Abstract
The Hippo signaling pathway regulates organ size and tissue homeostasis from Drosophila to mammals. At the core of the Hippo pathway is a kinase cascade extending from the Hippo (Hpo) tumor suppressor to the Yorkie (Yki) oncoprotein. The Hippo kinase cascade, in turn, is regulated by apical membrane-associated proteins such as the FERM domain proteins Merlin and Expanded (Ex), and the WW- and C2-domain protein Kibra. How these apical proteins are themselves regulated remains poorly understood. Here, we identify the transmembrane protein Crumbs (Crb), a determinant of epithelial apical-basal polarity in Drosophila embryos, as an upstream component of the Hippo pathway in imaginal disk growth control. Loss of Crb leads to tissue overgrowth and target gene expression characteristic of defective Hippo signaling. Crb directly binds to Ex through its juxtamembrane FERM-binding motif (FBM). Loss of Crb or mutation of its FBM leads to mislocalization of Ex to basolateral domain of imaginal disk epithelial cells. These results shed light on the mechanism of Ex regulation and provide a molecular link between apical-basal polarity and tissue growth. Furthermore, our studies implicate Crb as a putative cell surface receptor for Hippo signaling by uncovering a transmembrane protein that directly binds to an apical component of the Hippo pathway.
Keywords: apical-basal polarity, development, Drosophila, organ size, signal transduction
The Hippo signaling pathway, first discovered in Drosophila, has recently emerged as an evolutionarily conserved mechanism that regulates organ size in diverse species, including mammals (1, 2). In Drosophila, four tumor suppressor proteins including Hippo (Hpo), Salvador (Sav), Warts (Wts) and Mats form a kinase cascade that ultimately phosphorylates and inactivates the oncoprotein Yki (3). Although the molecular organization of this core kinase cascade is well established, signaling events upstream of Hpo remains less understood. Studies in Drosophila have implicated several tumor suppressor proteins as upstream regulators of the Hippo kinase cascade. Several apical membrane-associated cytoplasmic proteins, including the FERM-domain proteins Expanded (Ex) and Merlin (Mer) (4), and the WW- and C2-domain protein Kibra (5–7), have been suggested to function together in a protein complex that regulates Hippo signaling, at least in part, by recruiting the Hpo-Sav kinase complex to cell membrane (5). The atypical cadherin Fat (Ft) has been proposed as a cell surface receptor that regulates Hippo signaling by controlling the membrane localization and/or stability of Ex (8–10). However, no physical interactions have been reported between Ft and Ex, and this model was challenged with an alternative model wherein Ft and Ex function in parallel to regulate Wts stability and activity, respectively (11, 12). Overall, our understanding of these upstream pathway components remains incomplete, and additional cell surface proteins may exist that physically link the apical components such as Ex to the cell membrane.
Crumbs (Crb) is an apical transmembrane protein that is required for organizing apical-basal polarity and adherens junctions (AJs) in gastrulating Drosophila embryos (13). It contains 28 EGF-like and four laminin AG-like repeats in its extracellular domain and a short intracellular domain including a juxtamembrane FERM-binding motif (FBM) and a C-terminal PDZ-binding motif (PBM). Through its PBM, Crb forms a complex with the PDZ domain proteins Stardust (Sdt) and Patj (14–16). In regulating apical-basal polarity in Drosophila embryos, the Crb-Sdt-Patj complex functions together with another apical protein complex containing Bazooka (Baz), Par-6, and aPKC, and these apical complexes are antagonized by a basolateral protein complex containing Scribble (Scrib), Discs large (Dlg), and Lethal giant larvae (Lgl) (17, 18). Strikingly, the intracellular domain of Crb is required and sufficient for Crb's function in apical-basal polarity in Drosophila embryos (19). In contrast to its essential role in establishing apical-basal polarity in Drosophila embryos, Crb is not essential for apical-basal polarity or AJ integrity in third instar larval imaginal discs (20, 21). Rather, Crb is required for rhabdomere elongation and stalk membrane formation during photoreceptor morphogenesis (20, 21).
Here, we report the identification of Crb as a tumor suppressor and an upstream component of the Hippo signaling pathway. We further show that Crb directly binds to Ex through Crb's FBM and is required for apical localization of Ex in imaginal epithelium. Our studies uncover a transmembrane protein that directly binds to an apical component of the Hippo pathway and implicate Crb as a potential cell surface receptor for Hippo signaling.
Results
Identification of crb as a Tumor Suppressor Gene.
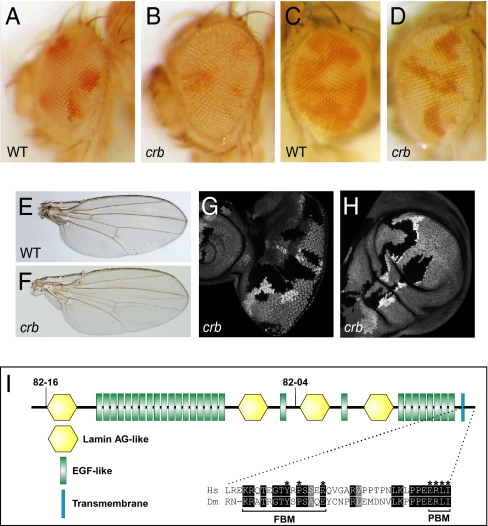
In a screen for growth regulators using the eyeless-FLP/recessive cell lethal technique (22), we identified two lethal mutations (82–04 and 82–16) defining a single complementation group on chromosome 3R that caused mild overgrowth of adult eyes and increased representation of mutant tissues (Fig. 1 A and B). To further compare the growth property of the mutant versus wild-type cells, we generated mosaic eyes using eyeless-FLP without the recessive cell lethal. Such mosaic eyes also showed increased representation of mutant tissues (Fig. 1 C and D). Adult wings comprised predominantly of mutant cells were also larger than normal wings (Fig. 1 E and F). Consistent with the overgrowth of adult tissues, mutant clones in larval eye and wing imaginal discs were larger than their twin spots (no cell lethal used) (Fig. 1 G and H).
Fig. 1.
Loss of crb results in tissue overgrowth. (A and B) A control (A) and a mutant eye composed predominantly of crb82-04 cells (B). The genotypes are y w ey-flp; FRT 82B/FRT 82B P[w+], L(3)c1-R3 (A) and y w ey-flp; FRT 82B crb82-04/FRT 82B P[w+], L(3)c1-R3 (B). (C and D) A control (C) and a mutant eye containing crb82-04 clones (D). The genotypes are y w ey-flp; FRT 82B /FRT 82B Ubi-GFP (C) and y w ey-flp; FRT 82B crb82-04 /FRT 82B Ubi-GFP (D). Note that crb82-04 mosaic eyes (D) contained predominantly mutant tissues (white), whereas mosaic eyes for a control chromosome contained far less white tissues (C). (E and F) A control (E) and a mutant wing composed predominantly of crb82-04 cells (F). The genotypes are y w MS1096-Gal4, UAS-flp; FRT 82B /FRT 82B P[w+], L(3)c1-R3 (G) and y w MS1096-Gal4, UAS-flp; FRT 82B crb82-04/FRT 82B P[w+], L(3)c1-R3 (H). crb82-04 wings were 112% of normal size (n = 20, t test: P = 0.0001). (G and H) An eye (G) or wing (H) disk containing crb82-04 clones (GFP-negative). Mutant clones were induced at 36 h after egg deposition. The average area of crb eye clones versus twin spots is 2.5 (n = 30). (I) Schematic diagram of the Drosophila Crb protein. The nonsense mutations in crb82-04 and crb82-16 predicted to truncate the protein are also shown. Sequence alignment shows the cytoplastic domains of Crb orthologs from Drosophila (Dm) and human (Hs).
We mapped 82–04 and 82–16 to molecular coordinates 20096927; 20124398 based on their noncomplementation with two overlapping deficiencies, Df(3R)Exel8178 (20096927;20353553) and Df(3R)BSC317 (20046218;20124398). Tests with available mutations in this interval showed that they failed to complement three independent alleles of crumbs (crb), crb1, crb2, and crb07207. 82–04 and 82–16 contained nonsense mutations that were predicted to truncate Crb in its extracellular domain: 1252Glu to STOP in 82–04 and 68Trp to STOP in 82–16 (Fig. 1I). As 82–16 truncates Crb close to its N terminus, and both alleles displayed similar overgrowth phenotypes, we consider both as null alleles.
Loss of crb Produces Signaling Defects Similar to Mutants of Upstream Regulators of the Hippo Pathway.
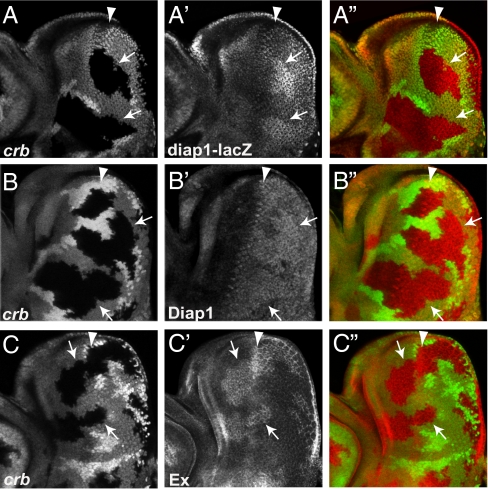
The overgrowth phenotype observed in crb mosaic eyes resemble that of defective Hippo signaling. To explore the relationship between Crb and Hippo signaling, we examined diap1 and ex, two well characterized Hippo target genes, in eye discs. crb clones showed a modest increase in diap1 transcription and Diap1 protein levels (Fig. 2 A–B″). crb clones also showed a significant increase in Ex protein levels (Fig. 2 C–C″), although we could not detect a visible increase in ex transcription (Fig. 3 A–A″). Such modest effects on target genes resemble upstream components of Hippo signaling but are weaker than the core components of the Hippo kinase cascade.
Fig. 2.
Crb regulates Hippo target genes. In all panels, crb clones (GFP-negative) are marked by arrows, and arrowhead marks the morphogenetic furrow (MF). (A–A″) An eye disk showing elevated diap1-lacZ levels in crb clones posterior to the MF. (B–B″) An eye disk showing elevated Diap1 protein levels in crb clones posterior to the MF. (C–C″) An eye disk showing elevated Ex protein levels in crb clones both anterior and posterior to the MF.
Fig. 3.
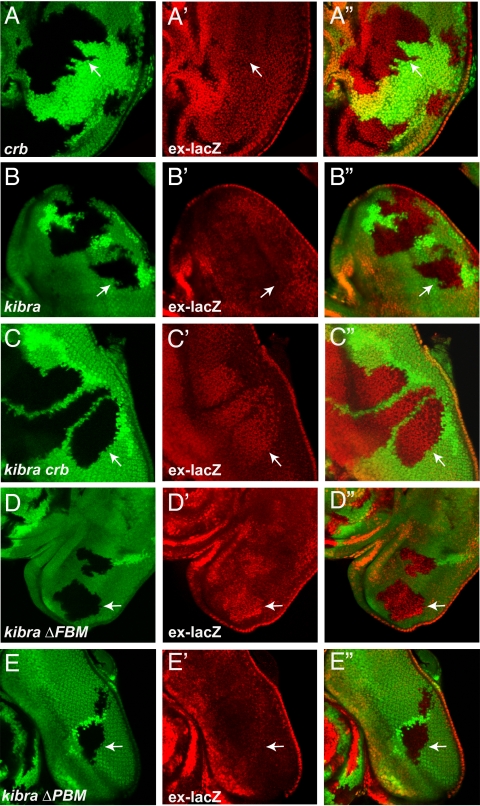
The FBM is required for Crb's function in regulating Hippo signaling. In all panels, mutant clones (GFP-negative) are indicated with arrows. Eye discs containing crb (A–A″), kibra (B–B″), kibra crb (C–C″), kibra crbΔFBM (D–D″), and kibra crbΔPBM (E–E″) clones were stained for ex-lacZ (red). Note elevated ex-lacZ levels in kibra crb and kibra crbΔFBM, but not in crb, kibra, or kibra crbΔPBM clones.
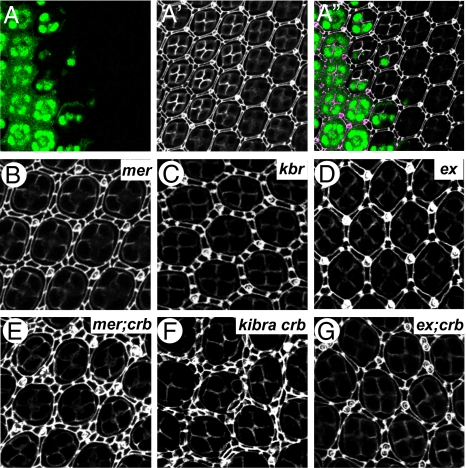
Another characteristic of defective Hippo signaling is alteration of interommatidial cell numbers in pupal retina. Inactivation of core components of the Hippo kinase cascade (such as hpo and sav) results in a massive increase in interommatidial cells [>40 extra cells per cluster, (ECPC)], whereas mutants of upstream regulators of the Hippo pathway show a much milder phenotype, with loss of ex, mer, and kibra resulting in 1.4, 8.4, and 5.8 ECPC, respectively (5). crb mutant pupal retina showed an average of 1.2 ECPC (Fig. 4 A–A″), which resembles ex but is milder than mer or kibra. To examine the relationship between crb and the known upstream regulators, we examined kibra crb, ex;crb and mer;crb double mutant clones. Although crb, mer, and kibra mutant eyes had an average of 1.2, 8.4, and 5.8 ECPC, respectively, mer;crb, and kibra crb eyes had 14.1 and 21.4 ECPC, respectively (Fig. 4). In contrast, ex;crb mutant eyes had 1.5 ECPC, which resembled crb or ex single mutants (Fig. 4). These observations strongly suggest that Crb might act through Ex to regulate Hippo signaling.
Fig. 4.
Crb and Ex function in a common pathway to regulate interommatidial cell number. (A–A″) A pupal eye containing crb82-04 clones (GFP-negative) (A), and stained for Dlg (A’). Superimposed image is shown in A″. (B–G) Pupal eyes of the indicated genotype and stained for Dlg. The genotypes are: (B) y w ey-flp Ubi-GFP FRT19A /mer4 FRT19A, (C) y w ey-flp; FRT 82B kibradel /FRT 82B Ubi-GFP, (D) y w ey-flp; FRT40A exe1/FRT40A Ubi-GFP, (E) y w ey-flp Ubi-GFP FRT19A /mer4 FRT19A; FRT 82B crb82-04 /FRT 82B Ubi-GFP, (F) y w hsp-flp; FRT 82B kibradel crb82-04 /FRT 82B Ubi-GFP, (G) y w ey-flp; FRT40A exe1/FRT40A Ubi-GFP; FRT 82B crb82-04 /FRT 82B Ubi-GFP. Twenty ommatidial clusters of each genotype were used for counting interommatidial cells.
Crb Directly Binds to Ex Through Its FBM.
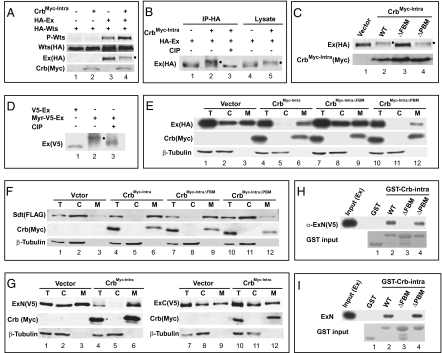
To substantiate a molecular link between Crb and Hippo signaling, we examined the effect of Crb on Wts T1077 phosphorylation (5). Because a truncated Crb construct encoding the membrane-bound cytoplasmic domain of Crb is sufficient for Crb's function in Drosophila embryos (19), we examined the activity of an analogous construct (CrbMyc-Intra) in S2R+ cells. Interestingly, although CrbMyc-Intra alone did not activate Wts phosphorylation, it enhanced Ex-mediated activation of Wts phosphorylation (Fig. 5A), thus revealing a positive influence of Crb on Hippo kinase cascade. In the course of this experiment, we noted that CrbMyc-Intra promoted Ex phosphorylation accompanied by a decrease of Ex protein levels (Fig. 5 A and B). Interestingly, mutation of the FBM (by mutating Y10P12E16 of the FBM to alanines), but not the PBM (by deleting the C-terminal residues ERLI), abrogated Crb-induced Ex phosphorylation (Fig. 5C). Given the presence of FERM domain in Ex, we hypothesized that in our S2R+ cell assay, Crb may recruit Ex to membrane via its FBM. Upon reaching the cell membrane, Ex may be phosphorylated by unknown kinase(s). Consistent with this hypothesis, targeting Ex to cell membrane by attaching a myristylation signal to it N terminus also induced Ex phosphorylation (Fig. 5D).
Fig. 5.
Crb binds to Ex through its FBM. (A–D) Hyperphosphorylated Ex is indicated by a filled circle next to the protein band. (A) CrbMyc-Intra promotes Wts phosphorylation in conjunction with Ex. S2R+ cells expressing the indicated constructs were probed with the indicated antibodies. Note the increased P-Wts signal in lane 4 compared with lanes 2 and 3. (B) HA-Ex immunoprecipitates were treated with or without Alkaline Phosphatase (CIP) (lanes 1–3). Note the reversal of CrbMyc-Intra-induced Ex mobility shift by CIP treatment. Ex in S2R+ cell lysates also showed Crb-induced mobility shift (lanes 4–5). (C) The FBM, but not the PBM, is required for CrbMyc-Intra to promote Ex phosphorylation. (D) Membrane targeting of Ex via fusion with a myristylation signal induced Ex phosphorylation (lanes 1–2), which was reversed by CIP treatment (lane 3). (E) The FBM is required for CrbMyc-Intra to recruit Ex to plasma membrane. S2R+ cells expressing HA-Ex with the indicated constructs were subjected to cell fractionation. Cytosol (C), membrane (M), and a portion of the total lysate (T) were probed with indicated antibodies. Note that CrbMyc-Intra (lanes 4–6) or CrbMyc-IntraΔPBM (lanes 10–12), but not CrbMyc-IntraΔFBM (lanes 7–9), relocated Ex from cytosol to membrane (compare with lanes 1–3). (F) Similar to (E) except that Sdt was analyzed. Note that CrbMyc-Intra (lanes 4–6) or CrbMyc-IntraΔFBM (lanes 7–9), but not CrbMyc-IntraΔPBM (lanes 10–12), relocated Sdt from cytosol to membrane (compare with lanes 1–3). (G) CrbMyc-Intra interacts with Ex's N-terminal half (ExN: aa1-709; lanes 1–6) but not with Ex's C-terminal half (ExC: aa710-1427; lanes 7–12) in recruitment assay. (H) Physical association between Crb and Ex in vitro. Cell lysates containing V5-ExN were incubated with glutathione beads containing purified GST (as a control), GST-Crbintra, GST-CrbintraΔFBM or GST-CrbintraΔPBM. V5-ExN associated with the beads was probed with α-V5 antibody. Note that GST-Crbintra and GST-CrbintraΔPBM bound to Ex, but GST and GST-CrbintraΔFBM did not. (I) Purified Crb and Ex bind to each other in vitro. Similar to H except that bacterially purified Myc-ExN was incubated with glutathione beads containing the respective GST fusion proteins, and Ex associated with the beads (pull-down) was probed with α-Myc antibody. Note that GST-Crbintra and GST-CrbintraΔPBM bound to Ex, but GST and GST-CrbintraΔFBM did not.
To investigate physical interactions between Crb and Ex, we used subcellular fractionation to examine the effect of CrbMyc-Intra on the relative distribution of Ex between cytosolic and membrane fractions. When expressed alone in S2R+ cells, Ex was equally distributed in membrane and cytosolic fractions (Fig. 5E). Strikingly, coexpression with CrbMyc-Intra resulted in a near complete relocation of Ex to membrane fraction (Fig. 5E). This interaction is specific, because CrbMyc-Intra did not affect the relative distribution of FERM-domain proteins Mer and Moesin (Moe) (Fig. S1 A and B). Of note, a similar construct expressing the membrane-bound intracellular domain of Ft (FtΔECD) (9) failed to recruit Ex to membrane fraction in our assay (Fig. S2). To further explore the specificity of Crb-Ex interaction, we conducted similar assays using CrbMyc-Intra with mutations in its FBM or PBM. Consistent with a critical role for Crb's FBM in Ex binding, mutation of the FBM, but not the PBM, abrogated Ex membrane recruitment (Fig. 5E). In contrast, Sdt, which is known to bind Crb through its PBM (14, 15), was recruited to the membrane by CrbMyc-Intra in a PBM-dependent, but FBM-independent manner (Fig. 5F). Further supporting a role for Crb's FBM in Ex binding, we mapped the Crb-interacting region in Ex to Ex's N-terminal half, which contains the FERM domain (Fig. 5G).
To examine direct binding between Ex and Crb, we conducted GST pull-down assays between GST-Crb-intra (with and without mutation of FBM or PBM) and cell lysates expressing epitope-tagged Ex (Fig. 5H) or bacterially purified Ex proteins (Fig. 5I). In both assays, GST-CrbIntra interacted with Ex in a FBM-dependent, but PBM-independent, manner (Fig. 5 H and I). In contrast, GST-CrbIntra did not interact with Mer (Fig. S1 C and D). Taken together, we suggest that the intracellular domain of Crb may be separated into two functional motifs, with the FBM and the PBM mediating Ex- and Sdt-binding, respectively.
Crb Is Required for Apical Membrane Localization of Ex in Epithelial Cells.
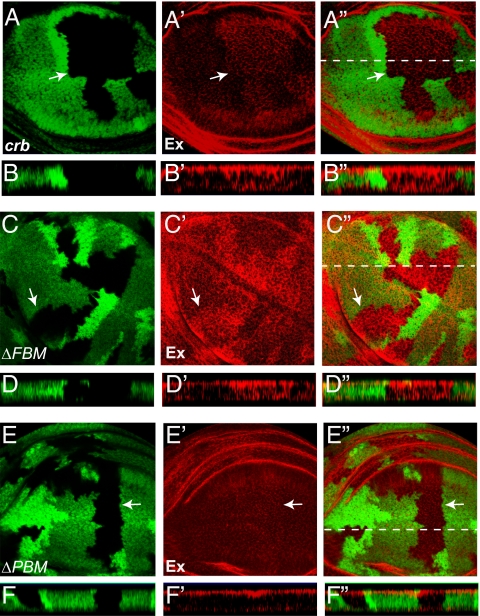
Given the apical localization of Crb (13) and Ex (23) and the Crb-Ex binding aforementioned, we examined whether Crb is required for the apical localization of Ex in imaginal discs. Indeed, in crb mutant clones, Ex staining was no longer restricted to apical surface; instead, Ex staining extended more basolaterally (Fig. 6 A–B”). Of note, loss of crb did not affect the apical localization of the related FERM protein Mer (Fig. S3). Because increased Ex levels per se do not perturb the apical restriction of Ex (4, 12), we conclude that the mislocalization of Ex in crb mutant clones reflects a specific requirement for Crb in localizing Ex to apical membranes of epithelial cells.
Fig. 6.
Loss of crb results in mislocalization of Ex in imaginal disk epithelial cells. In all panels, wing discs were stained with α-Ex antibody (red) and mutant clones (GFP-negative) are indicated with arrows. (A–A″) A horizontal section through a wing disk containing crb clones. The optical section was captured midway down from the apical surface of epithelium. Note increased Ex staining in crb clones. The dotted line in A″ indicates the position of vertical section in B–B″. (B–B″) A vertical section through the wing disk in A–A″ (apical is to the top). Ex localization extended more basolaterally in crb clones. (C–D″) Similar to A–B″ except that crbΔFBM clones were analyzed. Note the mislocalization of Ex to more basolateral position in crbΔFBM clones. (E–F″) Similar to A–B″ except that crbΔPBM clones were analyzed. Note the relatively normal apical localization of Ex in crbΔPBM clones.
Recently, we used genomic engineering to introduce mutation of the FBM or the PBM into the endogenous crb locus (24). These crb alleles (crbY10AP12AE16A and crbdelERLI) carry mutations identical to the FBM and the PBM mutations used in our biochemical analysis, and for simplicity, they will be referred to as crbΔFBM and crbΔPBM, respectively. These designer alleles allowed us to examine the respective contribution of Crb's FBM and PBM to Ex localization. Consistent with a role for Crb's FBM in concentrating Ex to apical membranes, Ex was mislocalized to basolateral domains in crbΔFBM clones in the wing (Fig. 6 C–D”). In contrast, crbΔPBM clones in the wing showed a relatively normal apical localization of Ex (Fig. 6 E–F”). In the eye disk, however, crbΔPBM clones did show a consistent mislocalization of Ex to basolateral domains, although this mislocalization was weaker than that observed in crbΔFBM clones (Fig. S4). The underlying reason for such tissue specificity is unclear and warrants further investigation. Taken together with our biochemical analysis, we conclude that Crb is required to concentrate Ex in the apical domain of epithelial cells, predominantly through direct binding between Crb's FBM and Ex's FERM domain.
FBM Is Required for Crb's Function in Hippo Signaling and Growth-Suppression.
Besides revealing a differential requirement for FBM and PBM in Ex localization, the crbΔFBM and crbΔPBM alleles allowed us to dissect the relative contribution of each motif in regulating Hippo signaling. For this purpose, we took advantage of the fact that although neither kibra nor crb single mutant clones exhibited visible up-regulation of ex transcription (Fig. 3 A–B”), kibra crb mutant clones showed robust elevation of ex transcription (Fig. 3 C–C”). Given the modest effect of crb mutation on Hippo target genes (Fig. 2), the kibra crb double mutant combination provided a more robust and reliable assay for crb function. Consistent with a critical role for the FBM in mediating Crb's input into the Hippo pathway, kibra crbΔFBM clones showed increased ex transcription similar to that observed in kibra crb clones (Fig. 3 D–D”). In contrast, kibra crbΔPBM clones did not show significant increase of ex transcription (Fig. 3 E–E”). These findings reinforce our biochemical and localization studies implicating Crb in Ex binding and subcellular localization.
Consistent with a role for Crb's FBM in Hippo signaling, we found that adult wings composed predominantly of crbΔFBM mutant cells were larger than normal (116% of normal; n = 20, t test: P = 0.01), whereas crbΔPBM had no significant effect on wing size (104% of normal; n = 20, t test: P = 0.19). These observations further support our conclusion that the growth-suppressive function of Crb is mediated predominantly through FBM-Ex interactions.
Discussion
Compared with the core kinase cascade leading from Hpo to Yki phosphorylation, signaling events upstream of Hpo are less well understood. Several apical membrane-associated cytoplasmic proteins function as tumor suppressors that act synergistically to regulate the Hippo kinase cascade, suggesting that the apical membrane represents a “subcellular niche” for Hippo pathway regulation. Understanding how these apical tumor suppressor proteins are targeted to the apical membranes may shed light on the molecular regulation of the Hippo signaling pathway.
Although the transmembrane protein Ft has been proposed as a potential receptor for the Hippo pathway by controlling the stability/localization of Ex (8–10), no direct physical interactions between Ft and Ex have been reported. Indeed, our own assays did not support a direct physical interaction between Ex and Ft, at least in S2R+ cells (Fig. S2). Using cell fractionation, in vitro binding and in vivo subcellular localization, we demonstrate direct binding between Crb and Ex. The physiological significance of this interaction is further supported by tissue overgrowth and elevated Hippo target genes in crbΔFBM clones. The specific biochemical and genetic link between Crb and Ex, but not between Crb and Mer or Crb and Kibra, is consistent with the view that these apical membrane-associated proteins may integrate distinct upstream signals. We also note that like kibra, ex, and mer, transcription of crb is increased in Hippo pathway mutant cells (25). Thus, negative-feedback regulation of upstream regulators appears to be a general feature of this pathway and may provide an important mechanism to maintain a constant level of Hippo signaling activity in vivo.
How does Crb regulate Ex and Hippo pathway activity? Crb may function as a scaffold that is merely required to recruit Ex to apical membrane, making it available for activation by another receptor such as Ft. Alternatively, Crb may function as a receptor that directly modulates Hippo signaling via its interaction with Ex. Yet another possibility is that Crb may function as a coreceptor for Ft. Although we cannot distinguish between the first two models at present, we can largely eliminate the third possibility as we found that the polarized distribution of Dachs, the most immediate known response to Ft signaling (26), is not abrogated by loss of Crb (Fig. S5).
Overexpression of full-length Crb or its intracellular domain was reported to drive tissue overgrowth characterized by loss of apical-basal polarity, elevated Yki activity and decreased Ex protein levels (27, 28). Although these observations are suggestive of an oncogenic role for Crb, our current study using loss-of-function analysis clearly uncovers a tumor suppressor function for Crb. This discrepancy may be due to distinct mutant-neighbor interactions encountered in the different studies. In the overexpression studies, Crb was expressed in whole compartments (27, 28), whereas our study examined isolated clones—the latter situation, but not the former, allows interactions between cells with differential Crb activity. It is also possible that Crb overexpression causes a dominant-negative or neomorphic phenotype. It is worth noting that Crb3, a mammalian homolog of Crb, has been implicated as a tumor suppressor in mammals (29). It will be interesting to examine whether Crb3 or other Crb homologs are required for Hippo signaling in mammals.
We note that Crb's FBM has been shown to mediate an inhibitory interaction wherein binding to the FERM-domain protein Yurt (a basolateral cytoskeleton protein) is required to restrict Crb to the apical domain (30). Accordingly, loss of Yurt results in expansion of the apical domain (30). The Crb-Ex interactions revealed in the current study is clearly distinct from the Crb-Yurt interaction as the former represents positive interactions. Besides the inhibitory Crb-Yurt interaction, Crb also positively regulates the Crb-Sdt-Patj complex via its PBM (14–16). How Crb coordinately interacts with the growth-regulatory Hippo pathway and the apical-basal polarity pathway remains to be determined. It will be especially interesting to determine whether and how these pathways crosstalk with each other, given the neoplastic tumor growth resulting from mutations of the Dlg-Lgl-Scrib polarity complex (31).
It is interesting to note that Hippo signaling can be influenced by Crb, an apical-basal polarity determinant, as well as Ft, a planar cell polarity (PCP) regulator. The fact that apical-basal polarity and PCP represent two orthogonal directions in an epithelium raises the intriguing possibility that signals representative of multiple dimensions may converge onto the Hippo pathway to define the final size of a 3D organ.
Materials and Methods
Drosophila Genetics.
EMS mutagenesis was conducted by crossing EMS-treated w−; FRT82B males with y w ey-flp, GMR-LacZ; FRT82B P[w+], L(3)c1-R3/TM6B females. Mutant flies with overgrown head were selected under microscope and maintained as stocks. For specific genotypes used for clonal analysis, see SI Materials and Methods.
Drosophila Cell Culture.
CrbMyc-Intra and CrbMyc-IntraΔPBM were constructed according to Wodarz et al. (19) and cloned into S2-expression vector pAc5.1/v5-HisB. CrbMyc-IntraΔFBM was generated by mutating residues Tyr10, Pro11, and Glu16 of the putative FERM-binding motif to Alanines. pAc-FtΔECD(V5) was kindly provided by Georg Halder (Baylor College of Medicine, Houston). Ex-HA, V5-Ex, V5-ExN(1-709), V5-ExC(710-1427), and HA-Mer have been described (5). N-tagged HA-Wts was made by PCR using pAc5.1/V5-HisB vector. FLAG-Sdt and FLAG-Moesin sequence were amplified from cDNA clones RE05272 and SD10366, respectively, by PCR and inserted into pAc5.1/V5-HisB vector. The myristylation signal from Drosophila c-Src (amino acids 1–10) was inserted into the N terminus of V5-Ex to generate the Myr-V5-Ex. All constructs were verified by DNA sequencing. Drosophila S2R+ cell culture, transfection, subcellular fractionation, and Western blotting were carried out as described (5).
GST Pull-Down Assay.
The intracellular domain of Crb and two mutants (CrbIntraΔPBM and CrbIntraΔFBM) were subcloned into pGEX-6p-1 and the GST fusion proteins were expressed in BL21-CodonPlus(DE3)-RIPL (Stratagene). Myc-ExN and Myc-Mer sequences were inserted into pGEX-6p-1 and used to express GST-Myc-ExN and GST-Myc-Mer fusion proteins. After purification by Glutathione Sepharose 4B, Myc-ExN and Myc-Mer were released from GST beads by Prescission protease cleavage. GST pull-down assay was carried out as described (32).
Supplementary Material
Acknowledgments
We thank R. Fehon (University of Chicago), G. Halder (Baylor College of Medicine), K. Irvine (Rutgers University), and J. Jiang (University of Texas Southwestern Medical Center) for providing antibodies, constructs, and fly strains. This work was supported by grants from the National Institutes of Health to D.J.P. (EY015708) and the National Key Scientific Program of China to S.W. (2010CB912204). D.J.P. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004279107/-/DCSupplemental.
References
- 1.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 2.Badouel C, Garg A, McNeill H. Herding Hippos: Regulating growth in flies and man. Curr Opin Cell Biol. 2009;21:837–843. doi: 10.1016/j.ceb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Irvine KD. Fat and expanded act in parallel to regulate growth through warts. Proc Natl Acad Sci USA. 2007;104:20362–20367. doi: 10.1073/pnas.0706722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- 16.Bhat MA, et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 17.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 18.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- 19.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 20.Pellikka M, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 21.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 22.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 23.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zhou W, Dong W, Watson AM, Hong Y. From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci USA. 2009;106:8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genevet A, et al. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 28.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp CM, et al. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–4115. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laprise P, et al. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilder D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.