 vaccine; they received a boost dose after 4 wk and were challenged after an additional 3 wk.
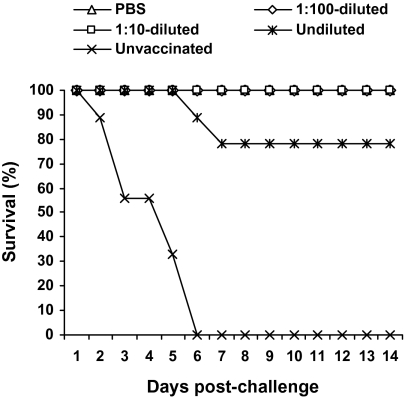
Fig. 2 shows the efficacy of the YEBIO Re-1 H5N1 vaccine in passively immunized and control chickens. Chickens that received PBS or 1:10 or 1:100 dilutions of H5 antisera before vaccination showed no disease signs after challenge with the lethal Egyptian H5N1 virus, whereas chickens that received the undiluted H5 antisera had severe disease signs (severe listlessness and depression with episodic neurological dysfunction) and 22% lethality. Four of nine birds passively immunized before vaccination shed the challenge virus from the trachea (titers ≤4.8 log EID50/mL) and cloaca (titers ≤5.0 log EID50/mL) (Table 4). All chickens in a control group that received the undiluted H5 antisera but no vaccine shed virus from the trachea (≤6.6 log EID50/mL) and cloaca (≤5.4 log EID50/mL) after showing typical disease signs. These results suggest that the transfer of H5 antibodies to chickens before vaccination interferes with induction of protective immunity by the commercial vaccine.
Fig. 2.
Survival curves of chickens passively immunized by retroperitoneal injection of pooled anti-H5N1 chicken sera (undiluted, 1:10-diluted, or 1:100-diluted) or PBS and vaccinated with two doses of YEBIO H5N1 Re-1 vaccine before challenge with Ck/Qal-Egypt/1/08 (H5N1) virus. A control group of chickens were injected i.p. with undiluted sera but were not vaccinated before challenge. The chickens were observed for 14 d after challenge.
Table 4.
Titers of Ck/Qal-Egypt/1/08 (H5N1) virus in chickens immunized passively with H5 antibodies before vaccination with the YEBIO H5N1 Re-1 vaccine
| i.p. injection with | Virus titers* | |||||
| Trachea | Cloaca | |||||
| 3 dpc | 5 dpc | 7 dpc | 3 dpc | 5 dpc | 7 dpc | |
| PBS | <(0/9)† | <(0/9) | <(0/9) | <(0/9) | <(0/9) | <(0/9) |
| 1:100-diluted sera | <(0/9) | <(0/9) | <(0/9) | <(0/9) | <(0/9) | <(0/9) |
| 1:10-diluted sera | <(0/9) | <(0/9) | <(0/9) | <(0/9) | <(0/9) | <(0/9) |
| Undiluted sera | 3.5 ± 2.3 (2/9) | 4.8 ± 1.9 (2/9) | 4.0 ± 2.0 (3/8) | 3.6 ± 1.4 (2/9) | 5.0 ± 0.7 (2/9) | 3.8 ± 0.0 (1/7) |
| Control‡ | 4.8 ± 1.6 (8/8) | 6.6 ± 0.8 (5/5) | nd | 5.4 ± 1.2 (3/8) | 4.8 ± 0.7 (5/5) | nd |
dpc, days postchallenge; nd, not determined because of death.
aLog10 EID50/mL determined in eggs. Data are the mean ± SD of positive (=0.75 log10 EID50/mL) titers. Values in parentheses are number shedding/number tested.
†<, the titer was below the limit of detection (<0.75 log10 EID50/mL).
‡Chickens were immunized passively with pooled anti-H5 chicken sera but not vaccinated before challenge.
Serologic Response to Vaccine after Passive Immunization.
Serologic responses to the YEBIO H5N1 Re-1 vaccine after passive immunization were determined by HI testing. CRBC agglutination was not inhibited (as shown in Table 3 at 3 wk postvaccination), but HRBC agglutination was inhibited (Table 5), demonstrating an antibody response. In a group given i.p. PBS rather than pooled anti-H5 sera, the vaccine induced protective levels of HI antibodies. In contrast, chickens that received undiluted H5 antisera showed attenuation of the antibody response: 4 wk after vaccination, the GMT was 17 and only four of the nine birds had detectable HI titers. Attenuation of the immune response continued after boost vaccination and after virus challenge; one of the two birds that died after challenge had shown no detectable HI titer, and the other bird had low HI titer (HI titer, 20). Chickens that received diluted antisera did not show attenuation of the antibody response; in fact, the GMTs in the group receiving 1:100-diluted antisera were higher than those in the PBS control group. Taken together, these results indicate that passive administration of a modest amount of H5 antibody (HI titer, 80) has a suppressive effect on the immune response to vaccine.
Table 5.
Influence of passively transferred H5 antibodies on the serological response of chickens to the commercial H5N1 vaccine
| i.p. injection with | HRBC HI GMT* | |||
| Prevac† | 4 wpv | 3 wpb | 2 wpc | |
| PBS | <10 (0/9) | 49 (7/9) | 86 (9/9) | 101 (9/9) |
| 1:100-diluted sera | <10 (0/9) | 59 (9/9) | 137 (9/9) | 209 (9/9) |
| 1:10-diluted sera | <10 (0/9) | 31 (8/9) | 80 (9/9) | 137 (9/9) |
| Undiluted sera | <10 (0/9) | 17 (4/9) | 47 (8/9) | 49 (7/7) |
| Control‡ | <10 (0/9) | <10 (0/9) | <10 (0/9) | ndd |
Prevac, prevaccination; wpv, weeks postvaccination; wpb, weeks postboost; wpc, weeks postchallenge; nd, not determined because of death.
*Boost vaccination with YEBIO H5N1 Re-1 vaccine occurred 4 wk after initial vaccination, and challenge with the lethal Ck/Qal-Egypt/1/08 (H5N1) virus occurred 3 wk later. HI titers against challenge virus (Ck/Qal-Egypt/1/08 [H5N1]) are the reciprocals of the highest dilutions of sera that inhibited hemagglutination by 4 HA units of virus. The results are the geometric mean titers of positive sera (≥10). Values in parentheses are number of chickens with positive sera/total number.
†The prevaccination samples were collected from 1-wk-old chickens on the day before i.p. injection of H5 antibodies.
‡Chickens were immunized passively with pooled anti-H5 chicken sera but not vaccinated before challenge.
Prevalence of Maternal Anti-H5 Antibodies on Egyptian Chicken Farms.
We performed HI testing of the yolk sacs from 20 eggs that had hatched within the previous day at an Egyptian poultry farm where the Yebio H5N1 Re-1 vaccine is used. PBS extracts of all 20 yolk sacs inhibited agglutination of HRBCs but not CRBCs (Table 6). The HI titers ranged from 10 to 160 (GMT, 48). Therefore, at least 50% of the day-old chicks had antibody titers ≥80, which could modulate the immune response to H5N1 vaccine.
Table 6.
Distribution of HI titers against A/Turkey/Egypt/7/06 (H5N1) virus in the yolk sacs of 20 1-d-old hatched chicken eggs from an Egyptian poultry farm
| HI antibody titer | Number (%) yolk sacs | |
| CRBCs | HRBCs | |
| <10 | 20 (100%) | 0 (0%) |
| 10 | 0 (0%) | 3 (15%) |
| 20 | 0 (0%) | 2 (10%) |
| 40 | 0 (0%) | 4 (20%) |
| 80 | 0 (0%) | 9 (45%) |
| 160 | 0 (0%) | 2 (10%) |
| GMT | — | 48 |
CRBCs, chicken red blood cells: HRBCs, horse red blood cells; GMT, geometric mean titer. Titers are the reciprocals of the highest serum dilutions that inhibited hemagglutination by 4 HA units of virus.
Discussion
Since early 2006, H5N1 HPAI has continued to spread in the domestic poultry farms of Egypt despite the implementation of quarantine, improved biosecurity, and vaccination (20, 22). We found that under laboratory conditions, three of the four commercially available vaccines used in Egypt provide complete protection of seronegative SPF chickens against the current Egyptian HPAI H5N1 isolate; we observed protective antibody levels and the absence of morbidity, mortality, and virus shedding (Fig. 1 and Tables 2 and 3). The Volvac H5N2 vaccine induced less antibody and provided less protection (two of the eight challenged birds shed virus and one died).
The failure of the Volvac vaccine containing A/Ck/Mexico/232/94 (H5N2) to provide complete protection was not surprising, because this virus is only distantly related antigenically to any of the HPAI H5N1 clades. We were very surprised, however, that the Yebio H5N2 vaccine, based on a 1973 H5N2 isolate (A/Turkey/England/N-28/73), provided complete protection. One of the continuing difficulties with agricultural vaccines is that they are not standardized for antigen content, and every batch must therefore be evaluated by immunization and challenge. Our findings do show that although agricultural vaccines administered with oil emulsion adjuvants provide extensive cross-protection between antigenically and phylogenetically distinct H5 viruses, the vaccine most closely related to the clade 2.2.1 H5N1 Egyptian viruses (Re-5) induced the highest levels of antibody to the challenge virus. Overall, our results show that most of the commercially available H5 vaccines should be efficacious in Egyptian chickens when administered under optimal conditions.
Although the H5N1 viruses isolated in Egypt in 2007 and 2008 showed minor antigenic drift (4-fold), most of the commercial vaccines were completely efficacious against the challenge virus, a 2008 Egyptian H5N1 isolate. Therefore, antigenic drift cannot completely explain the puzzling inefficiency of the commercial vaccines in the field. Because antibodies to the circulating H5N1 HPAI virus had been detected in day-old chickens in Egypt, and because transfer of maternal antibody through the yolk sac interferes with induction of immunity to infectious bursal disease virus (23, 24) and NDV (25, 26), we hypothesized that passive antibody transfer was a major underlying cause of the vaccine failure. We found that passive transfer of anti-H5 immunity via pooled chicken antisera markedly reduced the induction of HI antibody responses after a priming dose of antigen in 1-wk-old, seronegative, SPF chickens (Table 5). Only four of the nine vaccinated chicks developed detectable HI titers (HRBC HI GMT, 17; HRBC HI titer, 10, 20, 40, and 10, respectively). The suppressive influence of maternally transferred antibodies remained active 4 wk later, when the antibody response to a second dose of vaccine was attenuated; one bird failed to produce detectable antibody, and two died after challenge.
The mechanism by which passive transfer of antibodies suppresses vaccine induction of antibodies in chickens has not been addressed. Although it is possible that the initial antigen dose is reduced by formation of antigen–antibody complexes, such an effect is less likely 4 wk later, at the time of the boost vaccination. The one-week-old chicks received 0.5 mL of H5 antisera (HI titer, 80), which would be diluted ≈1:10 by the chick's body volume to yield an estimated serum titer of 8. After 4 wk, the antibody titer would have fallen at least to the same level observed in chickens receiving the 1:10 dilution of antisera—a level that did not interfere with antibody induction. We therefore hypothesize that the initial exposure of the chicks’ immature immune systems to H5 antibody–vaccine complexes caused a greater suppression of the immune response to vaccine than would be expected. An alternate explanation may be suppression of macrophages and/or other antigen-presenting cells in a manner similar to the antiinflammatory activity of therapeutic intravenous γ-globulin (IVIG). IVIG has been shown to provide antiinflammatory effects in mammals through differential sialylation of the Fc fragment of IgG (28, 29).
The effect of vaccine neutralization by passive antibody transfer is likely to be intensified by vaccination before full development of the immune system. Previous studies have demonstrated that the chicken immune response matures ≈14 d after hatching, which may explain the broad failure of immunization of chickens <2 wk of age (30). Although little is known about dendritic cells (DCs) in neonatal chickens, many studies have focused on these cells in mammals. It is well established that after antigen stimulation, both myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) from human cord blood express lower levels of MHC class II and the costimulatory molecules CD80 and CD86 than do adult peripheral blood DCs (31–33). Furthermore, neonatal mDCs and pDCs have a limited ability to produce IFN-α/β after pattern recognition receptor stimulation (34, 35). In mammals, antigen presentation by these immature DCs results in the development of anergy or tolerance by the responding lymphocytes. Therefore, the failure of vaccination of week-old chicks to produce high antibody titers may be due, at least in part, to targeting of an immature immune response.
Mass vaccination against HPAI in Egypt was adopted on the basis of the predicted efficacy of H5N1 vaccines in a number of avian species (36–40) and the recommendation of FAO/OIE to use vaccination as part of a control strategy for HPAI. Furthermore, field trials of oil emulsion–whole-virus H5 vaccines have shown promise in controlling H5N1 influenza outbreaks in Vietnam and the People's Republic of China (41–43). However, mass vaccination has failed to control the continuing H5N1 HPAI outbreaks in Egypt (22). Not only may maternally transferred antibody contribute to this failure, but the strategy of intensive countrywide vaccination used in Vietnam was not implemented, and other biosecurity measures are not yet fully realized (22). Additionally, backyard poultry that make up an estimated equivalent number of birds to commercial farms are largely not vaccinated. Our findings suggest that day-old chicks derived from immunized dames should not be vaccinated immediately. Studies now under way seek to determine the optimal time for vaccination of chickens with parentally transferred antibody and to elucidate the mechanism(s) involved. DNA vaccines are proposed as a strategy to circumvent maternal antibody suppression of the protective immune response of chickens to infectious bursal disease virus vaccine (44). DNA vaccines with or without cytosine-phosphate-guanine (CpG) oligodeoxynucleotide (an immune stimulant) hold the potential for in ovo vaccination and induction of protective immunity regardless of maternally transferred antibody (45–47). However, in ovo vaccination using DNA vaccines may not be practical under field conditions.
The ongoing circulation of HP H5N1 avian influenza in poultry in Egypt has caused >100 human infections and remains an unresolved threat to veterinary and public health. The decreased rate of fatality among human cases (from 60% to <20% as of January 2010) may reflect either a decline in the pathogenicity of the endemic H5N1 strain or improved clinical management. The recently reported reassortment between an avian HP H5N1 and a human seasonal H3N2 virus, generating hybrid viruses with substantial virulence (48), raises the specter of potential interhuman transmissibility of such reassortants. It is sobering to realize that ominous reassortment events remain possible as long as a virus continues to circulate; for example, swine H1N1 viruses recently reacquired pandemic-level transmissibility after an interval of nearly100 y. Therefore, HP H5N1 viruses in domestic poultry will always present a potential threat to humans. The cocirculation of the highly transmissible pandemic H1N1 2009 influenza virus raises concern about potentially transmissible reassortants.
In conclusion, parentally transferred anti-H5N1 antibodies appear to be modulating the efficacy of H5N1 inactivated virus vaccines in Egypt, thus facilitating the ongoing outbreaks of highly pathogenic H5N1 avian influenza in poultry and the continued infection of humans. Additional studies are needed to determine the mechanism(s) involved in this passive immunization and identify options to circumvent the problem.
Methods
Viruses.
A representative recent H5N1 HPAI virus isolate (Ck/Qal-Egypt/1/08; clade 2.2.1) was used as a challenge virus in this study. The virus was grown in 10-d-old embryonated chicken eggs for 36–48 h at 35 °C. Virus titer was determined by calculating the 50% egg infectious dose (EID50) per milliliter of virus. All experiments with the H5N1 virus were performed in biosafety level 3+ (BSL3+) facilities at St. Jude Children's Research Hospital and approved by the US Department of Agriculture and the US Centers for Disease Control and Prevention.
Chickens.
One-week-old SPF outbred white Leghorn chickens (Gallus domesticus) were purchased from McMurray Hatchery. The chickens were wing-banded, penned, and provided feed and water ad libitum in BSL3+ facilities. All animal experiments were approved by the Animal Care and Use Committee of St. Jude and complied with institutional, National Institutes of Health, and Animal Welfare Act policies and regulations.
Vaccines.
We tested four commercial inactivated oil emulsion–adjuvant H5 avian influenza vaccines that had been used at chicken farms in Egypt: (i) Volvac Avian Influenza Killed Virus (AI KV) H5N2 (A/Chicken/Mexico/232/94) vaccine (Boehringer Ingelheim Vetmedica); (ii) YEBIO inactivated H5N2 avian influenza (A/Turkey/England/N-28/73) vaccine (Yebio Bioengineering Co. Ltd.); (iii) YEBIO H5N1 (A/Goose/Guangdong/96) vaccine (Re-1); and (iv) reassortant H5N1 avian influenza virus (A/Duck/Anhui/1/06; clade 2.3) vaccine (Re-5) (First Bio-Products Manufactory of Heilongjiang Province, Harbin, China). A control inactivated oil emulsion–whole-virus H5N3 influenza vaccine (CkC58) containing the H5 HA of A/Chicken/Vietnam/C58/04 (H5N1), the N3 NA of A/Duck/Germany/1215/73 (H2N3), and the internal genes of A/Puerto Rico/8/34 (H1N1) (40, 49) was generated by reverse genetics. A placebo vaccine contained only virus-free allantoic fluid from 12-d-old embryonated chicken eggs.
Determination of the Lethal Dose of Ck/Qal-Egypt/1/08 (H5N1) Virus in Chickens.
Groups of three 8-wk-old chickens were inoculated by intranasal, intraocular, and intratracheal instillation of serial 10-fold dilutions of virus starting at 108.0 EID50 in a total volume of 1.0 mL. All birds were observed daily for morbidity and mortality.
Vaccination and Challenge.
Groups of eight 1-wk-old chickens were immunized with the vaccines and received a boost vaccination 4 wk later as instructed by the manufacturers. After an additional 3 wk, the chickens were challenged via intranasal, intraocular, and intratracheal instillation of 103.5 EID50 of Ck/Qal-Egypt/1/08 (H5N1) virus in a total volume of 1.0 mL All chickens were monitored daily for 2 wk for morbidity and mortality. Tracheal and cloacal swabs were collected daily from all living birds to examine shedding of the challenge virus.
Blood Sampling and Treatment.
All chickens were bled before the first immunization, boost vaccination, and challenge, and 14 d postchallenge. The serum samples were treated with a receptor-destroying enzyme (RDE) (Denka Seiken) and heat-inactivated at 56 °C for 30 min for HI assays.
Serologic Analysis.
PBS containing four agglutinating units of challenge virus was incubated with serial 2-fold dilutions of RDE-treated serum samples (starting at a 1:10 dilution) at room temperature for 30 min. The HI titers of the samples were determined by testing agglutination of 0.5% CRBCs or 1% HRBCs. HRBCs increased the sensitivity of the HI test by detecting sialic acid linked to galactose by α 2,3 linkage (SA α 2,3 Gal) as compared with CRBCs that detect primarily SA α 2,6 Gal (50).
Vaccination and Challenge After Passive Immunization with Pooled Anti-H5 Chicken Sera.
Groups of nine 1-wk-old chickens were injected i.p. with 0.5 mL of PBS or pooled anti-H5 chicken sera (undiluted, 1:10-diluted, or 1:100-diluted; HI titer, 80; determined against the challenge virus). The sera were pooled from groups of chickens immunized with two doses of one of the commercial H5 influenza vaccines (Volvac H5N2, YEBIO H5N2, YEBIO H5N1 Re-1, or First H5N1 Re-5). The chickens were immunized with the YEBIO H5N1 Re-1 vaccine 1 d after i.p. injection and received a boost vaccination 4 wk subsequently. Three weeks later, they were challenged via intranasal, intraocular, and intratracheal instillation of 103.5 EID50 of Ck/Qal-Egypt/1/08 (H5N1) virus in a total volume of 1.0 mL. All chickens were monitored daily for 2 wk for morbidity and mortality. Tracheal and cloacal swabs were collected daily from all living birds to examine shedding of the challenge virus.
Prevalence of Anti-H5N1 Maternal Antibodies in Egyptian Farm Chickens.
We obtained 20 yolk sacks from eggs hatched within the past day at a typical Egyptian poultry farm where the YEBIO H5N1 Re-1 vaccine was widely used. The yolks were diluted 1/2 in PBS and centrifuged to remove particulate matter. HI antibody titers in the supernatants were assayed against A/Turkey/Egypt/7/06 (H5N1) virus by using CRBCs and HRBCs.
Acknowledgments
We thank Sharon Naron for editorial assistance and James Knowles for manuscript preparation. This work was supported by National Institute of Allergy and Infectious Diseases (National Institutes of Health, Department of Health and Human Services) Contract HHSN266200700005C and the American Lebanese Syrian Associated Charities.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ungchusak K, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JSM, de Jong MD, Guan Y. Avian influenza virus (H5N1): A threat to human health. Clin Microbiol Rev. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Geneva, Switzerland: World Health Organization; 2010. [Accessed January 11, 2010]. Pandemic (H1N1) 2009—update 82 reported to WHO. Available at www.who.int/csr/don/2010_01_08/en/index.html. [Google Scholar]
- 4.World Health Organization . Geneva, Switzerland: World Health Organization; 2009. [Accessed January 11, 2010]. Cumulative number of confirmed human cases of avian influenza A/ (H5N1) reported to WHO. Available at www.who.int/csr/disease/avian_influenza/country/cases_table_2009_12_30/en/index.html. [Google Scholar]
- 5.World Health Organization . Geneva, Switzerland: World Health Organization; 2010. [Accessed April 9, 2010]. H5N1 avian influenza: timeline of major events reported to WHO. Available at www.who.int/csr/disease/avian_influenza/Timeline_10_01_04.pdf. [Google Scholar]
- 6.de Jong JC, Claas ECJ, Osterhaus ADME, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Subbarao K, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Geneva, Switzerland: World Health Organization; 2009. [Accessed January 11, 2010]. Continuing progress towards a unified nomenclature system for the highly pathogenic H5N1 avian influenza viruses reported to WHO. Available at www.who.int/csr/disease/avian_influenza/guidelines/nomenclature/en. [Google Scholar]
- 9.Ellis TM, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- 10.Choi YK, et al. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol. 2005;79:10821–10825. doi: 10.1128/JVI.79.16.10821-10825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keawcharoen J, et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, et al. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol. 2005;79:12058–12064. doi: 10.1128/JVI.79.18.12058-12064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Songserm T, et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006;12:681–683. doi: 10.3201/eid1204.051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Songserm T, et al. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis. 2006;12:1744–1747. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbarao K, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 16.Zitzow LA, et al. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, et al. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–192. doi: 10.1038/nature03974. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc Natl Acad Sci USA. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The World Organization for Animal Health Update on highly pathogenic avian influenza in animals. 2010. [Accessed April 9, 2010]. Available at www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm.
- 21.Bahgat MM, et al. Characterization of an avian influenza virus H5N1 Egyptian isolate. J Virol Methods. 2009;159:244–250. doi: 10.1016/j.jviromet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Peyre M, et al. Avian influenza vaccination in Egypt: Limitations of the current strategy. J Mol Genet Med. 2009;3:198–204. doi: 10.4172/1747-0862.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqi SA, Marquez B, Sahin N. Maternal antibody and its effect on infectious bursal disease immunization. Avian Dis. 1983;27:623–631. [PubMed] [Google Scholar]
- 24.Winterfield RW, Dhillon AS, Thacker HL, Alby LJ. Immune response of White Leghorn chicks from vaccination with different strains of infectious bursal disease virus and in the presence of maternal antibodies. Avian Dis. 1980;24:179–188. [Google Scholar]
- 25.Chu HP, Rizk J. The effect of maternal immunity, age at vaccination and doses or live vaccines on immune response to Newcastle disease. Dev Biol Stand. 1975;28:451–463. [PubMed] [Google Scholar]
- 26.Eidson CS, Thayer SG, Villegas P, Kleven SH. Vaccination of broiler chicks from breeder flocks immunized with a live or inactivated oil emulsion Newcastle disease vaccine. Poult Sci. 1982;61:1621–1629. doi: 10.3382/ps.0611621. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Moneim AS, et al. Sequence diversity of the haemagglutinin open reading frame of recent highly pathogenic avian influenza H5N1 isolates from Egypt. Arch Virol. 2009;154:1559–1562. doi: 10.1007/s00705-009-0461-2. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariyawasam S, Wilkie BN, Gyles CL. Resistance of broiler chickens to Escherichia coli respiratory tract infection induced by passively transferred egg-yolk antibodies. Vet Microbiol. 2004;98:273–284. doi: 10.1016/j.vetmic.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: Evidence for functional immaturity. Blood. 1994;84:4333–4343. [PubMed] [Google Scholar]
- 32.De Wit D, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Encabo A, Solves P, Carbonell-Uberos F, Miñana MD. The functional immaturity of dendritic cells can be relevant to increased tolerance associated with cord blood transplantation. Transfusion. 2007;47:272–279. doi: 10.1111/j.1537-2995.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 34.Danis B, et al. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- 35.Drohan L, et al. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum Immunol. 2004;65:1356–1369. doi: 10.1016/j.humimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Karunakaran D, Newman JA, Halvorson DA, Abraham A. Evaluation of inactivated influenza vaccines in market turkeys. Avian Dis. 1987;31:498–503. [PubMed] [Google Scholar]
- 37.Swayne DE. Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis. 2009;32:351–363. doi: 10.1016/j.cimid.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Swayne DE, Beck JR, Perdue ML, Beard CW. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 2001;45:355–365. [PubMed] [Google Scholar]
- 39.Tian G, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341:153–162. doi: 10.1016/j.virol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Webster RG, et al. The immunogenicity and efficacy against H5N1 challenge of reverse genetics-derived H5N3 influenza vaccine in ducks and chickens. Virology. 2006;351:303–311. doi: 10.1016/j.virol.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 41.Domenech J, et al. Experiences with vaccination in countries endemically infected with highly pathogenic avian influenza: The Food and Agriculture Organization perspective. Rev Sci Tech. 2009;28:293–305. doi: 10.20506/rst.28.1.1865. [DOI] [PubMed] [Google Scholar]
- 42.Ellis TM, et al. Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathol. 2004;33:405–412. doi: 10.1080/03079450410001724012. [DOI] [PubMed] [Google Scholar]
- 43.To TL, et al. Control of avian influenza: A vaccination approach in Viet Nam. In: Dodet B, editor. Vaccination:A Tool for the Control of Avian Influenza. Basel, Switzerland: Karger; 2007. [Google Scholar]
- 44.Haygreen L, Davison F, Kaiser P. DNA vaccines for poultry: The jump from theory to practice. Expert Rev Vaccines. 2005;4:51–62. doi: 10.1586/14760584.4.1.51. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood MS, Siddique M, Hussain I, Khan A, Mansoor MK. Protection capability of recombinant plasmid DNA vaccine containing VP2 gene of very virulent infectious bursal disease virus in chickens adjuvanted with CpG oligodeoxynucleotide. Vaccine. 2006;24:4838–4846. doi: 10.1016/j.vaccine.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Zhang F, Fang F, Chang H, Chen Z. Vaccination with hemagglutinin or neuraminidase DNA protects BALB/c mice against influenza virus infection in presence of maternal antibody. BMC Infect Dis. 2007;7:118. doi: 10.1186/1471-2334-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perozo F, et al. Protection against infectious bursal disease virulent challenge conferred by a recombinant avian adeno-associated virus vaccine. Avian Dis. 2008;52:315–319. doi: 10.1637/8122-100207-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 48.Li C, et al. Reassortment between avian H5N1 and human H3N2 influenza viruses creates hybrid viruses with substantial virulence. Proc Natl Acad Sci USA. Feb 22, 2010 doi: 10.1073/pnas.0912807107. 10.1073/pnas.0912807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JK, et al. Pathogenicity and vaccine efficacy of different clades of Asian H5N1 avian influenza A viruses in domestic ducks. J Virol. 2008;82:11374–11382. doi: 10.1128/JVI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol. 2003;70:391–398. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]