Abstract
Development of cancer cell resistance, low accumulation of therapeutic drug in the lungs, and severe adverse treatment side effects represent main obstacles to efficient chemotherapy of lung cancer. To overcome these difficulties, we propose inhalation local delivery of anticancer drugs in combination with suppressors of pump and nonpump cellular resistance. To test this approach, nanoscale-based delivery systems containing doxorubicin as a cell death inducer, antisense oligonucleotides targeted to MRP1 mRNA as a suppressor of pump resistance and to BCL2 mRNA as a suppressor of nonpump resistance, were developed and examined on an orthotopic murine model of human lung carcinoma. The experimental results show high antitumor activity and low adverse side effects of proposed complex inhalatory treatment that cannot be achieved by individual components applied separately. The present work potentially contributes to the treatment of lung cancer by describing a unique combinatorial local inhalation delivery of drugs and suppressors of pump and nonpump cellular resistance.
Keywords: inhalation, antisense oligonucleotides, liposomes, orthotopic lung cancer model, pump and nonpump resistance
Lung cancer is the leading cause of cancer-related death worldwide (1). Because of the size and distribution of lung cancer, cytoreductive surgery is not very effective for this disease, and therefore chemotherapy and/or radiation are the treatments of choice. However, despite the advances in cancer treatment and improvements in lifestyle and health care, death rates from lung cancer have not changed significantly over the last 50 years. In contrast, mortality from heart disease, the leading cause of death, declined almost 2.5-fold over the same period (2). The patient survival has been in a plateau for three decades (3), with a 5-year relative survival rate of less than 18% in most countries (4). The efficacy of chemotherapy in lung cancer is limited by the rapid development of cancer cell resistance during treatment. To overcome this resistance, higher doses of toxic anticancer drug(s) are administered, often producing adverse side effects in healthy organs. We hypothesize that a substantial enhancement in the efficiency of lung cancer treatment is possible by (i) local delivery of chemotherapeutic agent(s) by inhalation and (ii) simultaneous suppression of at least major mechanisms of lung cancer cell resistance. Local delivery of anticancer drugs directly into the lungs will increase their accumulation in tumor cells and will reduce adverse side effects on healthy organs by limiting drug concentration in the blood. Simultaneous suppression of cellular resistance in tumors will increase the intracellular concentration of the drug in cancer cells and enhance its cytolethality for cancerous cells.
Patients with asthma and chronic obstructive pulmonary disease commonly use inhaled drugs (5). Although some chemotherapeutic agents can be delivered through the pulmonary or intratracheal route (6–9), most anticancer drugs cannot be inhaled in their traditional form and require a special drug delivery system (DDS) to be deposited directly into the lungs. Recently, several different DDS have been developed for inhalation delivery of different drugs. Liposomes (10–13), lipid- and polymer-based nanoparticles (14–17), are the most frequently used carriers for local pulmonary delivery of different drugs, antisense oligonucleotides (ASO), and short-interfering RNA (siRNA). Comparison of the local pulmonary and systemic i.v. administration of drugs, ASO, and siRNA showed that pulmonary delivery provides for a better retention of active ingredients in the lungs and minimizes their penetration into the systemic circulation (6, 7, 12, 17), prerequisites for limiting adverse side effects of chemotherapy.
Two main mechanisms are responsible for the resistance of cancer cells against chemotherapy: pump and nonpump resistance (18–23). Pump resistance is caused by membrane efflux pumps that decrease the anticancer drug concentration inside the cells. It was found that, in most types of lung cancer cells, pump resistance does not depend on the expression of P-glycoprotein, the common drug efflux pump for most other types of cancer; rather, lung cancer cells overexpress proteins from the multidrug resistance-associated protein (MRP) family (24–26). Previously, we have shown that MRP1 transporter is expressed in untreated lung cancer cells and that this expression is up-regulated after the treatment of cancer cells with an anticancer agent, doxorubicin (DOX) (19–21). Another protein from the MRP cluster, MRP2, is not expressed at detectable levels in untreated lung cancer cells, whereas treatment with DOX leads to overexpression of this protein (19–21). Nonpump drug resistance is primarily attributed to the activation of antiapoptotic cellular defense, and BCL2 protein is a key player in this defense. Similarly to MRP1, the expression of BCL2 protein increases substantially after the treatment with anticancer drugs (19, 20, 23, 27–29).
Consequently, it is logical to hypothesize that local pulmonary delivery of anticancer agents, combined with simultaneous suppression of pump and nonpump resistance, would be capable of substantially enhancing the efficacy of treatment of lung cancer and limiting adverse side effects of chemotherapy on healthy organs. To test this hypothesis, we designed and examined, in an orthotopic murine model of human lung cancer, a complex system that allows inhalation local delivery of an anticancer drug (DOX) combined with suppressors of pump and nonpump resistance (ASO targeted to MRP1 and BCL2 mRNA, respectively).
Results
Cellular Cytotoxicity.
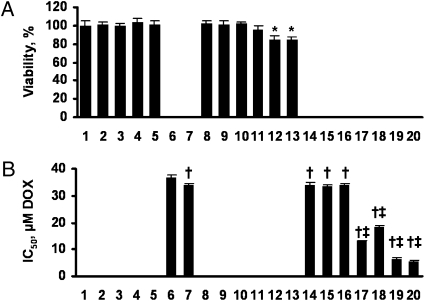
Empty liposomes, free (nonliposomal) ASO applied separately or in combination, were nontoxic (Fig. 1A, bars 1–5). Similarly, sense oligonucleotides targeted to MRP1 and BCL2 mRNA, and their combination did not influence on viability of lung cancer cells (Fig. 1A, bars 8–10). Liposomal ASO targeted to MRP1 mRNA slightly and statistically nonsignificantly (P > 0.05) decreased cell viability. Liposomal ASO targeted to BCL2 mRNA and combination of these ASO with MRP1 ASO slightly but statistically significantly (P < 0.05) decreased the viability of cancer cells (Fig. 1A, bars 11–13). It appears that such a decrease induced by the combination of liposomal ASO was almost completely attributed to BCL2 ASO (compare bars 13 and 12 in Fig. 1A). As expected, delivery of DOX by liposomes slightly (~10%) but statistically significantly increased its toxicity compared with free DOX (Fig. 1B, bars 6 and 7). A further escalation in toxicity of liposomal DOX was achieved by incorporating MRP1, BCL2 ASO and their combination (Fig. 1B , bars 17–20). At the same time, inclusion of sense oligonucleotides in the liposomal drug delivery system containing DOX did not significantly change the IC50 of liposomal DOX (Fig. 1B, bars 14–16). Therefore, enhancement of the cytotoxicity was attributed to the sequence of ASO but not to the presence of oligonucleotides in the liposomal formulations. Both a mixture (“cocktail”) of liposomal formulations containing different ASO combined with DOX or a single complex liposomal DDS containing all active components in one liposome demonstrated a comparable effect, with the complex system demonstrating higher cytotoxicity (IC50 doses for mixture and complex system were 6.64 ± 0.30 μM and 5.58 ± 0.30 μM, respectively, P < 0.05; Fig. 1B, bars 19 and 20).
Fig. 1.
Viability of human lung cancer cells incubated 48 h with the indicated formulations. (A) Cytotoxicity of formulations that do not contain DOX. (B) Cytotoxicity of formulations that contain DOX. Concentration of DOX, antisense oligonulcleotides (ASO), and sense oligonucleotides (SO) and composition of liposomes in all formulations and their mixtures were the same. Mean ± SD are shown. 1, control (fresh media); 2, empty liposomes; 3, MRP1 ASO; 4, BCL2 ASO; 5, MRP1 ASO+BCL2 ASO; 6, DOX; 7, liposomal-DOX; 8, liposomal-MRP1 SO; 9, liposomal-BCL2 SO; 10, liposomal-MRP1 SO-BCL2 SO; 11, liposomal-MRP1 ASO; 12, liposomal-BCL2 ASO; 13, liposomal-MRP1 ASO-BCL2 ASO; 14, liposomal-DOX-MRP1 SO; 15, liposomal-DOX-BCL2 SO; 16, liposomal-DOX-MRP1 SO-BCL2 SO; 17, liposomal-DOX-MRP1 ASO; 18, liposomal-DOX-BCL2 ASO; 19, mixture: liposomal-DOX-MRP1 ASO + liposomal-DOX-BCL2 ASO; 20, complex DDS: liposomal-DOX-MRP1 ASO-BCL2 ASO. *P < 0.05 when compared with control; †P < 0.05 when compared with DOX; ‡P < 0.05 when compared with liposomal DOX.
Development, Visualization, and Characterization of Lung Tumor.
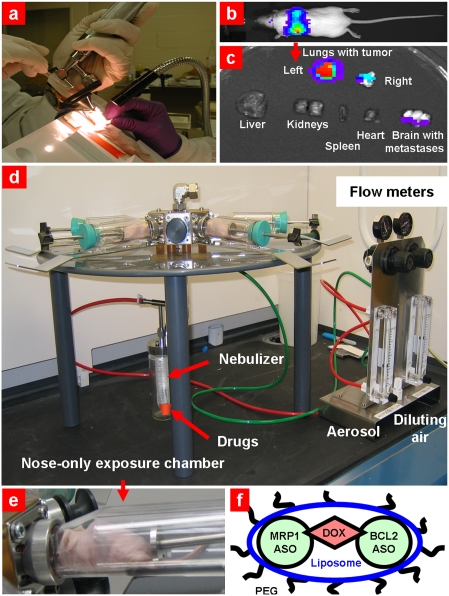
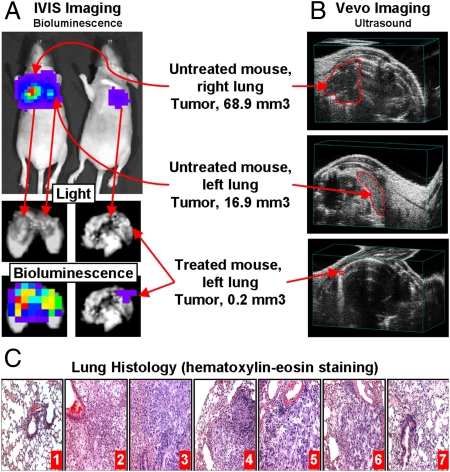
To create an orthotopic murine model of lung cancer, A549 human lung cancer cells were transfected with luciferase and injected intratracheally into lungs of nude mice (Fig. 2A). The initial deposition of cancer cells in the lungs was confirmed by demonstrating the bioluminescence of transfected cancer cells in live, anesthetized animals using an IVIS imaging system. The subsequent progression of the lung tumor was monitored using bioluminescent IVIS and ultrasound Vevo imaging systems in live animals (Fig. 2B and Fig. 3 A and B); the cancer cells in lungs and other organs were visualized using bioluminescence after the mice were killed and the lungs and other organs were excised (Fig. 2C). These data demonstrated that the tumor was induced in both lungs of mice; some mice (~5%) showed brain metastases.
Fig. 2.
Orthotopic lung tumor model and inhalation treatment of lung caner. (A) Human A549 lung cancer cells transfected with luciferase were intratracheally injected into the lungs of nude mice. (B) Typical bioluminescent image of a mouse with lung tumor 4 weeks after instillation of cancer cells. Intensity of bioluminescence is expressed by different colors, with blue reflecting the lowest intensity and red indicating the highest intensity. (C) Bioluminescence of excised organs from mice. (D) Installation for inhalation treatment. (E) Mouse inside the nose-only exposure chamber. (F) PEGylated liposomal drug delivery system containing anticancer drug (DOX) and suppressors of pump (antisense oligonucleotides targeted to MRP1 mRNA) and nonpump (antisense oligonucleotides targeted to MRP1 mRNA) resistance.
Fig. 3.
Inhalation treatment of mice with orthotopic human lung tumor by DOX combined with inhibitors of pump and nonpump cellular resistance significantly decreases tumor size. Typical bioluminescent (A, IVIS imaging system) and ultrasound (B, Vevo imaging system) images of mice (untreated and treated within 4 weeks). Untreated mouse had tumors in both left and right lungs (16.9 and 68.9 mm3, respectively). Treated mouse had one small (~0.2 mm3) tumor in left lung only. (C) Lung tissue histology (H&E staining). 1, control (no tumor); 2, untreated tumor; 3, DOX (i.v.); 4, liposomal-DOX (i.v.); 5, liposomal-DOX (inhalation); 6, mixture: liposomal-DOX-MRP1 ASO + liposomal-DOX-BCL2 ASO (inhalation); 7, complex DDS: liposomal-DOX-MRP1-BCL2 ASO (inhalation).
The quantitative analysis of lung tumor size is presented in Fig. 4C (details of the estimation are presented in SI Materials and Methods and Fig. S1). In addition to tumor progression, we used the IVIS system to evaluate the organ distribution of DDS after i.v. and inhalation instillations using liposomes labeled with the near-infrared cyanine dye Cy5.5.
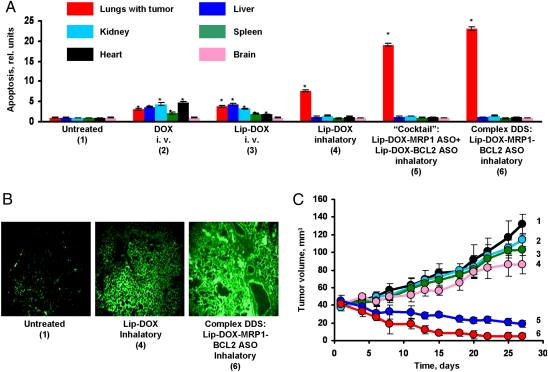
Fig. 4.
Local inhalation codelivery of anticancer drug and suppressors of pump and nonpump cellular resistance enhances apoptosis induction in the lung tumor, decreases tumor size, and prevents adverse side effects of treatment on healthy organs. An orthotopic lung tumor model was created by intratracheal instillation of human A549 lung cancer cells in nude mice. Untreated mice (1), mice treated by i.v injection of DOX (2), by i.v. injection of liposomal DOX (3), by inhalation with liposomal DOX (4), by inhalation with a mixture of the two systems (liposomal DOX + ASO targeted to MRP1 mixed with liposomal DOX + ASO targeted to BCL2 mRNA) (5), and by inhalation with one complex liposomal delivery system containing DOX, ASO targeted to MRP1, and BCL2 mRNA (6). (A) Apoptosis induction in the lungs with tumor and other organs 4 weeks after beginning of treatment. Enrichment of histone-associated DNA fragments (mono- and oligonucleosomes) per gram tissue in the tumor and organs of control animals was set to unit 1, and the degree of apoptosis was expressed in relative units. Apoptosis measurements were performed 24 h after last treatment. (B) Typical fluorescent microscopy images of tumor tissue slides labeled by TUNEL 24 h after last treatment. (C) Changes in tumor volume after beginning of treatment. Mice were treated on days 0, 3, 7, 11, 14, 17, 21, and 24. Mean ± SD are shown. *P < 0.05 when compared with untreated animals.
Histological examination of lung tissues showed that lungs of untreated mice bearing tumor demonstrated the presence of multiple tumor nodules compressing bronchi and adjacent alveoli (Fig. 3C). The tumors were composed of large pleomorphic cells with abundant eosinophilic cytoplasm and large irregular nuclei, and contained multiple prominent nucleoli. Mitotic figures, many of which were atypical, were frequent. In areas, the tumor cells formed ribbons and ill-defined glandular structures. Masses of tumor cells were interlaced with a fine connective tissue stroma and small capillary networks. Lungs of healthy control mice were well defined with well-preserved epithelial linings; alveoli were patent and clear of inflammation.
Body Distribution of Inhaled DDS.
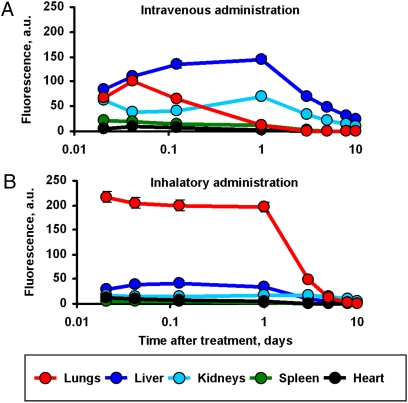
A Collison nebulizer (BGI) connected to four-port, nose-only exposure chambers (CH Technologies) (Fig. 2 D and E) was used for inhalation codelivery of drugs and ASO by complex liposomal DDS (Fig. 2F). The solution of DDS was aerosolized at a flow rate of 2 L/min and then diluted by an additional 2 L/min air. The liposomes were labeled with Cy5.5; ASO were labeled with FITC and DOX possessing intrinsic red fluorescence. Organ distribution of all DDS components was studied using the IVIS system within 10 days after a single inhalation or i.v. administration (Fig. 5A). The data show that inhalation delivery substantially enhanced lung exposure to DDS and limited the accumulation of DDS in other organs. In contrast, inhalation administration of ASO substantially enhanced the exposure of the lungs to ASO and minimized the accumulation of ASO in other organs (Fig. 5B). Similar profiles of organ distribution were found also for DOX and liposomes (12). The calculation of content of ASO per gram tissue in different organs also showed their preferential accumulation in the lungs after inhalation administration (Fig. S2).
Fig. 5.
Inhalation delivery enhances lung exposure to liposomal ASO and limits their content in other organs. ASO were labeled with FITC and delivered by i.v. route (A) or by inhalation (B) into the mice. Fluorescence of ASO in different organs was measured using IVIS Xenogen imaging system. Mean ± SD are shown.
Suppression of Targeted mRNA and Proteins.
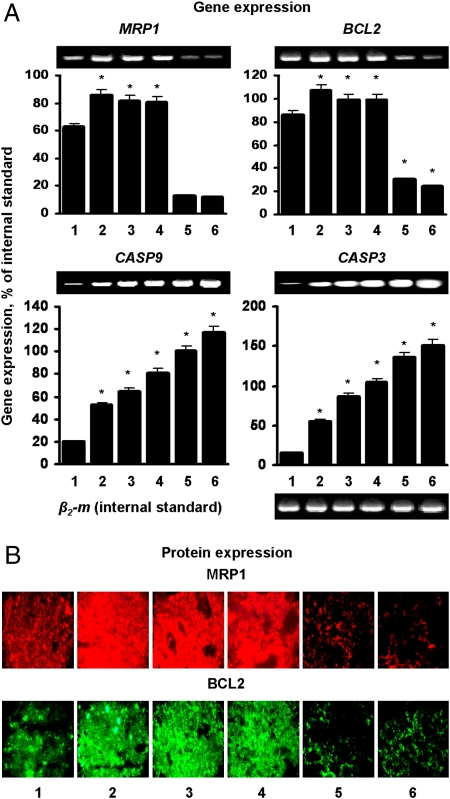
Expression of MRP1 and BCL2 mRNA and proteins responsible for pump and nonpump cellular resistance, respectively, was studied in the tumors (Fig. 6). Free and liposomal DOX delivered by the i.v. route or by inhalation up-regulated the expression of examined genes and proteins. Inhalation treatment of mice with a mixture of two DDS (liposomal DOX + MDR1 ASO and liposomal DOX + BCL2 ASO) or one complex DDS (liposomal DOX + MDR1 and BCL2 ASO) led to the simultaneous suppression of MRP1 and BCL2 mRNA and proteins in cancerous lungs (Fig. 6 A and B). The analysis of the expression of two main participants in caspase-dependent apoptosis pathway, caspase 9 (initiator of apoptosis) and caspase 3 (effector of apoptosis), showed that i.v. as well as inhalation treatments with all studied preparations activated this apoptotic pathway (Fig. 6A). However, i.v. liposomal DOX was more effective than free DOX after i.v. injection in terms of activating apoptotic cell death signal; inhalation treatment was more effective than i.v. treatment, and simultaneous suppression of pump and nonpump resistance led to the significantly higher activation of proapoptotic mechanisms. It is interesting to note that a complex DDS was more effective in activating both studied caspases than the mixture approach despite the comparable suppression of MRP1 and BCL2 genes and proteins (Fig. 6, bars 5 and 6).
Fig. 6.
Local inhalation codelivery of anticancer drug and antisense oligonucleotides enhances suppression of targeted mRNA and proteins and activation of caspase-dependent signaling pathways of apoptosis in lung tumor. Untreated mice (1), mice treated by i.v. injection of DOX (2), by i.v. injection of liposomal DOX (3), by inhalation with liposomal DOX (4), by inhalation with a mixture of the two systems (liposomal DOX + ASO targeted to MRP1 mixed with liposomal DOX + ASO targeted to BCL2 mRNA) (5), and by inhalation with one complex liposomal delivery system containing DOX, ASO targeted to MRP1, and BCL2 mRNA (6). (A) Gene expression was measured by RT-PCR and calculated as a ratio of band intensity of studied gene to that in internal standard [β2-microglobulin (β2-m)]. (B) Protein expression was examined using immunohistochemistry. Measurements were performed 24 h after last treatment. Means ± SD are shown.*P < 0.05 when compared with untreated animals.
Lung Histology.
Representative histopathology samples are presented on Fig. 3C. Tumors of mice treated with DOX were composed of large cells with eosinophilic cytoplasm; commonly formed ribbons and ill-formed glandular profiles were frequent. Tumor cell nuclei were large and irregular, with multiple prominent nucleoli. Multinucleated tumor cells and mitotic figures were abundant. Invasion of blood vessels was noted in multiple samples. Apoptotic tumor cells, characterized by apoptotic bodies and nuclear condensation, were scattered throughout the tumor masses. A thin fibrovascular stroma within the tumors was readily identified. Scattered macrophages filled with a granular brown–gold material, possibly the test material, were presented.
Lung tumors from mice treated with liposomal DOX revealed several distinct patterns of response. Small tumors appeared particularly to be affected by the treatment and displayed extensive cell death best characterized as confluent apoptosis. The nuclei were highly condensed and the cytoplasm was fragmented. The nuclei of surviving cells in small tumors were basophilic and showed early condensation. Larger tumors were less severely altered by the treatment. Tumor cells demonstrated the formation of abortive glands and ribbons of malignant cells. The tumor cells were highly atypical, with a high mitotic rate. Apoptotic cells were distributed throughout the tumor mass.
Liposomal DOX administered by inhalation resulted in alterations of normal pulmonary parenchyma characterized by alveolar hemorrhage and peri-bronchial accumulation of chronic inflammatory cells, primarily lymphocytes and macrophages. Multiple small tumors were present within the parenchyma. These tumors were composed primarily of loose aggregates of large cells with foamy cytoplasm, and showed little evidence of glandular formation. Mitoses were relatively uncommon. Apoptotic cells were observed diffusely throughout the tumor.
The mixture therapy appeared to induce focal damage to both the alveoli and proliferation of bronchial-associated lymphatic tissue. Hemorrhage and congestion of alveolar capillaries was noted in multiple areas of the lung. Large bronchi were surrounded by aggregates of chronic inflammatory cells, including lymphocytes, macrophages, and plasma cells. Several small tumors were noted within the parenchyma, usually immediately below the pleura. These tumors were composed of loosely organized masses of large epithelial cells with prominent foamy cytoplasm. The cells showed no tendency to form glands or epithelial ribbons. Although nuclei were large and cytologically atypical, mitoses were rare.
Treatment of mice with complex DDS induced some alveolar injury and some mild stimulation of bronchial-associated lymphatic tissue. Tumors in lungs of these animals were small and loosely organized and demonstrated considerable amounts of apoptotic changes. The tumor cells showed abundant foamy cytoplasm. Mitoses were uncommon, but some large syncytial tumor cells were noted. A diffuse fibrovascular connective tissue was noted throughout the tumor.
Antitumor Effect.
The apoptosis evaluation by cell death ELISA showed that local inhalation delivery of DOX enhanced apoptosis induction in the lungs with the tumors and limited adverse side effects of the treatment on healthy organs (Fig. 4A). Further enhancement of apoptosis was achieved by simultaneous suppression of pump and nonpump resistance in combination with DOX. It was found that a complex system containing all active components (DOX, MDR1 ASO, and BCL2 ASO) was more effective when compared with a mixture of two DDS each containing DOX in combination with only one type of ASO. The high effectiveness of the anticancer drug in inducing cancerous cell death and simultaneous suppression of cellular resistance was also confirmed by the other method of apoptosis detection, TUNEL (Fig. 4B). The suppression of tumor growth after inhalation treatment with the complex DDS was documented by bioluminescent and ultrasound measurements (Fig. 3 A and B and Fig. 4C). It can be seen that DOX delivered by the i.v. route or by inhalation partially decreased tumor growth. However, only inhalation treatments with a mixture of two DDS and complex DDS were able to significantly induce the regression of lung tumors (Fig. 4C). Again, the complex DDS containing DOX, MDR1 ASO, and BCL2 ASO was more effective than the mixture approach.
Discussion
The present data clearly demonstrate that we were able to verify the hypothesis and show the following: (i) inhalation delivery of DOX and ASO substantially increased the exposure of the lungs to active components of DDS (drug and ASO) and prevented cell death induction in healthy organs, thereby limiting adverse side effects of the treatment; (ii) ASO delivered in DDS by inhalation was able to down-regulate their targets, namely, MRP1 and BCL2 proteins, which are mainly responsible for the development of cellular resistance in tumor cells; and (iii) concurrent induction of tumor cell death by DOX and the suppression of cancer cell resistance enhanced apoptosis induction in the cancerous lungs and resulted in the regression of tumor growth. Such a degree of regression of tumor growth could not be achieved by the individual components of DDS applied separately.
The present study shows that both mixture and complex delivery systems successfully enhanced the efficiency of the lung cancer treatment and limited toxicity of the chemotherapy to other organs. However, the complex delivery systems showed a more pronounced effect when compared with separate, simpler systems combined as a mixture. Theoretically, simultaneous cell death induction and suppression of cellular resistance can be achieved by the delivery of each active ingredient (an anticancer drug, ASO targeted to MRP1 and BCL2 mRNA) by a mixture of simpler systems. It should be stressed that to maximize the efficacy of the treatment, all active components should be delivered to the tumor at the same time and should be released inside the cancer cell with a comparable profile. Such spatial–temporal synchronization can be achieved using one complex DDS or a mixture of several DDS that provides the same pharmacokinetic profile, delivers all drugs to the tumor at the same time, and offers similar cell-penetrating ability and release of all active components inside the cells.
In the present study, we constructed three types of DDS: one complex liposomal DDS containing all active components (DOX, MRP1, and BCL2 ASO), and two simpler liposomal systems containing DOX with ASO targeted to MRP1 mRNA and DOX combining with ASO targeted to BCL2 mRNA. Experimental testing of such systems showed a clear advantage of the complex DDS. Despite similar degrees of suppression of targeted proteins, the complex DDS induced apoptosis in cancerous lungs and prevented growth of lung tumors more effectively than a mixture of several DDS. The complex DDS often provoked the regression of overall tumor volume. We propose the following explanation of this unexpected phenomenon. First, to accumulate the same amount of active ingredients (DOX and both types of ASO), twice as many liposomes (liposomes with DOX and MRP1 ASO + liposomes with DOX and BCL2 ASO) should be applied and allowed to enter the cancer cell by endocytosis when compared with one complex system. However, the passing capacity of cellular endocytosis is limited because endocytosis is an energy-dependent process, and internalization of twice as many liposomes requires more energy. Such a limitation can, at least partially, be responsible for a lower effectiveness of the mixture when compared with one complex system. This explanation is supported by in vitro IC50 data that show that a mixture of DDS demonstrated slightly but statistically significantly lower toxicity when compared with complex DDS. Although other mechanisms can possibly play roles in this phenomenon, the data obtained clearly show advantages of the combination of all active components into one complex, multifunctional delivery system over a mixture approach.
In summary, the data obtained showed that the developed DDS suppressed the growth of lung adenocarcinoma to an extent that could not be achieved by individual components applied separately. The present work potentially contributes to the treatment of lung cancer by describing a unique combinatorial local inhalation delivery of drugs and suppressors of pump and nonpump cellular resistance.
Materials and Methods
Materials.
DOX was obtained from Sigma. P-ethoxy modified antisense oligonucleotides targeted to MRP1 (5′–TGC TGT TCG TGC CCC CGC CG–3′) and BCL2 (5′–CAG CGT GCG CCA TCC TTC CC–3′) mRNA were synthesized according to our design by Oligos Etc. PEGylated liposomes containing DOX and ASO were prepared as previously described (12). The selection of neutral PEGylated liposomes was based on the following considerations. First, we had to incorporate neutral P-ethoxy modified antisense oligonucleotides and doxorubicin in one liposome; consequently, we had to use neutral liposomes because loading efficiency of neutral ASO and DOX into charged liposomes is low. Second, our previous data (12) showed that PEGylated neutral liposomes provided for the most favorable accumulation and retention of liposomes and their payload in the lungs after inhalation delivery.
Cytotoxicity, Apoptosis, Expression of Genes and Proteins, and Histopathological Analysis.
Previously described methods (19, 20, 30, 31) were used for the analysis of these parameters (details in SI Materials and Methods).
In Vivo Tumor Growth and Treatment.
A modified orthotopic model of lung cancer originally developed for nude rats was used (32). We developed and tested a similar procedure in mice. Briefly, nude nu/nu mice, 6–8 weeks old, were purchased from Taconic. A549 human lung adenocarcinoma epithelial cell line transfected with luciferase was purchased from Xenogen. Cancer cells (5–8 × 106) were resuspended in 0.1 mL of RPMI medium containing 20% FBS, mixed with 5 μM EDTA and were administered to the murine lung through a catheter (Fig. 2A). It was shown (32) that a slight disruption of the pulmonary epithelium or the surfactant layer by coadministration of either pancreatic elastase or EDTA allowed significantly better tumor engraftment. At the same time, cancer cell suspensions tolerate this concentration of EDTA, and animals subjected to EDTA at this concentration exhibit no histological pulmonary abnormalities (32). Rapid growth of lung tumor developed in 80% of the animals. The progression of tumor growth was monitored by bioluminescent IVIS (Xenogen) and ultrasound Vevo 2100 (VisualSonics) imaging systems. Four weeks after the instillation of tumor cells, mice were treated on days 0, 3, 7, 11, 14, 17, 21, and 24 with free DOX (i.v. injection), liposomal DOX (i.v. and inhalation administrations), inhalation instillation of liposomal DDS containing DOX and ASO targeted to MRP1 mRNA mixed with liposomal DDS containing DOX and ASO targeted to BCL2 mRNA (mixture), as well as with a complex liposomal DDS containing DOX, MRP1, and BCL2 ASO. The dose of all formulations containing doxorubicin for i.v. and inhalation administration was 2.5 mg/kg for the single injection. This dose corresponds to the maximum tolerated dose of free DOX. The maximum tolerated dose of free DOX was estimated in separate experiments based on animal weight change after the injection of increasing doses of DOX as previously described (33, 34). The dose of each ASO was 0.125 mg/kg.
Antitumor Activity and Adverse Side Effects of Treatment.
Apoptosis induction in healthy organs was used as an index of adverse side effects of the treatment. Total volume of lung tumor was measured using IVIS and Vevo imaging systems. The degree of apoptosis in lungs with tumor and tumor volume were used as indicators of antitumor activity of the tested substances.
Statistical Analysis.
Data obtained were analyzed using descriptive statistics and single-factor ANOVA, and are presented as a mean ± SD from five independent measurements. We analyzed data sets for significance with Student's t test and considered P values of less than 0.05 as statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. D. C. Reimer from Rutgers Lab Animal Services for his help with the development and implementation of orthotopic mouse model of lung cancer and T. Conte and T. Coulthard from VisualSonics for assistance with obtaining and analysis of ultrasound images of animals using the Vevo 2100 imaging system. This work was supported in part by National Institutes of Health Grants CA111766 and CA100098.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004604107/-/DCSupplemental.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous . Health, United States, 2004. Hyattsville, MD: National Center for Health Statistics; 2004. p. 498. [Google Scholar]
- 3.Yang P. Epidemiology of lung cancer prognosis: Quantity and quality of life. Methods Mol Biol. 2009;471:469–486. doi: 10.1007/978-1-59745-416-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: Geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 5.Melani AS. Inhalatory therapy training: A priority challenge for the physician. Acta Biomed. 2007;78:233–245. [PubMed] [Google Scholar]
- 6.Valle MJ, González López F, Sánchez Navarro A. Pulmonary versus systemic delivery of levofloxacin. The isolated lung of the rat as experimental approach for assessing pulmonary inhalation. Pulm Pharmacol Ther. 2008;21:298–303. doi: 10.1016/j.pupt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.de Jesús Valle MJ, López FG, Hurlé AD, Navarro AS. Pulmonary versus systemic delivery of antibiotics: Comparison of vancomycin dispositions in the isolated rat lung. Antimicrob Agents Chemother. 2007;51:3771–3774. doi: 10.1128/AAC.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jesús Valle MJ, Uranga NS, López FG, Hurlé AD, Navarro AS. Disposition of linezolid in the isolated rat lung after systemic and pulmonary drug delivery. J Antimicrob Chemother. 2007;60:1074–1079. doi: 10.1093/jac/dkm306. [DOI] [PubMed] [Google Scholar]
- 9.Gagnadoux F, et al. Aerosol delivery of chemotherapy in an orthotopic model of lung cancer. Eur Respir J. 2005;26:657–661. doi: 10.1183/09031936.05.00017305. [DOI] [PubMed] [Google Scholar]
- 10.Anabousi S, et al. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur J Pharm Sci. 2006;29:367–374. doi: 10.1016/j.ejps.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Anabousi S, et al. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J Nanosci Nanotechnol. 2006;6:3010–3016. doi: 10.1166/jnn.2006.461. [DOI] [PubMed] [Google Scholar]
- 12.Garbuzenko OB, et al. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm Res. 2009;26:382–394. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 13.Koshkina NV, et al. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7:3258–3262. [PubMed] [Google Scholar]
- 14.Beck-Broichsitter M, et al. Pulmonary drug delivery with aerosolizable nanoparticles in an ex vivo lung model. Int J Pharm. 2009;367:169–178. doi: 10.1016/j.ijpharm.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Borm PJ, Kreyling W. Toxicological hazards of inhaled nanoparticles—potential implications for drug delivery. J Nanosci Nanotechnol. 2004;4:521–531. doi: 10.1166/jnn.2004.081. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen J, Steele TW, Merkel O, Reul R, Kissel T. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J Control Release. 2008;132:243–251. doi: 10.1016/j.jconrel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramesh R, et al. Local and systemic inhibition of lung tumor growth after nanoparticle-mediated mda-7/IL-24 gene delivery. DNA Cell Biol. 2004;23:850–857. doi: 10.1089/dna.2004.23.850. [DOI] [PubMed] [Google Scholar]
- 18.Pakunlu RI, Cook TJ, Minko T. Simultaneous modulation of multidrug resistance and antiapoptotic cellular defense by MDR1 and BCL-2 targeted antisense oligonucleotides enhances the anticancer efficacy of doxorubicin. Pharm Res. 2003;20:351–359. doi: 10.1023/a:1022687617318. [DOI] [PubMed] [Google Scholar]
- 19.Pakunlu RI, et al. Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: Novel multicomponent delivery system. Cancer Res. 2004;64:6214–6224. doi: 10.1158/0008-5472.CAN-04-0001. [DOI] [PubMed] [Google Scholar]
- 20.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Minko T. A novel cancer therapy: Combined liposomal hypoxia inducible factor 1 alpha antisense oligonucleotides and an anticancer drug. Biochem Pharmacol. 2004;68:2031–2042. doi: 10.1016/j.bcp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Minko T, Dharap SS, Pakunlu RI, Wang Y. Molecular targeting of drug delivery systems to cancer. Curr Drug Targets. 2004;5:389–406. doi: 10.2174/1389450043345443. [DOI] [PubMed] [Google Scholar]
- 23.Pakunlu RI, et al. In vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drug. J Control Release. 2006;114:153–162. doi: 10.1016/j.jconrel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Hsia TC, Lin CC, Wang JJ, Ho ST, Kao A. Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung. 2002;180:173–179. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M, et al. A case of pulmonary adenocarcinoma with overexpression of multidrug resistance-associated protein and p53 aberration. Anticancer Res. 2000;20(3B):1921–1925. [PubMed] [Google Scholar]
- 26.Scagliotti GV, Novello S, Selvaggi G. Multidrug resistance in non-small-cell lung cancer. Ann Oncol. 1999;10(Suppl 5):S83–S86. doi: 10.1093/annonc/10.suppl_5.s83. [DOI] [PubMed] [Google Scholar]
- 27.Chandna P, et al. Targeted proapoptotic anticancer drug delivery system. Mol Pharm. 2007;4:668–678. doi: 10.1021/mp070053o. [DOI] [PubMed] [Google Scholar]
- 28.Dharap SS, et al. Molecular targeting of BCL2 and BCLXL proteins by synthetic BCL2 homology 3 domain peptide enhances the efficacy of chemotherapy. J Pharmacol Exp Ther. 2006;316:992–998. doi: 10.1124/jpet.105.094243. [DOI] [PubMed] [Google Scholar]
- 29.Zalipsky S, et al. Antitumor activity of new liposomal prodrug of mitomycin C in multidrug resistant solid tumor: Insights of the mechanism of action. J Drug Target. 2007;15:518–530. doi: 10.1080/10611860701499946. [DOI] [PubMed] [Google Scholar]
- 30.Jayant S, et al. Targeted sialic acid-doxorubicin prodrugs for intracellular delivery and cancer treatment. Pharm Res. 2007;24:2120–2130. doi: 10.1007/s11095-007-9406-1. [DOI] [PubMed] [Google Scholar]
- 31.Lu G, et al. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–11501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 32.March TH, Marron-Terada PG, Belinsky SA. Refinement of an orthotopic lung cancer model in the nude rat. Vet Pathol. 2001;38:483–490. doi: 10.1354/vp.38-5-483. [DOI] [PubMed] [Google Scholar]
- 33.Dharap SS, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci USA. 2005;102:12962–12967. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minko T, Kopecková P, Kopecek J. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int J Cancer. 2000;86:108–117. doi: 10.1002/(sici)1097-0215(20000401)86:1<108::aid-ijc17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.