Abstract
Multiple sclerosis (MS) is a human demyelinating disease characterized by multifocal regions of inflammation, progressive myelin loss within the central nervous system (CNS), and eventual failure to remyelinate damaged axons. These problems suggest deficiencies in recruiting and/or maturation of oligodendrocyte progentior cells (OPCs) and highlight cell replacement therapies to promote remyelination. We have used a model of viral-induced demyelination to characterize signaling cues associated with positional migration of transplanted remyelination-competent cells. Although successful transplantation of rodent-derived glial cell types into models of MS has been performed, the mechanisms by which these cells navigate within an inflammatory environment created by a persistent virus has not been defined. Infection of the mouse CNS with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in an immune-mediated demyelinating disease with clinical and histologic similarities to MS. Surgical engraftment of GFP+ neural stem cells (NSCs) into spinal cords of JHMV-infected mice with established demyelination results in migration, proliferation, and differentiation of the cells into OPCs and mature oligodendrocytes that is associated with increased axonal remyelination. Treatment with anti-CXCL12 [stromal derived factor–1α, (SDF-1α)] blocking serum resulted in a marked impairment in migration and proliferation of engrafted stem cells. Moreover, small molecule–mediated antagonism of CXCR4, but not CXCR7, impaired migration and proliferation, to an extent similar to that with anti-CXCL12 treatment. These data highlight the importance of the CXCL12:CXCR4 pathway in regulating homing of engrafted stem cells to sites of tissue damage within the CNS of mice persistently infected with a neurotropic virus undergoing immune-mediated demyelination.
Keywords: chemokine receptors, chemokines, demyelination, trafficking, glia
The etiology of the human demyelinating disease multiple sclerosis (MS) is not known, although numerous factors including both genetic and environmental influences are considered important in initiation and maintenance of disease. Viral infection has long been considered a potential triggering mechanism involved in demyelination, and numerous human viral pathogens have been suggested to be involved in eliciting myelin-reactive lymphocytes and/or antibodies that subsequently infiltrate the CNS and damage the myelin sheath (1–4). Therefore, viral models of demyelination are clearly relevant and have provided important insight into mechanisms associated with disease initiation, neuroinflammation and demyelination.
An important clinical aspect related to the pathogenesis of the human demyelinating disease MS is the eventual remyelination failure by endogenous neural stem cells (NSCs). Among the various experimental approaches undertaken to promote remyelination, cell replacement therapies using both mouse and human NSCs have emerged as a clinically relevant and increasingly practical method for promoting remyelination (5, 6). However, the vast majority of these studies have used either autoimmune models of neuroinflammatory-mediated demyelination or chemical-induced demyelination to assess the remyelination potential of NSCs. Given the possibility of viral infection in initiating demyelination as well as the fact that numerous neurotropic viruses exist that are capable of persisting within the CNS, it is imperative to evaluate the remyelination potential of stem cells in the presence of a persistent viral infection that is correlative with chronic neuroinflammation and demyelination. We have recently demonstrated that transplantation of NSCs into mice persistently infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) is well tolerated and is associated with axonal sparing accompanied by extensive remyelination while not significantly dampening either neuroinflammation or T cell responses (7, 8). Evident from this work is the ability of engrafted cells to preferentially migrate to regions of demyelination, indicating that specific signals associated within white matter lesions are able to attract transplanted cells. The molecular mechanisms governing NSC migration within an inflammatory environment resulting from demyelination derived from a persistent viral infection have not been defined. Therefore, the present study uses a viral model of demyelination to identify the signaling mechanisms required by transplanted NSCs to selectively home to areas of myelin damage.
Results
Engraftment of GFP-NSCs into JHMV-Infected Mice Promotes Remyelination.
Following plating of GFP-NSCs (9–11) onto matrigel-coated substrates and concomitant growth factor withdrawal, cells started to display complex cellular morphologies. By 5 d of differentiation, the majority (~85%) of cells expressed the oligodendrocyte marker GalC, with the remaining cells representing either astrocytes or neurons (Fig. 1 A–D). Oligodendrocytes and astrocytes could be identified based upon morphology and immunolabeling. Oligodendrocytes displayed a multipolar morphology and GalC immunoreactivity (Fig. 1A), whereas astrocytes exhibited a stellate morphology and stained positive for GFAP (Fig. 1B). Very few Map-2 reactive neurons were detected within cultures (Fig. 1 C and D). Following differentiation, cells continued to constitutively express GFP (Fig. S1). To assess the potential of GFP-NSC-mediated remyelination, mice were infected i.c. with JHMV and 2.5 × 105 cells were engrafted at day 14 p.i., which represents a time in which surviving mice have established demyelinating lesions (12). Mice have cleared virus below levels of detection by plaque assay (<100 PFU/g tissue), and we have previously determined that NSC engraftment does not alter viral titers (7, 8). Constitutive expression of GFP by the engrafted cells was clearly evident under FITC illumination, and graft origin was confirmed based on anti-GFP immunoreactivity as well as by BrdU labeling of transplanted cells. Transplantation of lysed cells ruled out resident cell uptake of GFP label. Based upon these findings, subsequent studies were therefore based on identification of transplanted cells by GFP fluorescence alone, as reported previously (10). Transplantation of NSCs resulted in extensive migration up to 12 mm rostral and 8 mm caudal from the implantation site by 4 d posttransplantion (p.t.). Proliferation and selective colonization of demyelinated white matter tracts were apparent by 3 wk p.t. (Fig. 1 E–G). It is important to note that migration occurred early following engraftment, and the subsequent increase in total GFP+NSC numbers argues that migration precedes extensive proliferation and the majority of engrafted cells replicate upon arrival to areas of demyelination. Systematic analysis of spinal cords of nontransplanted mice revealed the presence of numerous demyelinated axons (Fig. 1J). In marked contrast, transplantation of GFP-NSCs resulted in widespread remyelination characterized by thin myelin sheaths (Fig. 1K) distributed primarily throughout ventral and lateral columns within areas of transplanted cell migration. Immunohistochemical staining was performed on spinal cords at day 4 and 21 p.t. to determine the differentiation fate of implanted cells. By day 4 p.t., ~7% of GFP-NSCs were GST-π+ (marker expressed by mature oligodendrocytes), and ~29% were positive for NG2, indicating an oligodendrocyte progenitor cell (OPC) (Table S1). Moreover, very few GFP+ cells expressing either the astrocyte marker GFAP or neuron marker Map-2 were detected. At 3 wk p.t., the majority of engrafted cells had differentiated into cells of the oligodendrocyte lineage, e.g., mature oligodendrocytes (~22%) and OPCs (~76%) (Fig. 1 L–P and Table S1). These findings support earlier studies from our laboratory (7, 8).
Fig 1.
Transplanted GFP-NSCs survive, migrate toward areas of demyelination, and are associated with increased remyelination. Following 5 d in differentiation culture conditions, GFP-NSCs acquire the morphology and express markers specific for oligodendrocytes (GalC, A), astrocytes (GFAP, B, arrows), and neurons (Map-2, C, arrow). (D) NSC differentiation consistently resulted in ~85% of cells expressing GalC (P < 0.01), ~10% GFAP, and ~5% Map-2. (E) Representative coronal section from a JHMV-infected mouse spinal cord at 3 wk p.t. stained with toluidine blue revealing normally myelinated (dark blue staining, solid arrow) and demyelinated white matter (unfilled arrow). Demyelination is concentrated primarily in ventral and lateral white matter columns. (F) Sequential coronal tissue section from spinal cord shown in E demonstrating that GFP+ NSCs preferentially migrate toward sites of demyelination in white matter tracts. G, gray matter; W, white matter. (G) Distribution of GFP+ cells rostral and caudal from site of implantation (arrow) within the spinal cords of JHMV-infected mice at 4 d and 3 wk p.t. shows the rapid migration by the cells toward lesioned areas. Data are representative of at least three experiments with n > 3. Error bars represent SD. (H) Increased remyelination in transplanted animals extended 8 mm rostral and 6 mm caudal to the site of implantation (arrow) and was significantly (*P < 0.05, **P < 0.01) greater than the degree of remyelination in nontransplanted animals. Representative images of toluidine blue–stained transverse section of spinal cords from a healthy noninfected mouse (I), a JHMV-infected nontransplanted mouse at day 35 p.i. (J), or a JHMV-infected transplanted mouse at day 21 p.t. (day 35 p.i.) (K). Normally myelinated axons are depicted with solid arrows (I), whereas representative demyelinated and remyelinated axons in J and K are indicated with open arrowheads and arrows, respectively. Representative confocal images of GFP fluorescence by engrafted cell and GST-π immunostaining from transplanted spinal cord sections of a JHMV-infected mouse at day 21 p.i. reveal that engrafted cells are capable of differentiating into mature oligodendrocytes (L). (M) Representative confocal images demonstrating engrafted GFP-positive cells expressing NG2 (yellow in M and N–P) within the spinal cord of a JHMV-infected mouse at day 21 p.t. (Scale bars, 20 μm in A, B, I and L; 10 μm in N; and 50 μm in M.)
GFP-NSCs Express Chemokine Receptors CXCR4 and CXCR7.
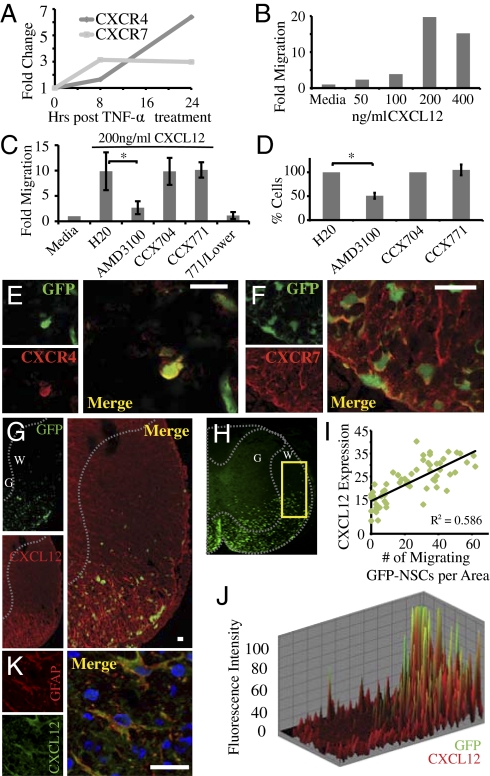
Previous studies have demonstrated that NSCs express chemokine receptors and have suggested their involvement in homing to areas of damage in models of CNS injury (13–15). Therefore, to better understand the underlying mechanisms associated with positional migration of engrafted GFP-NSCs, we examined chemokine receptor expression following exposure to the cytokine TNF-α, as cells transplanted into JHMV-infected mice will encounter this cytokine following transplantation into the inflammatory environment present in mice persistently infected with JHMV (16). Treatment with TNF-α resulted in a selective increase in transcripts specific for CXC chemokine receptors 4 (CXCR4) and 7 (CXCR7) (Fig. 2A), which are signaling receptors for the ligand CXCL12 (17–19). Addition of recombinant CXCL12 resulted in dose-dependent migration of cytokine-treated cells using an in vitro chemotaxis assay (Fig. 2B). Inclusion of the small-molecule compounds AMD3100 or CCX771 were used to evaluate whether CXCL12-mediated migration was dependent upon CXCR4 or CXCR7, respectively. The presence of AMD3100 resulted in >75% reduction (P < 0.05) in GFP-NSC migration of cells in response to CXCL12, whereas the presence of CCX771 had no effect on in vitro migration (Fig. 2C). In assessing proliferative roles, treatment with AMD3100 hampered GFP-NSC growth in culture by ~50% (P < 0.05), whereas treatment with CCX771 had no effect (Fig. 2D). Immunohistochemical staining of spinal cords from engrafted mice at 21 d p.t. indicated that transplanted GFP-NSCs within white matter tracts express both CXCR4 (~92%) and CXCR7 (~79%) (Fig. 2 E and F). In addition, staining revealed that CXCL12 was detected in white matter tracts of spinal cords of infected mice (Fig. 2 G) and that CXCL12 staining positively correlated with GFP signal (Fig. 2 H–J). Furthermore, staining for GFAP and CXCL12 revealed that astrocytes were the predominant source of CXCL12 during chronic disease (Fig. 2K), supporting previous findings that astrocytes express CXCL12 within the inflamed CNS (14, 20). These data suggest that NSC preferentially home to areas of demyelination following JHMV infection by migrating to enriched areas of CXCL12 expression derived from activated astrocytes.
Fig. 2.
GFP-NSCs express CXCR4 and CXCR7 and respond to CXCL12 signaling. (A) Cultured GFP-NSCs up-regulate mRNA transcripts specific for CXCL12 receptors, CXCR4, and CXCR7, following treatment with TNF-α (100 U). (B) Cultured GFP-NSCs migrate in a dose-dependent response to recombinant mouse CXCL12. Data shown are representative of at least three independent experiments. (C) CXCL12-mediated migration is hampered by treatment with the CXCR4 antagonist (AMD3100, 10 μM, P < 0.05) but not with the CXCL12:CXCR7 binding blocker CCX771 (100 nM), which also does not induce migration when alone in the lower wells. CCX704 is a close CCX771 analog that has no CXCR7 binding affinity. (D) Inclusion of AMD3100 (10 μM) reduced proliferation by ~50% (*P < 0.05) compared with control cultures, whereas CCX771 (500 nM) had no effect on NSC proliferation. Representative confocal microscopy confirmed that engrafted cells express both CXCR4 (~92%) (E) and CXCR7 (~79%) (F) within the spinal cords of JHMV-infected mice at day 21 p.t. (G) Engrafted GFP-NSCs migrate to areas undergoing demyelination that are enriched for CXCL12 staining (red). (H) Representative spinal cord from JHMV-infected mouse at day 21 p.t. used for analysis to confirm the association of GFP signal with CXCL12 staining. Increased GFP signal correlated with elevated staining for CXCL12 as determined by regression analysis (I). Data were collected from 10 images from three separate spinal cords and (J) merging fluorescence intensity imaging maps. (K) Astrocytes (determined by GFAP staining, red) represent the predominant cellular source for CXCL12 expression (green) as measured by confocal microscopy in JHMV-infected spinal cord (DAPI-stained nuclei). (Scale bars, 20 μm.)
Blocking CXC12 Signaling Limits Migration and Proliferation of Engrafted GFP-NSCs.
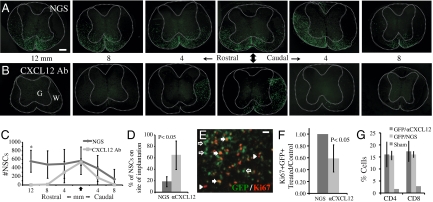
To test whether CXCL12 is important in homing of engrafted cells to sites of demyelination, JHMV-engrafted mice were treated i.p. with a blocking antibody specific for mouse CXCL12 (21). We confirmed penetration of blocking antiserum through the blood–brain barrier by goat IgG immunostaining. Blocking CXCL12 signaling resulted in a dramatic reduction in migration of engrafted GFP-NSCs from the site of transplantation (Fig. 3 A–D). Quantification of cell migration within engrafted spinal cords indicated that administration of anti-CXCL12 resulted in a significant reduction (P < 0.05) in migration of GFP-NSCs rostral to the site in implantation and a significantly larger proportion of cells remaining on the site of implantation (Fig. 3 C and D). Furthermore, anti-CXCL12 treatment also significantly (P < 0.05) reduced GFP-NSC proliferation as determined by Ki67 expression on transplanted cells (Fig. 3 E and F). No significant differences in caspase-3 staining by positive GFP-NSCs were detectable by immunohistochemistry, indicating that treatment with anti-CXCL12 did not increase apoptosis of transplanted cells. Moreover, blocking CXCL12 did not affect recruitment of inflammatory CD4+ or CD8+ T cells into the CNS as compared with experimental mice treated with control serum (Fig. 3G). Staining with H&E revealed that anti-CXCL12 treatment did not inhibit inflammatory cell migration and infiltration from the microvasculature into the parenchyma (Fig. S2). Finally, blocking CXCL12 did not alter the differentiation of engrafted NSCs into cells of the oligodendrocyte lineage, as similar frequencies were observed compared with control mice (Table S2).
Fig. 3.
CXCL12 enhances the migration and proliferation of transplanted GFP-NSCs. (A) Representative sections of spinal cord rostral and caudal to the site of implantation (arrow) depict the extent of migration of engrafted GFP-NSCs within spinal cords of JHMV-infected mice treated with control normal goat serum (NGS). (B) Equivalent spinal cord sections as those in A from JHMV-infected mice treated with CXCL12-blocking serum show the diminished migration away from the implant site as well as reduced total numbers of GFP-NSCs. (C) Quantification of cellular migration of engrafted GFP-NSCs following treatment with either anti-CXCL12 or NGS at 21 d p.t. into spinal cords of JHMV-infected mice. Distribution of GFP-NSCs in representative sections along the length of transplanted cords reveal impaired migration of NSCs away from the site of implantation (arrow) in animals treated with CXCL12-blocking antibody when compared with NGS-treated controls. A significant difference (P < 0.05) in number of GFP-NSCs was observed at 12 mm rostral from implantation site (n = 4, NGS-treated mice; n = 4, anti-CXCL12–treated mice). (D) On average, 65% of GFP-NSCs in treated mice remained on the site of transplantation compared with 18% in control mice (*P < 0.05) 3 wk p.t. (E) Ki67 immunostaining of transplanted cord sections illustrate GFP and Ki67 double-positive cells (solid arrows), nonproliferating transplanted cells (open arrows), and proliferating resident cells (arrowheads). (F) Proportion of GFP-NSCs within spinal cords of JHMV-infected mice treated with CXCL12-blocking antibody expressing the proliferative marker Ki67 21 d p.t. was reduced ~40% (P < 0.05) compared with that in mice treated with NGS. Analysis was performed at day 21 p.t., n = 4, NGS-treated mice; n = 6, anti-CXCL12–treated mice. (G) Trafficking of CD4+ and CD8+ T cells to spinal cords of JHMV-infected mice is not impaired following anti-CXCL12 treatment (n = 3) compared with NGS treatment (n = 3). Data presented are derived from FACS analysis and presented as percentage of positive cells within gated population. (Scale bar, A, 200 μm; D, 20 μm.)
CXCL12 Enhances Engrafted NSC Migration and Proliferation via CXCR4 Without an Apparent Need for CXCR7.
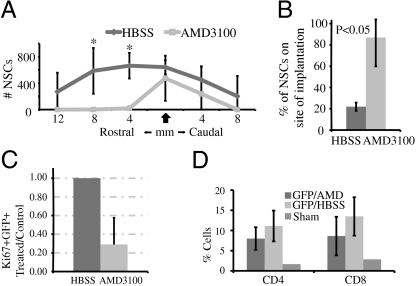
As CXCL12 is a ligand for both CXCR4 and CXCR7 (17–19), we next sought to determine the roles played by each receptor in migration and/or proliferation following engraftment. To test this, JHMV-infected mice were transplanted with GFP-NSCs and treated with either AMD3100 via continuous s.c. osmotic infusion (22) or injected s.c. daily with CCX771 (a small compound antagonist of CXCR7), and the effects on migration were determined at 3 wk p.t. Treatment with AMD3100 was associated with inhibited migration of engrafted GFP-NSCs from the site of implantation (Fig. 4 A and B and Fig. S3). Furthermore, administration of AMD3100 also dampened proliferation of engrafted cells by ~70% as determined by Ki67 staining (Fig. 4C). Remyelination of axons was diminished in sites distal to implantation in mice treated with AMD3100 compared with control animals, and this correlated with impaired migration of engrafted cells. However, there were similar levels of remyelination at the site of implantation in AMD3100-treated mice compared with control mice, suggesting that remyelination potential of engrafted cells was not dramatically reduced. Previous reports have shown that AMD3100 treatment during experimental autoimmune encephalomyelitis results in worsening of disease that correlated with elevated T cell infiltration into the CNS as a result of increased permeability of the blood–brain barrier (23). However, AMD3100 treatment did not result in significant change in T cell migration to the CNS (Fig. 4D) or increased inflammatory cell infiltration to the parenchyma (Fig. S2 C and D) during the chronic stage in our model. Daily administration of 30 mg/kg of CCX771 had no effect on GFP-NSC migration subsequent to implantation into JHMV-infected mice (Fig. S4A). Proliferation of engrafted cells was not affected in animals treated with CCX771 when compared with transplanted mice receiving vehicle alone (Fig. S4B). The treatment did result in efficient penetration of CCX771 into CNS parenchyma as determined by LCMS (Fig. S4C). In addition, inflammatory leukocyte infiltration into the CNS was not affected by blocking CXCR7 binding compared with such infiltration in mice treated with vehicle alone (Fig. S2 E and F). Taken together, these data suggest that engrafted NSC migration to sites of demyelination is dependent upon CXCL12 signaling through CXCR4 alone. Finally, the differentiation of GFP-NSCs did not seem to be affected by treatment with either AMD3100 or CCX771 signaling (Table S2).
Fig. 4.
CXCL12 enacts its role in the migration and proliferation of engrafted NSCs through CXCR4. (A) Distribution of transplanted NSCs along length of spinal cords of animals treated with AMD3100 and in HBSS-treated (control) mice 21 d p.t. shows impaired migration (*P < 0.05) in AMD3100-treated animals to an extent similar to that seen in mice treated with CXCL12-blocking antibody. (B) As in blocking antibody experiments, a significantly greater (*P < 0.05) proportion of GFP-NSCs in AMD3100-treated mice remained in the site of implantation 3 wk p.t. (C) The proportion of GFP-NSCs in mice treated with AMD3100 expressing the proliferative marker Ki67 21 d p.t. was reduced by ~70% compared with NGS-treated animals (P < 0.05) (n = 5 for HBSS-treated and n = 4 for AMD3100-treated animals). (D) Frequency of cells staining for CD4 and CD8 by FACS following percol extraction of treated and control spinal cords 1 wk after transplantation are not significantly different (n = 3 per group).
Discussion
Following transplantation, stem cells need to migrate to successfully engraft and repopulate target tissue. The molecular and cellular events involved in stem cell homing are regulated by a complex series of events that are gradually being characterized. Components considered critical in these processes include cell adhesion molecules interacting with target ligands as well as signaling of chemokine ligands with specific target chemokine receptors. Indeed, CXCL12 signaling through CXCR4 has previously been identified as a key step in homing of hematopoietic stem cells within the bone marrow compartment, as numerous studies have highlighted the importance of this signaling pathway (24–26). More recent investigations have revealed that chemokines are often up-regulated within areas of brain pathology and may provide crucial signaling cues for stem cell migration necessary for repair. For example, following experimental stroke in mice, there is localized increase in expression of CXCL12 within the site of injury, and use of in vitro slice cultures have demonstrated that migration of NSCs is mediated by CXCR4 (13, 15, 27). These findings emphasize the importance of chemokines in participating in NSC-mediated tissue remodeling following hypoxic ischemic cerebral injury. The therapeutic potential of NSCs in promoting clinical recovery and repair has been recognized, and the positional migration of engrafted cells is an essential part of the process. Specific migratory pathways have not been clearly elucidated in models of autoimmune demyelination or in models of viral-induced neurologic disease. With this in mind, we have investigated the chemokine signaling pathways involved in regulating stem cell homing to white matter pathology using a viral model of demyelination. The rationale for the use of a viral model of neurologic disease include (i) the long-standing theory that viruses are considered important in initiating and/or maintaining disease, and (ii) the fact that an understanding of the navigational cues used by engrafted NSCs required for successful migration and accumulation in white matter tracts within an inflammatory environment created by a persistent virus has not been defined.
The findings presented support and extend earlier work from our laboratory that demonstrated transplantation of glial-committed stem cells in JHMV-infected mice was associated with extensive migration and remyelination of axons (7). Importantly, our current studies have used GFP-labeled stem cells that allow greater ease in visualizing transplanted cells in that there is minimal loss of GFP signal following differentiation, and this approach minimizes toxicity associated with alternative trafficking dyes such as BrdU. Similarly, neural stem cells derived from GFP transgenic mice have been used successfully in models of retinal transplantation and of Alzheimer's disease to study regeneration (11, 28, 29). The findings presented here demonstrate that cultured NSCs increase transcript expression of the chemokine receptors CXCR7 and CXCR4 in response to treatment with the proinflammatory cytokine TNF-α. NSCs responded in vitro to CXCL12 by migrating in a dose-dependent manner, and migration was inhibited using the CXCR4 receptor antagonist AMD3100. Examination of spinal cords from mice persistently infected with JHMV revealed that CXCL12 was possibly expressed by astrocytes and was enriched in white matter tracts undergoing demyelination. Furthermore, engrafted NSCs expressed CXCR4 and CXCR7. Blocking studies confirmed in vitro observations indicating the importance of CXCL12:CXCR4 signaling in promoting the positional migration of engrafted cells. Antibody targeting of CXCL12 muted NSC migration from the site of engraftment. Similarly, blocking CXCR4 signaling through administration of AMD3100 resulted in impaired migration of implanted NSCs. This migratory impairment was sustained to 21 d p.t. even though cells are able to reach areas 12 mm away from implantation site by 4 d p.t. In marked contrast, blocking CXCL12 binding to CXCR7 did not affect the migration of NSCs. Our findings also imply that CXCR4 signaling is important in proliferation of implanted cells, as blocking either CXCL12 or CXCR4 resulted in a significant reduction in GFP-NSC proliferation. However, CCX771 treatment, which blocks CXCL12 binding to CXCR7, did not affect engrafted cell proliferation further, emphasizing that CXCR4 is an important receptor responsible for NSC proliferation. These results are consistent with previous in vitro studies demonstrating that CXCL12 can enhance proliferation of neural progenitor cells through CXCR4 signaling (30). We conclude from our data that CXCR4 signaling is important for both proliferation and migration of engrafted NSCs to regions in which CXCL12 expression is enriched within spinal cords of JHMV-infected mice. Furthermore, CXCL12 signaling does not influence T cell accumulation within the CNS during chronic disease, which is in contrast with other models of acute neuroinflammatory disease (22, 23).
We believe that the findings derived from these studies further highlight the therapeutic potential of remyelination-competent cells in promoting myelin repair. Furthermore, these in vivo signaling-blocking studies reinforce previous findings regarding the importance of CXCR4 signaling in the migration of NSCs in other models of CNS injury. Importantly, this chemokine signaling pathway appears to be crucial in the homing of engrafted NSCs to sites of persistent neurotropic virus–mediated injury, a first step in promoting repair within an inflammatory environment relevant in MS.
Materials and Methods
Animals and Virus.
Age-matched (5–7 wk) C56BL/6 male mice were used for all studies (National Cancer Institute). Following anesthetization, mice were injected intracranially (i.c.) with 200 PFU JHMV (strain V2.2–1) suspended in 30 μL sterile HBSS. Control animals (sham) were injected with 30 μL HBSS alone. For further detail, refer to SI Materials and Methods.
Cell Culture and Transplantation.
GFP-NSCs (kindly provided by M. Young, Harvard University) were cultured as described previously (11). For transplantation studies, JHMV-infected mice received an injection of 2.5 × 105 GFP-NSCs (suspended in 2.5 μL HBSS) at T9 of the spinal cord on day 14 p.i. Control animals infected with virus were transplanted with HBSS alone at T9 of the spinal cord (7). For further detail, refer to SI Materials and Methods.
In Vivo Chemokine and Chemokine Receptor Blocking.
CXCL12-blocking antibody or control serum was administered i.p. three times per week. AMD3100 (130 μg/d) or HBSS was administered via s.c. infusion with implantation of an osmotic pump (Durect). CCX771 (ChemoCentryx) (30 mg/kg) or an equal volume of vehicle (10% captisol) were administered by daily s.c. injection. All treatments were started on the day of transplantation. CCX771 levels in the brain were analyzed by LCMS on a Sciex 3000. For further detail, refer to SI Materials and Methods.
Histopathology.
Animals were killed by inhalation of halothane (Sigma) and fixed by cardiac profusion. The spinal cord was extracted and processed for OCT and resin-embedded sections as previously described (7). The number of GFP-positive cells was counted on at least two sections 80 μm apart from each tissue block for each animal. Counts from experimental mice were averaged, and data are presented as mean ± SD. For further detail, refer to SI Materials and Methods
Statistical Analysis.
Statistical analysis was carried out by one-way ANOVA, with P ≤ 0.05 considered significant. For further detail, refer to SI Materials and Methods
Supplementary Material
Acknowledgments
We thank Dr. Mark Penfold (ChemoCentryx, Inc.) for the kind gift of CCX771 and CCX704. This work was funded by National Institutes of Health Grant NS41249 and National Multiple Sclerosis Society Grant RG3857A5/1 (to T.E.L.). K.S.C. and C.S. were supported by California Institute for Regenerative Medicine Training Grant T1-00008.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006375107/-/DCSupplemental.
References
- 1.Ebers GC, Sadovnick AD, Risch NJ, Canadian Collaborative Study Group A genetic basis for familial aggregation in multiple sclerosis. Nature. 1995;377:150–151. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 2.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 5.Pluchino S, Zanotti L, Brini E, Ferrari S, Martino G. Regeneration and repair in multiple sclerosis: The role of cell transplantation. Neurosci Lett. 2009;456:101–106. doi: 10.1016/j.neulet.2008.03.097. [DOI] [PubMed] [Google Scholar]
- 6.Sher F, Balasubramaniyan V, Boddeke E, Copray S. Oligodendrocyte differentiation and implantation: New insights for remyelinating cell therapy. Curr Opin Neurol. 2008;21:607–614. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- 7.Totoiu MO, Nistor GI, Lane TE, Keirstead HS. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardison JL, Nistor G, Gonzalez R, Keirstead HS, Lane TE. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp Neurol. 2006;197:420–429. doi: 10.1016/j.expneurol.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 10.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, et al. Transplantation of EGF-responsive neurospheres from GFP transgenic mice into the eyes of rd mice. Brain Res. 2002;943:292–300. doi: 10.1016/s0006-8993(02)02906-2. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: Host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robin AM, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 16.Sun N, Grzybicki D, Castro RF, Murphy S, Perlman S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213:482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boldajipour B, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Mazzinghi B, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 21.Phillips RJ, et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 22.McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci USA. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- 24.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 26.Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schönemeier B, Schulz S, Hoellt V, Stumm R. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol. 2008;198:39–45. doi: 10.1016/j.jneuroim.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Mizumoto H, Mizumoto K, Shatos MA, Klassen H, Young MJ. Retinal transplantation of neural progenitor cells derived from the brain of GFP transgenic mice. Vision Res. 2003;43:1699–1708. doi: 10.1016/s0042-6989(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 29.Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, et al. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem. 2009;109:1157–1167. doi: 10.1111/j.1471-4159.2009.06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.