Abstract
In persistent hepadnavirus infections, a distinctive feature of woodchuck hepatitis virus (WHV) is the coupling of frequent viral integrations into myc family genes with the rapid onset of primary liver tumors. We have investigated the patterns of WHV DNA insertion into N-myc2, a newly identified retroposed oncogene, in woodchuck hepatomas resulting from either natural or experimental infections. In both cases, integrated viral sequences were preferentially associated with the N-myc2 locus. In more than 40% of the woodchuck tumors analyzed, viral insertion sites were clustered in a 3-kb region upstream of N-myc2 or in the 3' noncoding region. Insertion of WHV sequences homologous to the human hepatitis B virus enhancers, either upstream or downstream of the N-myc2 coding domain, was associated with the production of normal N-myc2 mRNA or hybrid N-myc2-WHV transcripts, initiated at the normal N-myc2 transcriptional start site. Transient-transfection assays with different N-myc2-WHV constructs in HepG2 cells demonstrated that the viral enhancers could efficiently activate the N-myc2 promoter. These results, showing that cis activation of preferred cellular targets through enhancer insertion is a common strategy for tumor induction by WHV, emphasize the previously noted similarities between hepadnaviruses and nonacute oncogenic retroviruses.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Friedman J. M. Regulation of N-myc gene expression: use of an adenovirus vector to demonstrate posttranscriptional control. Mol Cell Biol. 1990 Dec;10(12):6700–6708. doi: 10.1128/mcb.10.12.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Murphey-Corb M. Targeted integration of baboon endogenous virus in the BEVI locus on human chromosome 6. Nature. 1983 Jan 13;301(5896):129–132. doi: 10.1038/301129a0. [DOI] [PubMed] [Google Scholar]
- Couturier J., Sastre-Garau X., Schneider-Maunoury S., Labib A., Orth G. Integration of papillomavirus DNA near myc genes in genital carcinomas and its consequences for proto-oncogene expression. J Virol. 1991 Aug;65(8):4534–4538. doi: 10.1128/jvi.65.8.4534-4538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- DePinho R. A., Schreiber-Agus N., Alt F. W. myc family oncogenes in the development of normal and neoplastic cells. Adv Cancer Res. 1991;57:1–46. doi: 10.1016/s0065-230x(08)60994-x. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dolcetti R., Rizzo S., Viel A., Maestro R., De Re V., Feriotto G., Boiocchi M. N-myc activation by proviral insertion in MCF 247-induced murine T-cell lymphomas. Oncogene. 1989 Aug;4(8):1009–1014. [PubMed] [Google Scholar]
- Dény P., Zignego A. L., Rascalou N., Ponzetto A., Tiollais P., Bréchot C. Nucleotide sequence analysis of three different hepatitis delta viruses isolated from a woodchuck and humans. J Gen Virol. 1991 Mar;72(Pt 3):735–739. doi: 10.1099/0022-1317-72-3-735. [DOI] [PubMed] [Google Scholar]
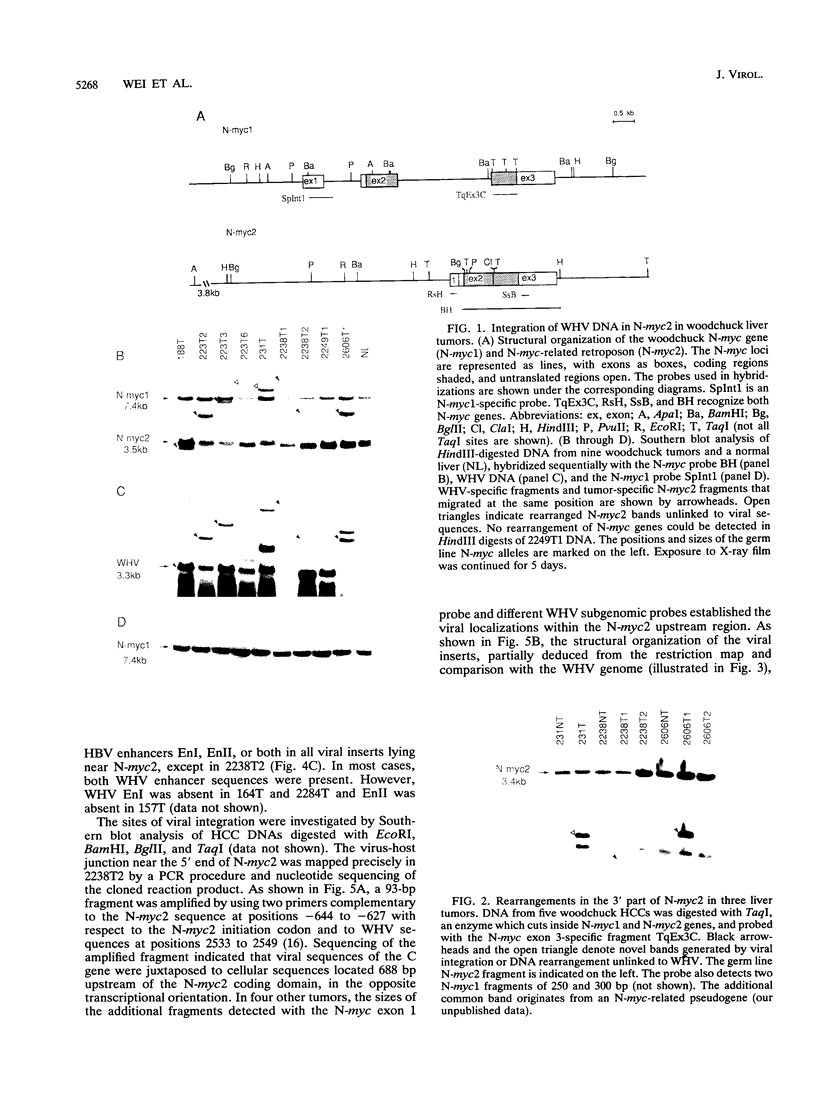
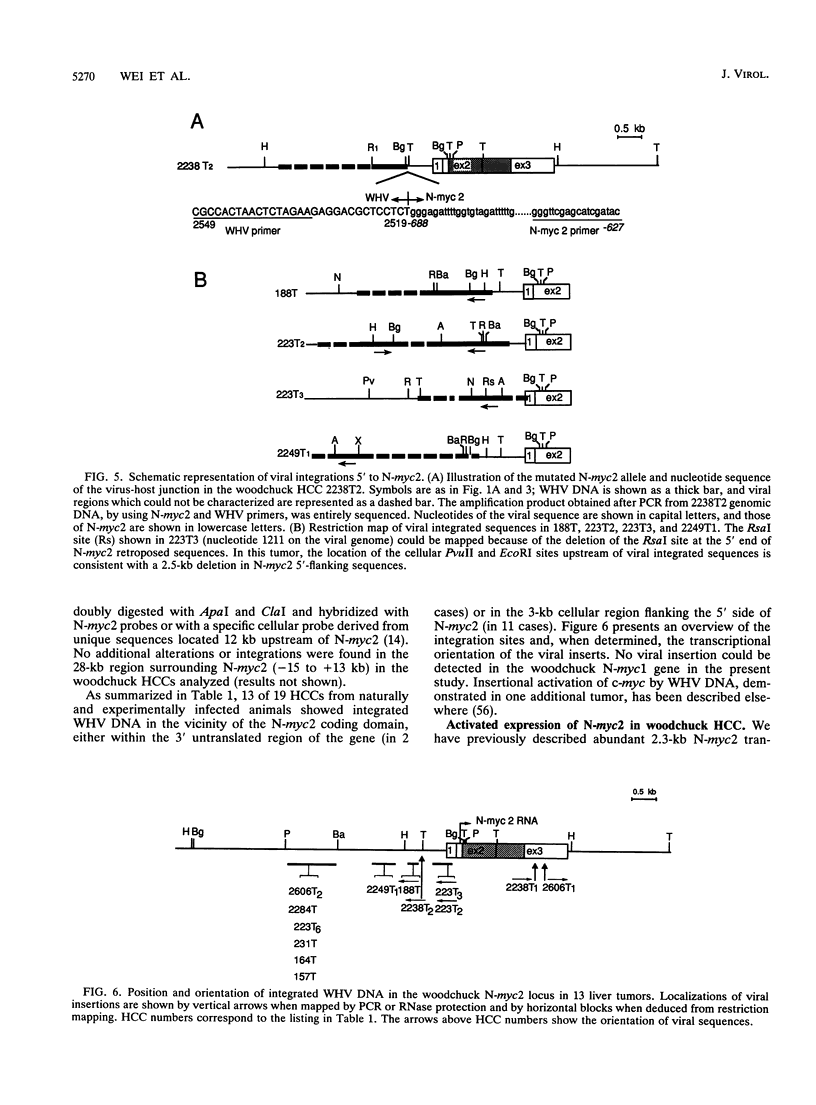
- Fourel G., Trepo C., Bougueleret L., Henglein B., Ponzetto A., Tiollais P., Buendia M. A. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990 Sep 20;347(6290):294–298. doi: 10.1038/347294a0. [DOI] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hsu T., Möröy T., Etiemble J., Louise A., Trépo C., Tiollais P., Buendia M. A. Activation of c-myc by woodchuck hepatitis virus insertion in hepatocellular carcinoma. Cell. 1988 Nov 18;55(4):627–635. doi: 10.1016/0092-8674(88)90221-8. [DOI] [PubMed] [Google Scholar]
- Joliot V., Martinerie C., Dambrine G., Plassiart G., Brisac M., Crochet J., Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992 Jan;12(1):10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Cole M. D. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3' untranslated sequences. Mol Cell Biol. 1987 Dec;7(12):4513–4521. doi: 10.1128/mcb.7.12.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski W., Porter J. C. A novel non-enzymatic procedure for removing DNA template from RNA transcription mixtures. Biotechniques. 1991 Feb;10(2):210–214. [PubMed] [Google Scholar]
- Kekulé A. S., Lauer U., Meyer M., Caselmann W. H., Hofschneider P. H., Koshy R. The preS2/S region of integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature. 1990 Feb 1;343(6257):457–461. doi: 10.1038/343457a0. [DOI] [PubMed] [Google Scholar]
- Kelly J. H., Darlington G. J. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989 Feb;25(2):217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Cote P. J., Wells F. V., Baldwin B., Popper H., Purcell R. H., Tennant B. C., Gerin J. L. Natural history of woodchuck hepatitis virus infections during the course of experimental viral infection: molecular virologic features of the liver and lymphoid tissues. J Virol. 1989 Mar;63(3):1360–1370. doi: 10.1128/jvi.63.3.1360-1370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F. V., Baldwin B., Cote P. J., Tennant B. C., Popper H., Gerin J. L. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989 Mar;9(3):461–470. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990 Jun;7(3):243–260. [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Negro F., Korba B. E., Forzani B., Baroudy B. M., Brown T. L., Gerin J. L., Ponzetto A. Hepatitis delta virus (HDV) and woodchuck hepatitis virus (WHV) nucleic acids in tissues of HDV-infected chronic WHV carrier woodchucks. J Virol. 1989 Apr;63(4):1612–1618. doi: 10.1128/jvi.63.4.1612-1618.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Popper H., Hoyer B. H., London W. T., Ford E. C., Bonino F., Purcell R. H., Gerin J. L. Transmission of the hepatitis B virus-associated delta agent to the eastern woodchuck. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2208–2212. doi: 10.1073/pnas.81.7.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto A., Negro F., Gerin J. L., Purcell R. H. Experimental hepatitis delta virus infection in the animal model. Prog Clin Biol Res. 1991;364:147–157. [PubMed] [Google Scholar]
- Popper H., Roth L., Purcell R. H., Tennant B. C., Gerin J. L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987 Feb;84(3):866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherdin U., Rhodes K., Breindl M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J Virol. 1990 Feb;64(2):907–912. doi: 10.1128/jvi.64.2.907-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Baldwin B., Hornbuckle W. E., Yeager A. E., Tennant B. C., Cote P., Ferrell L., Ganem D., Varmus H. E. Woodchuck hepatitis virus is a more efficient oncogenic agent than ground squirrel hepatitis virus in a common host. J Virol. 1991 Apr;65(4):1673–1679. doi: 10.1128/jvi.65.4.1673-1679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi M., Higuchi Y., Yoshida S., Nasu N., Miyazaki Y., Akizuki S., Yamamoto S. Insertional activation of N-myc by endogenous Moloney-like murine retrovirus sequences in macrophage cell lines derived from myeloma cell line-macrophage hybrids. Mol Cell Biol. 1989 Oct;9(10):4515–4522. doi: 10.1128/mcb.9.10.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Su H., Yee J. K. Regulation of hepatitis B virus gene expression by its two enhancers. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2708–2712. doi: 10.1073/pnas.89.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tokino T., Matsubara K. Chromosomal sites for hepatitis B virus integration in human hepatocellular carcinoma. J Virol. 1991 Dec;65(12):6761–6764. doi: 10.1128/jvi.65.12.6761-6764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Robinson H. L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J Virol. 1986 Nov;60(2):683–692. doi: 10.1128/jvi.60.2.683-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Wei Y., Ponzetto A., Tiollais P., Buendia M. A. Multiple rearrangements and activated expression of c-myc induced by woodchuck hepatitis virus integration in a primary liver tumour. Res Virol. 1992 Mar-Apr;143(2):89–96. doi: 10.1016/s0923-2516(06)80086-5. [DOI] [PubMed] [Google Scholar]
- Wollersheim M., Debelka U., Hofschneider P. H. A transactivating function encoded in the hepatitis B virus X gene is conserved in the integrated state. Oncogene. 1988 Nov;3(5):545–552. [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Breuer M., Berns A. N-myc is frequently activated by proviral insertion in MuLV-induced T cell lymphomas. EMBO J. 1989 Jan;8(1):133–136. doi: 10.1002/j.1460-2075.1989.tb03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]