Abstract
Formation of the vascular system within organs requires the balanced action of numerous positive and negative factors secreted by stromal and epithelial cells. Here, we used a genetic approach to determine the role of SLITs in regulating the growth and organization of blood vessels in the mammary gland. We demonstrate that vascularization of the gland is not affected by loss of Slit expression in the epithelial compartment. Instead, we identify a stromal source of SLIT, mural cells encircling blood vessels, and show that loss of Slit in the stroma leads to elevated blood vessel density and complexity. We examine candidate SLIT receptors, Robo1 and Robo4, and find that increased vessel angiogenesis is phenocopied by loss of endothelial-specific Robo4, as long as it is combined with the presence of an angiogenic stimulus such as preneoplasia or pregnancy. In contrast, loss of Robo1 does not affect blood vessel growth. The enhanced growth of blood vessels in Robo4−/− endothelium is due to activation of vascular endothelial growth factor (VEGF)-R2 signaling through the Src and FAK kinases. Thus, our studies present a genetic dissection of SLIT/ROBO signaling during organ development. We identify a stromal, rather than epithelial, source of SLITs that inhibits blood vessel growth by signaling through endothelial ROBO4 to down-regulate VEGF/VEGFR2 signaling.
Keywords: angiogenesis, ROBO, SLIT, mammary gland, organogenesis
Recent studies on the SLIT family of axon guidance molecules have demonstrated a conserved role in regulating development of the vascular system. However, a comprehensive understanding of their vascular function has been hampered by contradictory findings (1). SLITs have been shown to both attract (2–6) and repel (7–9) endothelial cells. ROBO1, which binds directly to SLITs, has been shown to promote endothelial cell motility, either alone (2, 6, 10) or as a heterodimeric partner with ROBO4 (5, 9). In contrast, ROBO4 binds SLITs at either very low affinity or not at all and likely requires a coreceptor, such as ROBO1 or a Syndecan, to signal (5, 11, 12). ROBO4 has been assigned the repellent functions of SLITs (7, 8) and, more recently, an alternative role in countering the effects of vascular endothelial growth factor (VEGF) to provide vascular stabilization (13, 14). One experimental variable that may be responsible for these contradictory findings is that many studies were performed in vitro using recombinant SLIT protein prepared in a variety of ways (2, 5, 6, 8, 13, 15). Here, we circumvent the requirement for recombinant protein by taking a genetic approach to address the function of SLIT in a biological context using the mammary gland as a model system. Such an approach provides insight into the role of endogenous SLIT/ROBO signaling in mammary development and angiogenesis.
During postnatal mammary gland development, the epithelium elaborates a bilayered, tree-like structure as it grows from the nipple subdermally through the surrounding stromal environment (16). The stroma is composed of adipocytes, fibroblasts, immune cells and a limited number of principal arteries supplying the capillary plexuses that envelop ducts. The gland undergoes stereotyped cycles of cell growth and differentiation under the influence of estrus and pregnancy hormones. In the virgin, the estrus cycle does not cause expansion of the vasculature, but pregnancy is accompanied by robust capillary sprouting that provides increased blood supply to promote lobulo-alveolar expansion (17, 18). Classic studies on vascular patterning of the gland demonstrated the importance of the epithelium because its absence resulted in only the major vessels and none of the duct-associated capillary plexuses (17). One explanation for this observation is that the epithelium acts as an important source of vascular endothelial growth factor (VEGF) (19–22) and possibly other factors, such as guidance cues. VEGF is also up-regulated in breast tumors, stimulating angiogenesis and fueling cancer cell growth (23). Thus, epithelial VEGF plays an important role in regulating angiogenesis in the mammary gland. In contrast, little is known about the role of guidance cues such as SLITs in directing blood vessel growth and organization during organ development.
We previously demonstrated the expression of SLITs in mammary gland epithelium (24). Here, we identify a second source of SLIT, mural cells associated with blood vessels. We use transplantation experiments to determine the compartment, epithelial or stromal, in which SLIT/ROBO signaling occurs. We show that stromal, but not epithelial, SLITs inhibit vessel growth by downregulating VEGFR signaling through ROBO4; ROBO1 is not required for this inhibition. However, loss of the inhibitory action of SLIT, alone, does not stimulate vessel growth. This requires additional positive factors such as SDF1 or VEGF, and we demonstrate that preneoplasia or pregnancy supplies these proangiogenic molecules. Together, these studies elucidate a role for SLIT/ROBO signaling in maintaining vascular homeostasis during mammary morphogenesis.
Results
Loss of Global, but Not Epithelial, Slit Expression Leads to Increased Blood Vessel Number and Complexity.
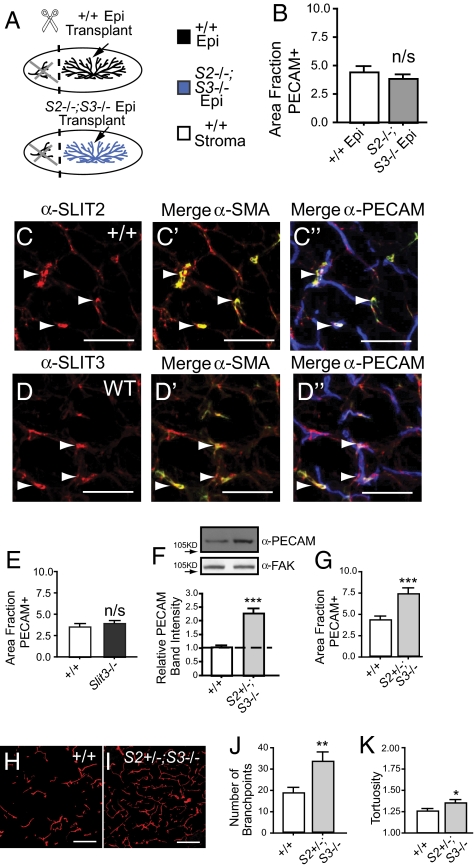
To determine SLIT function during mammary gland development, we initially focused on epithelial SLITs as a target-derived source because previous studies have reported directional migration of endothelial cells in response to exogenous or tumor-supplied SLIT protein (2, 4, 7, 8). Only Slit2 and Slit3 are expressed by mammary epithelia (24); therefore, to evaluate the consequences of losing epithelial SLIT expression, we generated Slit2−/−;Slit3−/− chimeric mammary outgrowths by transplantation because the Slit2−/− mutation is perinatal lethal (Fig. 1A) (25). This technique involved placing small fragments of adult epithelium into contralateral fat pads of immunocompromised (Foxn1nu−/−) host mice that have been precleared to remove endogenous epithelium (26). After 10 weeks, the fragments have grown into mature epithelial trees and the entire gland was harvested. Blood vessel density was analyzed by immunostaining for blood vessel marker PECAM and quantified. We observed no significant difference in blood vessel density between transplants containing WT or Slit2−/−;Slit3−/− epithelium (Fig. 1B and Fig. S1 A and B), suggesting that endothelial cells are refractory to the loss of epithelial SLIT.
Fig. 1.
Loss of global, but not epithelial Slits, enhances blood vessel growth. (A) Diagram illustrating transplants that generate chimeric mammary glands with Slit2−/−;Slit3−/− epithelium (blue) and contralateral WT epithelium (black), transplanted into immunocompromised (Foxn1nu) hosts (white) that have been precleared of their WT epithelium (black). (B) Lack of Slit in the epithelium does not alter blood vessel density in outgrowths. Quantitative analysis of PECAM-positive pixel area (n = 3 contralateral outgrowths, 15 fields of view (FOV)/outgrowth). Error bars = SEM. n/s = not significant. (C and D) Mural cells express SLIT2 and SLIT3. Representative images of sections immunostained for PECAM (blue), SMA (green), and SLIT2 or SLIT3 (red). Arrows indicate mural cell localization. (Scale bar, 50 μm.) (E) Lack of Slit3 does not increase blood vessel number in the mammary gland. Quantitative analysis of PECAM-positive pixel area (n = 3 animals, 15 FOV/gland). Error bars = SEM. n/s = not significant. (F–K) Global lack of Slit significantly increases blood vessel number and network complexity. (F) Representative PECAM immunoblots on WT and Slit2+/−;Slit3−/− mammary lysates (50 μg loaded; FAK immunoblot is loading control). Bar graph represent quantitative analysis of PECAM band intensity (ImageJ) (n = 3). Error bars = SEM, ***P < 0.001 unpaired t test. (G) Quantitative analysis of PECAM-positive pixel area (n = 3 animals, 15 FOV/animal). Error bars = SEM. *** P < 0.001 unpaired t test. (H and I) Representative images of WT (H) and Slit2+/−;Slit3−/− (I) mammary sections immunostained with anti-PECAM (red). (Scale bar = 50 μm.) (J) Number of branchpoints and (K) tortuosity of blood vessels were quantified. Error bars = SEM, ***P < 0.001, **P < 0.005, *P < 0.01 unpaired t test.
It has been reported that Slit2 and Slit3 are expressed in cells surrounding the vasculature (6, 13), suggesting the presence of a stromal source for SLITs, at least in some tissues. We performed immunohistochemistry on sections of adult mammary gland with antibodies directed against SLIT2 or SLIT3, PECAM, and the mural cell marker, SMA. We observe strong colocalization of SMA with both SLITs, demonstrating expression of SLIT in support cells surrounding blood vessels. We also observe weaker colocalization of SLITs with PECAM that may reflect cell-associated SLIT, either secreted from surrounding support cells or a consequence of low level SLIT expression by endothelial cells (Fig. 1 C and D and Fig. S1 C and D). These studies identify a stromal source of SLITs that may exert a local effect on vessel growth and organization.
To evaluate the consequences of knocking out Slit3 in both the epithelia and stroma, we examined intact, adult glands of mice that were homozygous null for Slit3 and observed no changes in blood vessel density (Fig. 1E and Fig. S1 E and F). This suggests that, unlike the embryonic diaphragm (6), SLIT3 functions redundantly with SLIT2 in the adult mammary gland. To evaluate the consequences of depleting both Slits, we examined intact glands of mice that are homozygous for Slit3 and heterozygous for Slit2 because the Slit2 null mutation is lethal (Slit2+/−; Slit3−/−). In these glands, we observe an approximately two-fold increase in blood vessel density and a significant increase in the complexity of the vessel network (Fig. 1 F–K). This analysis shows that a single functional allele of Slit2 is insufficient to supply the SLIT required to restrict blood vessel growth in the mammary gland. Together with the absence of phenotype in transplanted Slit2−/−;Slit3−/− glands in which Slits are knocked out in the epithelium alone (Fig. 1B), the data suggest that stromal SLITs function at short-range to restrain the growth of mammary gland endothelial cells.
Combined Loss of Robo1 and Robo4 Leads to Increased Vessel Density.
To evaluate the roles of ROBO1 and ROBO4 in mammary gland vasculature, we examined their loss-of-function phenotypes because phenocopy of Slit2+/−;Slit3−/− defects provides strong genetic evidence that one, or both, of these receptors functions in the same pathway. ROBO4 has been identified as an endothelial specific mediator of SLIT signaling and its removal is not lethal to the animal (13, 14). To evaluate its loss-of-function phenotype, we analyzed intact, adult Robo4−/− and WT glands and did not observe a significant difference in the number of blood vessels (Fig. 2A and Fig. S2 A and B).
Fig. 2.
Loss of ROBO1 and ROBO4 enhances blood vessel growth. (A) Lack of Robo4 does not alter blood vessel density in the mammary gland. Quantitative analysis of PECAM-positive pixel area (n = 3 animals, 15 FOV/animal). Error bars = SEM. n/s = not significant. (B) ROBO1 is expressed by blood vessels. Representative images of ROBO1 (green) and PECAM (red) immunostaining on WT mammary sections. (Scale bar, 20 μm.) (C) Lack of Robo1 does not alter blood vessel density in the mammary gland. Quantitative analysis of PECAM-positive pixel area (n = 3 animals, 15 FOV/animal). Error bars = SEM. n/s = not significant. (D–I) Lack of both Robo1 and Robo4 significantly increases blood vessel density and network complexity in the mammary gland. (D) Representative PECAM immunoblots on WT, Robo1−/−, Robo4−/−, and Robo1−/−;Robo4−/− mammary lysates (50 μg loaded; FAK immunoblot is loading control). Bar graph represent quantitative analysis of PECAM band intensity (ImageJ) (n = 3). Error bars = SEM, ***P < 0.001 ANOVA. (E) Quantitative analysis of PECAM-positive pixel area (n = 4 animals, 15 FOV/animal). ***P < 0.001 unpaired t test. (F and G) Representative images of WT (F) and Robo1−/−;Robo4−/− (G) mammary sections immunostained with anti-PECAM (red). (Scale bar, 50 μm.) (H and I) Quantification of branchpoint number (H) and tortuosity (I). Error bars = SEM. ***P < 0.001, **P < 0.005 unpaired t test.
Next, we examined the expression of ROBO1 in blood vessels because it is unclear whether it is expressed by all types of endothelial cells (2, 7). We performed immunohistochemical analysis on WT glands using anti-ROBO1 (27) and found it colocalized in a membrane-associated pattern with PECAM (Fig. 2B). We confirmed these results by taking advantage of the expression of LacZ in knockout tissue under the control of the endogenous Robo1 promoter and found positive staining in Robo1−/− blood vessels (Fig. S2 C and D). Thus, in our system, ROBO1 is expressed on blood vessels and may serve as a SLIT receptor. To investigate, we evaluated the loss-of-function phenotype in intact, adult Robo1−/− and WT glands and did not observe a significant difference in blood vessel number (Fig. 2C).
Because analysis of the single knock-out Robo1 and Robo4 glands did not yield a phenotype, we generated and analyzed Robo1−/−;Robo4−/− mice. We discovered an approximately twofold increase in blood vessel density and complexity in Robo1−/−;Robo4−/− glands (Fig. 2 D–I and Fig. S2 E and F) that was similar to the increase observed in Slit2+/−;Slit3−/− glands (Fig. 1 F–K). These results demonstrate that loss of both SLIT receptors is required to achieve increased blood vessel density.
Robo4−/− Blood Vessels Display Enhanced Angiogenesis in Response to SDF1 and VEGF.
Our studies show that generating a blood vessel surplus, similar to that observed in Slit2+/−;Slit3−/− glands, requires loss of both Robo1 and Robo4. One explanation for this requirement is that each receptor compensates for the other in restraining vessel growth and only loss of both ROBO receptors leads to increased density. Alternatively, there may be an epithelial effect because these analyses were performed on intact, rather than transplanted, glands. ROBO1 is expressed in the epithelium (24), as well as the endothelium (Fig. 2B and Fig S2 C and D), raising the possibility that loss of Robo1 in the epithelium contributes to the observed increase in blood vessel density in the Robo1−/−;Robo4−/− mice. Indeed, we previously showed that epithelial loss of Robo1 generates disorganized, hyperplastic tissue that is characterized by up-regulation of the chemokine CXCL12, also known as stromal derived factor-1 (SDF1) (27).
SDF1 induces the expression of VEGF in breast cancer cell lines (28) and normal breast epithelium (Fig. S3A). Therefore, we evaluated the expression of SDF1 and VEGF in Robo1−/− tissue and found that loss of Robo1, either alone or in combination with Robo4, resulted in up-regulation of both SDF1 and VEGF-A in mammary epithelium (Fig. 3 A and B and Fig. S3 B–J). To determine whether the angiogenic phenotype in Robo1−/−;Robo4−/− glands is attributable to the loss of Robo1 in the epithelium and consequent up-regulation of proangiogenic factors, we generated chimeric mammary glands by transplantation. First, we transplanted Robo1−/− and WT epithelial fragments into WT fat pads (Fig. 3C). After 10 weeks of outgrowth, we examined the number and complexity of blood vessels and observed no difference between the outgrowths (Fig. 3D and Fig S3 K and L), suggesting that loss of epithelial Robo1, alone, is insufficient to increase blood vessel density.
Fig. 3.
The proangiogenic factor, VEGF, emanating from Robo1−/− and pregnant epithelium increases angiogenesis in Robo4−/− glands. (A and B) SDF1 (A) and VEGF-A (B) are expressed at higher levels by Robo1−/− and Robo1−/−;Robo4−/−, compared to WT or Robo4−/−, mammary glands. Immunostained sections were scored according to cell percent positivity and staining intensity (n = 3 animals, 10 FOV/animal). Scores were plotted on a vertical scatter plot and red bars indicate average score. ***P < 0.001 ANOVA. (C) Diagram illustrating generation of chimeric glands with Robo1−/− epithelium (red) and contralateral WT epithelium (black), transplanted into immunocompromised (Foxn1nu) hosts (white) that have been cleared of their WT epithelium (black) (D) Lack of Robo1 in the epithelium does not alter blood vessel density in outgrowths. Quantification of PECAM-positive pixel area [n = 7 contralateral outgrowths, 15 FOV/outgrowth]. Error bars = SEM. n/s = not significant. (E) Diagram illustrating generation of chimeric glands with Robo1−/− epithelium (red) and contralateral WT epithelium (black) into syngeneic Robo4−/− background (light gray) that have been cleared of their host Robo4−/− epithelium (dark gray). (F–H) Increased blood vessel density with loss of Robo1 in the epithelium combined with the loss of Robo4. (F) Quantitative analysis of PECAM-positive pixel area (n = 3 contralateral outgrowths, 15 FOV/outgrowth). Quantification of branchpoint number (G) and tortuosity (H). Error bars = SEM, **P < 0.005, *P < 0.01 unpaired t test. (I) Robo4 is necessary for SLIT2N-mediated inhibition of VEGF-induced human microvascular endothelial cells-lung (HMVEC-L) migration. HMVEC-L cells were subjected to control, Robo1, or Robo4 siRNA and allowed to migrate in response to VEGF in the presence of Mock or SLIT2N (n > 3, *** P < 0.001 ANOVA). Western blot analysis confirmed knockdown of Robo1 or Robo4 expression. (J–M) Pregnancy increases blood vessel density in Robo4-/ glands compared to WT. (J) Representative immunoblots of anti-PECAM on WT and Robo4−/−mammary lysates (50 μg loaded; FAK immunoblot is loading control). Bar graph represents quantification of PECAM band intensity (ImageJ) (n = 3). Error bars = SEM, ***P < 0.001 unpaired t test. (K) Quantitative analysis of PECAM-positive pixel area (n = 3 animals, 10 FOV/animal). Error bars = SEM, **P < 0.005 unpaired t test. (L and M) Representative images of WT (L) and Robo4−/− (M) mammary sections from animals at pregnancy day 12.5 immunostained with anti-PECAM (red) and anti-CK14 (green), a marker for myoepithelial cells. Arrowheads indicate capillary baskets surrounding alveoli. (Scale bar, 50 μm.)
Next, we examined the angiogenic phenotype in glands that combined loss of epithelial Robo1 with loss of stromal Robo4. We generated these chimeric glands by transplanting Robo1−/− and contralateral WT epithelium into Robo4−/− fat pads and examined the number and complexity of blood vessels after 10 weeks of outgrowth (Fig. 3E). We found a significant increase in blood vessel density in glands containing Robo1−/− epithelium combined with Robo4−/− stroma (Fig. 3 F–H and Fig S3 M and N), similar to the increase observed in Robo1−/−;Robo4−/− (Fig. 2 D–I) and Slit2+/−;Slit3−/− glands (Fig. 1 F–K). Together, these data show that the presence of ROBO1 in the endothelium does not compensate for the loss of ROBO4. Instead, ROBO4 appears to function alone in the endothelium as an angiogenesis inhibitor. To examine whether there are other contexts in which ROBO4 mediates SLIT signaling in the absence of ROBO1, we performed migration assays on Human Lung MicroVascular Endothelial Cells-Lung (HMVEC-L) (Fig. 3I). These cells express low levels of Robo1 and robust levels of Robo4, both of which could be selectively knocked down using siRNAs. We observed that VEGF stimulated migration of these cells was reduced by the N-terminal fragment of SLIT2, a reduction that occurred upon knockdown of Robo1, but not Robo4, providing another example where ROBO4 transduces a SLIT signal, even when Robo1 expression is greatly diminished or absent.
Together, our studies suggest that ROBO1 contributes to the Robo1−/−;Robo4−/−angiogenic phenotype through its role in the epithelium as a negative regulator of SDF1 and VEGF-A (Fig. 3 A and B and Fig. S3 B–J). The up-regulation of proangiogenic cues that occurs in Robo1−/− mammary epithelium generates a prepathological environment. However, this alone was insufficient to increase angiogenesis because we found that, in addition, loss of Robo4 was also necessary (Figs. 2 D–I and 3D–I). This process of pathological angiogenesis in response to proangiogenic cues has previously been documented in the visual system of Robo4−/− animals (13). However, it is unknown whether the loss of Robo4, alone, will result in increased angiogenesis during normal developmental processes, in part because there are few examples of robust blood vessel growth in the adult animal. In the mammary gland, however, there is a normal developmental event, pregnancy, associated with exuberant sprouting angiogenesis (18) that is driven by VEGF-A (19, 20) (Fig. S3O). To evaluate the consequences of Robo4 loss in this context, we analyzed midpregnant Robo4−/− glands and found a significant increase in blood vessel density (Fig. 3 J–M). These data show that a normal developmental event, pregnancy, results in excessive sprouting angiogenesis in the absence of Robo4.
ROBO4 Restrains VEGF/VEGFR Signaling.
One model proposed for SLIT/ROBO4 signaling is that it functions to restrain pathologic angiogenesis by inhibiting VEGF/VEGFR2 signaling (13). We examined the activation of VEGFR2 by evaluating its autophosphorylation status in Robo4−/− glands under two proangiogenic conditions: hyperplasia, due to loss of Robo1−/−, and pregnancy. We observed an approximately twofold increase in phosphorylation in extracts from Robo1−/−;Robo4−/− glands, compared to WT, Robo1−/−, or Robo4−/− gland extracts (Fig. 4A). Moreover, a similar increase in VEGFR2 activation was observed in extracts from pregnant Robo4−/−, compared to pregnant WT, glands (Fig. 4B). We confirmed this increase in VEGFR2 signaling by immunohistochemistry using anti-PY1175 VEGFR2 (Fig. 4C and Fig. S4 A–D). Next, we examined whether this increase in VEGFR phosphorylation activated downstream signaling pathways by immunoblotting for phospho-Src (PY416) (Fig. 4D) and immunostaining for phospho-FAK (PY-397) (Fig. 4E and Fig. S4 E–H). We found up-regulated VEGFR2 signaling in hyperplastic Robo1−/−;Robo4−/− and pregnant Robo4−/− glands. Altogether the data show that loss of Robo4 under conditions that favor angiogenesis, tissue hyperplasia or pregnancy, leads to increased VEGF/VEGFR2 signaling (Fig. 4) and increased angiogenesis (Figs. 2 D–I and 3 F–H, L, and M).
Fig. 4.
ROBO4 functions to restrain VEGF/VEGFR2 signaling in the mammary gland. (A) Increased activation of VEGFR2 in Robo1−/−;Robo4−/− but not WT, Robo1−/− or Robo4−/− glands. Immunoblotting for VEGFR2 and phosphotyrosine (4G10) after immunoprecipitation of VEGFR2 from adult gland lysates. Bar graph represents quantification of phospho-VEGFR2 relative to total VEGFR2 (ImageJ) (n = 4 per stage and per genotype). Error bars = SEM, **P < 0.005 unpaired t test. (B) Increased activation of VEGFR2 in Robo4−/− in pregnant glands (day 12.5) compared to WT. Bar graph represents quantification of phospho-VEGFR2 relative to total VEGFR2 (ImageJ) (n = 3). Error bars = SEM, **P < 0.005 unpaired t test. (C) Increased activation of VEGFR2 in Robo1−/−;Robo4−/− adult glands and Robo4−/− pregnant glands, compared to WT controls. Bar graphs represent area fraction of pixels positive for PY1175-VEGFR2 divided by the area fraction positive for PECAM (n = 4 animals/genotype, 10 FOV/animal). Error bars = SEM, **P < 0.005 ANOVA. (D) Increased activation of Src in Robo1−/−;Robo4−/− adult virgin glands and Robo4−/− pregnant (day 12.5) glands, compared to WT controls. Representative immunoblots for Src and P-Y416-Src on mammary lysates (50 μg loaded). Bar graph represent quantitative analysis of Src and P-Y416-Src band intensity (ImageJ) (n = 3). Error bars = SEM, *P < 0.01 unpaired t test. (E) Increased activation of FAK in Robo1−/−;Robo4−/− adult virgin glands and Robo4−/− pregnant (day 12.5) glands compared to WT controls. Bar graphs represent the area fraction of pixels positive for PY397-FAK divided by the area fraction positive for PECAM (n = 4 animals, 10 FOV/animal). Error bars = SEM, *P < 0.01, **P < 0.005 ANOVA.
Discussion
Here, we took advantage of a relatively simple, but highly manipulable, model system of organ development to examine the role of SLIT guidance cues in regulating vascular development during postnatal mammogenesis. This involves the elaboration of an extensive vascular bed and the generation of ductal capillary plexuses, concomitant with expansive growth of the epithelial mammary tree (18). There have been many conflicting reports describing the response of cultured endothelial cells to SLIT, but few studies examining the role of SLIT/ROBO signaling in regulating angiogenesis and vascular remodeling in vivo (6, 13). Our data show that blood vessels respond to a stromal source of SLIT that signals through a ROBO4-mediated pathway to counter VEGF/VEGFR signaling and restrain angiogenesis (Fig. S5). In contrast, ROBO1 on endothelial cells does not appear to restrain vessel growth. Taken together, our studies support a recently proposed model for SLIT/ROBO4 function based on studies of pathologic angiogenesis in the retina (13). Both in this context and in the mammary gland, there are two requirements for increased angiogenesis (1): elimination of the restraining function of ROBO4 and (2) provision of a proangiogenic cue such as VEGF (Figs. 2 D–G, 3 F–H and J–M, 4, and Fig S5).
The identity of the SLIT receptor on blood vessels is unclear because both ROBO1 and ROBO4 have been implicated in endothelial cell migration (2, 7, 10, 29). Surprisingly, we found in mammary gland that loss of neither Robo4 nor Robo1, alone, affected blood vessel growth but, instead, the absence of both ROBO receptors was required to generate the increased angiogenesis observed in Slit2+/−;Slit3−/− glands. This was perplexing because the current model for SLIT/ROBO signaling in endothelium proposes the formation of a heterodimeric complex of receptors, with ROBO1 responsible for SLIT binding and ROBO4 functioning in signal transduction (5). If this heterodimeric complex were present on mammary blood vessels, then we would expect loss of either Robo1 or Robo4 to yield a phenotype, because both would be required to transduce the SLIT signal.
One of the authors (D.Y.L.) and coworkers, however, recently showed using the retina as an in vivo model system that loss of Robo4, alone, yielded a phenotype in the adult animal during the process of pathological angiogenesis (13). In this study, no phenotype was found in Robo4−/− animals during development that occurred normally with no apparent defects in vasculogenesis or angiogenesis. When evaluating the mammary gland phenotypes generated by loss of both Robo1 and Robo4, we realized that loss of either Slit or Robo1 in our mammary model system causes a secondary, potentially proangiogenic effect: up-regulation of SDF1 and VEGF (Fig. 3 A and B and Fig. S3 A–J) (27). There is growing evidence that SDF1 and VEGF collaborate to stimulate neoangiogenesis occurring in response to tumors, wounds, and chronic inflammatory disorders (28, 30, 31). Together, these factors contribute to the rapid proliferation of blood vessels observed during pathological angiogenesis. Our data show that up-regulation of these proangiogenic cues, due to loss of Slit or Robo1, functions as a “stimulatory cue” in our model system (Fig. 3 A, B, F–H, J–M and Fig. S3). Absent of this effect, ROBO1 does not appear to play a significant role transducing the inhibitory SLIT signal in blood vessels as evidenced by (i) the lack of phenotype in Robo1−/− glands (Figs. 2C and 3D and Figs. S2 E and F and S3 K and L), and (ii) the increase in vessel density in chimeric glands containing Robo1−/− epithelium and Robo4−/− endothelium (Fig. 3 F–H and Fig. S3 M and N).
Our data show a clear genetic interaction between SLITs and ROBO4. Moreover, there is recently published evidence that SLITs activate a ROBO4-initiated downstream signaling cascade (6, 14). However, it is still unclear whether SLITs bind directly to ROBO4. Direct interactions have been demonstrated by coimmunoprecipitation assays (6, 7), but the interaction cannot be duplicated with recombinant protein in Biacore assays (15). Thus, it seems likely that a coreceptor is required to transmit SLIT binding into ROBO4 activation. In some contexts, ROBO1 may fulfill this function (5), whereas in other contexts it may be served by receptors such as a Syndecan (11, 12).
Datasets from microarray analyses on human breast tumor samples show decreased Robo4 expression in human breast cancer (32), colorectal cancer (33), and prostate tumors (34). Our study suggests one explanation for this finding. Environments that require growth, such as tumor microenvironments, may down-regulate Robo4 expression to enhance the blood supply to cancerous cells because SLIT/ROBO4 signaling inhibits VEGF-mediated angiogenesis. These studies suggest that one way a proangiogenic tumor environment reduces SLIT/ROBO4 signaling and releases the brake on VEGF/VEGFR signaling is by downregulating Robo4 expression.
The recent model proposed for ROBO4 action, in which it counters the activation of VEGF/VEGFR signaling, limited its role to pathological processes in the retina. Here, we present evidence that ROBO4 also restrains blood vessel growth during the nonpathological expansion of epithelium and endothelium occurring in mammary gland in preparation for milk production and delivery. VEGF-A is the prime candidate for mediating this rapid increase in capillary number achieved by sprouting angiogenesis (19, 20, 35), but an unanswered question is how its actions are regulated during this short burst of pregnancy-associated angiogenesis that is coupled with rapid epithelial expansion. This period of development must be tightly regulated to prevent loss of growth control that would contribute to tumor development (36). We find that loss of Robo4 during midpregnancy, when VEGF-A expression is at its highest (19), leads to a significant increase in the vascular density of the gland (Fig. 3 J–M). This corresponds to increased VEGFR2 autophosphorylation and activation of downstream signaling pathways (Fig. 4). Thus, down-regulation or silencing of Robo4 expression during pregnancy or involution, periods of active tissue remodeling, could contribute to a tumor microenvironment and may play a role in the transient increase in breast cancer risk observed following pregnancy (37). Taken together, our results indicate a guardianship role for Robo4 in normal development, when we propose it functions to restrain VEGF/VEGFR2 signaling during sprouting angiogenesis that generates alveolar blood supply.
In conclusion, the findings presented in this report identify the importance of locally-derived SLIT in restraining vascular growth during pregnancy and early stages of breast transformation. This study comprehensively addresses the contribution of both SLIT and their ROBO receptors to vascular development during mammalian organogenesis. Our data support a role for this signaling axis in inhibiting endothelial cell proliferation by downregulating the activation of downstream Src and FAK family kinases and, consequently, counteracting VEGF-VEGFR signaling.
Materials and Methods
Animals.
All mice were harvested as adults (10- to 12-wk-old). The study conformed to guidelines set by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Santa Cruz. Slit2, Slit3, Robo1, and Robo4 null mice were generated as described (13, 24). Transplant techniques, antibodies, immunohistochemistry, migration assays, RT-PCR, immunoprecipitation, immunoblotting, image processing, statistical analyses and determination of blood vessel density, branchpoints and tortuosity are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Hector Macias and Jennifer Compton for critical reading of the manuscript and Jennifer Compton and Angel Moran for genotyping. Slit3−/− mice were generously provided by Dr. Ornitz (Washington University, St. Louis, MO) and Slit2−/− and Robo1−/− mice by Dr. Tessier-Lavigne (Genentech Inc., South San Francisco, CA). This research was funded by the National Institutes of Health (RO1 CA-128902), Congressionally Directed Medical Research Program (W81XWH-08-1-0380), and Santa Cruz Cancer Benefit Group.
Footnotes
Conflict of interest statement: D.Y.L. is employed by the University of Utah, which has filed intellectual property surrounding the therapeutic uses of targeting Robo4 and with the intent to license this body of intellectual property for commercialization.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001896107/-/DCSupplemental.
References
- 1.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Wang B, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, et al. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J Biol Chem. 2006;281:11347–11356. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- 4.Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheldon H, et al. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, et al. Repulsive Axon Guidance Molecule Slit3 Is a Novel Angiogenic Factor. 2009;Blood 114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park KW, et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 8.Seth P, et al. Magic roundabout, a tumor endothelial marker: Expression and signaling. Biochem Biophys Res Commun. 2005;332:533–541. doi: 10.1016/j.bbrc.2005.03.250. [DOI] [PubMed] [Google Scholar]
- 9.Kaur S, et al. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol. 2008;9:61. doi: 10.1186/1471-2121-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LJ, et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci. 2008;99:510–517. doi: 10.1111/j.1349-7006.2007.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- 12.Steigemann P, Molitor A, Fellert S, Jäckle H, Vorbrüggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Jones CA, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones CA, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005;19:121–123. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- 16.Hinck L, Silberstein GB. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soemarwoto IN, Bern HA. The effect of hormones on the vascular pattern of the mouse mammary gland. Am J Anat. 1958;103:403–435. doi: 10.1002/aja.1001030305. [DOI] [PubMed] [Google Scholar]
- 18.Djonov V, Andres AC, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001;52:182–189. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Pepper MS, et al. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn. 2000;218:507–524. doi: 10.1002/1097-0177(200007)218:3<507::AID-DVDY1012>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Hovey RC, Goldhar AS, Baffi J, Vonderhaar BK. Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol Endocrinol. 2001;15:819–831. doi: 10.1210/mend.15.5.0635. [DOI] [PubMed] [Google Scholar]
- 21.Rossiter H, et al. Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 2007;21:3994–4004. doi: 10.1096/fj.07-8720com. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Y, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 23.Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216. doi: 10.1186/bcr1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strickland P, Shin GC, Plump A, Tessier-Lavigne M, Hinck L. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development. 2006;133:823–832. doi: 10.1242/dev.02261. [DOI] [PubMed] [Google Scholar]
- 25.Plump AS, et al. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 26.Robinson GW, Accili D, Hennighausen L. Rescue of Mammary Epithelium of Early Lethal Phenotypes by Embryonic Mammary Gland Transplantation as Exemplified with Insulin Receptor Null Mice. New York: Kluwer Academic/Plenum Press; 2000. pp. 307–316. [Google Scholar]
- 27.Marlow R, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res. 2008;68:7819–7827. doi: 10.1158/0008-5472.CAN-08-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Z, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 30.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Lima e Silva R, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 32.Richardson AL, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Gröne J, et al. Robo1/Robo4: Differential expression of angiogenic markers in colorectal cancer. Oncol Rep. 2006;15:1437–1443. [PubMed] [Google Scholar]
- 34.Latil A, et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer. 2003;103:306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 35.Goldhar AS, Vonderhaar BK, Trott JF, Hovey RC. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol Cell Endocrinol. 2005;232:9–19. doi: 10.1016/j.mce.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.McDaniel SM, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168:608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.