Abstract
Perception and response to nutritional iron availability by bacteria are essential to control cellular iron homeostasis. The Irr protein from Bradyrhizobium japonicum senses iron through the status of heme biosynthesis to globally regulate iron-dependent gene expression. Heme binds directly to Irr to trigger its degradation. Here, we show that severe manganese limitation created by growth of a Mn2+ transport mutant in manganese-limited media resulted in a cellular iron deficiency. In wild-type cells, Irr levels were attenuated under manganese limitation, resulting in reduced promoter occupancy of target genes and altered iron-dependent gene expression. Irr levels were high regardless of manganese availability in a heme-deficient mutant, indicating that manganese normally affects heme-dependent degradation of Irr. Manganese altered the secondary structure of Irr in vitro and inhibited binding of heme to the protein. We propose that manganese limitation destabilizes Irr under low-iron conditions by lowering the threshold of heme that can trigger Irr degradation. The findings implicate a mechanism for the control of iron homeostasis by manganese in a bacterium.
Keywords: Bradyrhizobium, heme, regulated degradation, oxidative stress
Iron is required for many cellular processes, but can be toxic at high concentrations. Thus, iron homeostasis is strictly regulated so that iron acquisition, storage, and consumption are geared to iron availability, and that intracellular levels of free iron do not reach toxic levels (reviewed in ref. 1). Recently, the roles of manganese and its control in cells have been investigated, and it is becoming clear that some aspects of the metabolism of iron and manganese are interrelated.
Numerous members of the Nramp family of eukaryotic and prokaryotic proteins transport both Mn2+ and Fe2+ into cells (2–5). Growth of an Escherichia coli iron-transport mutant is inhibited in an mntH background, suggesting that manganese may substitute for iron in cells (6). The mntH and sitABCD genes are transcriptionally regulated by iron as well as by manganese in the model organisms E. coli, Salmonella enterica, and Bacillus subtilis (7–9). However, a regulatory role for manganese in controlling cellular processes other than its own transport is less well understood, and is addressed in the present study.
Manganese protects cells against oxidative stress, and some aspects of this property are also related to iron. Ferrous iron can react with H2O2 to form the very reactive hydroxyl radical. It has been proposed that manganese may substitute for iron in mononuclear enzymes to prevent iron-catalyzed oxidative damage at those sites (6). The peroxide sensor PerR from B. subtilis senses H2O2 through a bound Fe2+ moiety that catalyzes the oxidation of a histidine residue to inactivate the protein and derepress the expression of genes needed to manage oxidative stress (10). PerR is much less sensitive to H2O2 under low-iron conditions, when Mn2+ occupies the regulatory metal site (11).
Divalent iron and manganese share similar sizes and coordination geometries, but the total cellular iron content is in excess of manganese in E. coli cells (6, 12). A regulatory role for manganese must accommodate this discrepancy, otherwise it would be out-competed by iron. The simplest explanation is that the availability of each metal to control cellular processes is not proportional to the total content. However, specific mechanisms have not been explored.
Bradyrhizobium japonicum resides as a free-living organism or as an endosymbiont of soybean. B. japonicum is a model for iron metalloregulation in the α-Proteobacteria, an extremely diverse taxonomic group that includes pathogens, symbionts, photosynthetic organisms, bacteria that degrade environmental pollutants, and the abundant marine organism Pelagobacter ubique (13). The regulation of iron metabolism in B. japonicum, and the α-Proteobacteria as a whole, differs substantially from that described in other model systems (13).
The Irr protein is a global iron regulator in B. japonicum (14, 15). Most genes that are strongly regulated by iron are directly or indirectly controlled by Irr (14, 15). Irr functions under iron limitation, and binds to the promoters of target genes to positively or negatively regulate gene expression (14–17). Positively controlled genes include those required for high-affinity iron transport, whereas many negatively regulated genes encode iron-containing proteins. Irr is a conditionally stable protein that degrades rapidly upon cellular exposure to iron (18, 19). This iron-dependent turnover is mediated by heme, which binds directly to the protein to trigger its degradation (19–21). As a result, Irr is stable and independent of iron in heme biosynthesis-mutant cells. Irr interacts with ferrochelatase, the enzyme that catalyzes the final step of the heme biosynthetic pathway, and responds to heme locally at the site of its synthesis (22). Thus, heme is a signaling molecule that reflects the cellular iron status. By contrast, E. coli and many other bacteria respond directly to iron through the Fur protein, which complexes with Fe2+ and binds to the promoters of genes under its control (23).
In the present study, we show that manganese controls iron homeostasis in B. japonicum. The data support the conclusion that manganese interferes with the role of heme as a degradation signal under iron limitation to affect iron-dependent gene expression.
Results
Manganese Deficiency Results in a Cellular Iron Deficiency.
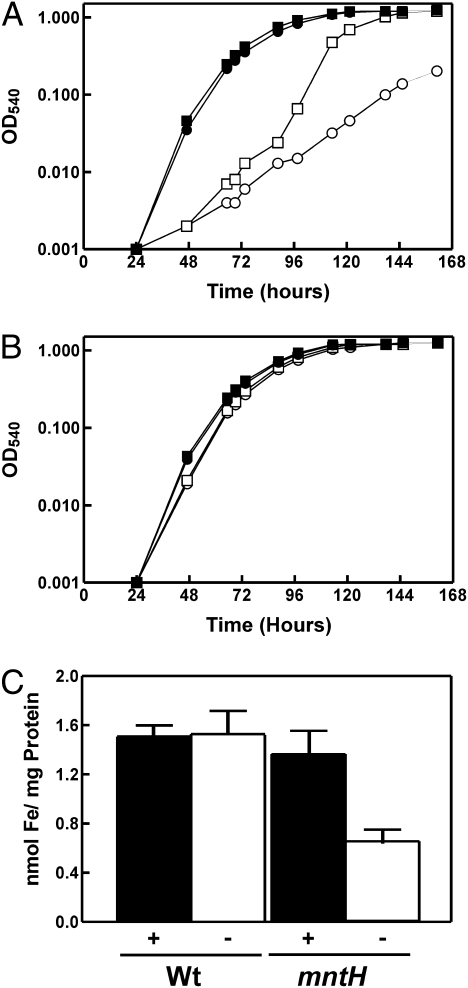
MntH is the major Mn2+ transporter in B. japonicum (24). The mntH gene is induced under manganese limitation, and an mntH mutant has a growth phenotype and contains less intracellular manganese than the parent strain (24). B. japonicum MntH does not transport iron, nor is the mntH gene regulated by it (24). Here, we found that the mntH mutant grows well in a low-manganese growth medium (0.4 μM Mn) that is supplemented with 20 μM FeCl3 (Fig. 1A). Under these conditions, there was a substantial lag in the initial growth, but the subsequent rate of growth and final optical density was similar to the parent strain. However, cells of the mntH strain grew much less well under manganese limitation if iron was also limiting (0.3 μM Fe) (Fig. 1A). In the presence of manganese, the mntH mutant grew similar to the wild type independently of the iron status (Fig. 1B). These data show that iron substantially enhances growth of an mntH strain under manganese limitation.
Fig. 1.
Effect of iron and manganese on the growth and cellular iron content of B. japonicum parent and mntH strains. Growth media were inoculated with 5 × 105 cells mL−1 of parent strain (closed symbols) or the mntH mutant (open symbols) and grown in media using pyruvate as the carbon source containing 20 μM FeCl3 (squares) or no exogenous iron (circles) and (A) no exogenous manganese or (B) 50 μM MnCl2. Unsupplemented media contained 0.4 μM manganese and 0.3 μM iron. (C) Cellular iron content of the parent strain and mntH mutant grown in low-iron and low- or high-manganese media. Cells were grown in iron-limited media containing either no added manganese (−) or 50 μM MnCl2 (+). Iron content of whole cells was determined by atomic absorption spectroscopy. The data are presented as the average of three replicates ± the standard deviation.
Manganese limitation may somehow result in iron deficiency, thus requiring supplementation of iron to the growth medium. To address this possibility, we measured the intracellular iron content of the parent and mntH strains grown in low-iron media with no added Mn (0.4 μM final concentration) or supplemented with 20 μM MnCl2 (Fig. 1C). Because the mntH mutant only grew to an OD (540 nm) of about 0.1, all cultures were grown to that density. The parent strain maintained a nearly constant intracellular iron level in both high- and low-manganese media, and was similar to that observed in the mntH mutant grown in high-manganese media. However, the iron level of the mntH strain grown under manganese limitation was decreased by about 50% compared with the controls. We showed previously that MntH is not an Fe2+ transporter in B. japonicum (24), and thus the iron deficiency cannot be explained by a defect in MntH-catalyzed iron transport. These findings suggest that manganese affects the cellular iron status.
Manganese Affects the Expression of Iron-Regulated Genes.
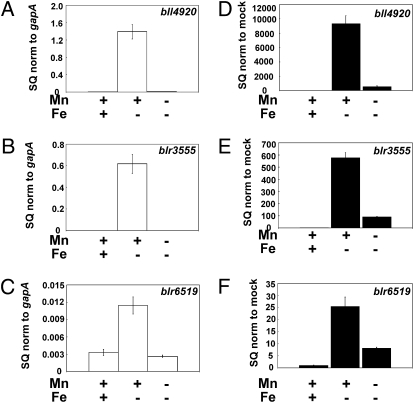
The extremely poor growth of the mntH strain in low-iron and -manganese media limited additional analysis of the mutant. To further address the effects of manganese on iron metabolism, we examined the expression of several genes known to be regulated by iron at the mRNA level in B. japonicum wild-type cells. blr3555 and bll4920 encode iron siderophore transport outer-membrane receptors, and blr6519 encodes an iron-independent fumarase, which replaces the iron-sulfur fumarase when the metal is limiting (15, 17, 25). Cells were grown under various iron or manganese conditions, and mRNA encoding each gene was examined by quantitative real-time PCR (qPCR). In media supplemented with manganese, all three genes were strongly regulated by iron, with high expression under iron limitation (Fig. 2 A–C). In previous studies, we routinely cultured cells in media supplemented with trace elements that include manganese (15, 17), and thus our observations agree with previous findings. However, those genes were not induced under iron limitation in cells grown in low-manganese media (Fig. 2 A–C). Thus, manganese contributes to the expression of these iron-regulated genes, and further suggests that manganese affects iron-dependent metabolism.
Fig. 2.
Effect of manganese on the expression of Irr-regulated genes and promoter occupancy by Irr. (A–C). mRNA transcripts of bll4920, blr3555, and blr6519 obtained from cells grown under different metal conditions were analyzed by qPCR. The data are expressed as the relative starting quantity (SQ) of the respective mRNAs normalized to the housekeeping gene gapA, and presented as the average of three replicates ± the standard deviation. (D–F) Cross-linking of parent strain cells grown under different conditions of iron and manganese, followed by co-IP using anti-Irr antiserum was carried out as described in Materials and Methods. The mock experiment was carried out without antibody. Immunoprecipitated DNA was analyzed by qPCR using primers delimiting the promoter regions of the respective genes. The data are expressed as the relative SQ of the respective pull-down DNA normalized to the mock pull-down samples and presented as the average of three replicates ± the standard deviation. The normalized SQ values obtained were considered to be directly proportional to the Irr promoter occupancy of the respective genes.
Cellular Irr Protein Level Is Attenuated by Manganese Limitation.
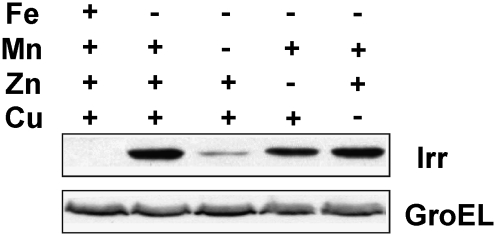
Irr is a global regulator of iron metabolism in B. japonicum that functions under iron limitation. Irr positively controls iron transport, as well as the genes shown in Fig. 2 in iron-limited cells (15, 17), and thus we wanted to address whether Irr expression or activity was affected under manganese limitation. To do this, cells were grown in media containing either all of the trace metals normally added or in media where iron alone or in combination with another metal was omitted. Irr levels were examined by Western blot analysis (Fig. 3). In the presence of iron, Irr was nearly undetectable, but accumulated to a high level when iron was omitted, in good agreement with previous studies (18, 19). However, when manganese was removed from the iron-limited media, Irr levels were much lower. This diminution is consistent with low induction of the blr3555, bll4920, and blr6519 genes (Fig. 2). Removal of Zn had only a slight effect on the Irr level, and removal Cu had no effect. These data show that manganese is needed for high expression of Irr level under iron limitation.
Fig. 3.
Effect of metals on Irr levels in B. japonicum. Cells were grown under different metal conditions and steady-state levels of Irr were detected by immunoblotting using anti-Irr antibodies. GroEL was used as a control for an unregulated protein, and was detected using anti-GroEL antibodies. Thirty micrograms of protein was loaded per lane.
If the level of diminution of Irr in response to manganese limitation is sufficient to alter gene expression, we would expect to observe a manganese-dependent change in the occupancy of target-gene promoters by Irr. Promoter occupancy of the blr3555, bll4920, and blr6519 genes was assessed in vivo by cross-linking/immunoprecipitation analysis, as described previously (16). mntH was used as a negative control for a gene regulated by manganese, but not by iron (24) (Fig. S1). Cells were grown under various iron or manganese conditions, followed by cross-linking of protein to DNA. DNA that coprecipitated with anti-Irr antibodies in cell extracts was analyzed by qPCR using primers that amplify the promoter of interest (Fig. 2 D–F). High promoter occupancy was observed for the target genes under low-iron, high-manganese conditions (Fig. 2 D–F), consistent with high mRNA levels (Fig. 2 A–C). However, occupancy by Irr was low in iron-limited cells when manganese was also limiting. Thus, the level of Irr was sufficiently diminished to decrease promoter occupancy of the genes under study.
Manganese Responsiveness of Irr Is Heme-Dependent.
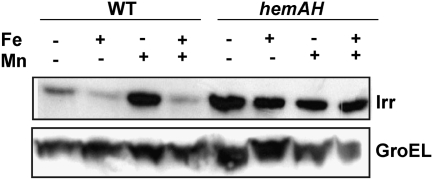
We sought to examine how manganese affects Irr levels in cells. The irr gene is modestly controlled by iron at the transcriptional level, but is strongly regulated by iron at the level of protein stability (19, 22). Moreover, iron-dependent Irr degradation requires binding of the protein to heme, thus this control is lost in heme-deficient mutants (19, 20, 22). Here, we found that manganese did not affect irr mRNA levels in iron-deficient media (Fig. S2B), showing that manganese exerts its effect at a posttranscriptional step. To elucidate whether heme is necessary for manganese responsiveness, Irr levels were analyzed in a heme-deficient strain or the wild type grown under various iron and manganese conditions (Fig. 4). In the parent strain, Irr accumulated to a high level only under low-iron, high-manganese conditions, but was greatly diminished under manganese limitation. However, in the heme-deficient strain, Irr levels remain high independent of the manganese status. Thus, heme is required for responsiveness to manganese as well as to iron, and this suggests that manganese limitation promotes heme-dependent Irr degradation in wild-type cells.
Fig. 4.
Effect of different iron and manganese conditions on Irr levels in cells of the wild-type or a heme-deficient strain. Cells were grown in media supplemented (+) or unsupplemented (−) with FeCl3 (Fe) or MnCl2 (Mn). Media were also supplemented with 60 nM heme, which is necessary for growth of the mutant strain. Steady-state levels of Irr and GroEL were determined as by immunoblotting as described in Fig. 3.
Manganese Inhibits the Binding of Heme to Irr in Vitro.
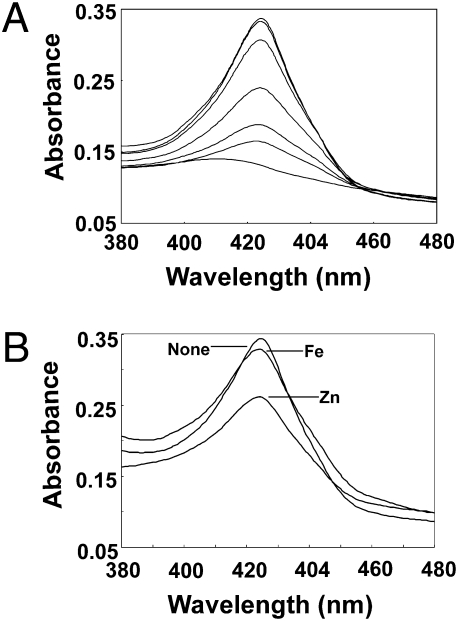
Degradation of Irr in vivo requires direct binding of heme to the protein (19, 20, 22), therefore we determined whether manganese affects heme binding in vitro (Fig. 5). Ferrous heme gives an absorption peak at 423 nm when bound to Irr, but absorbs little in that region when unbound to the protein (Fig. 5A). Titration of MnCl2 into the binding reaction decreased the absorption peak at 423 nm, demonstrating that manganese inhibits the binding of Irr to heme. The intracellular manganese concentration is about 80 μM (24), and 20 μM MnCl2 was sufficient to abrogate heme binding in vitro. When the binding experiment was repeated with ZnCl2 or FeSO4 in place of MnCl2, the absorption of bound heme was diminished to a much lesser extent by those metals (Fig. 5B), and thus the effect of manganese is specific. Heme is required for Irr degradation, and thus, the inhibition of heme binding to Irr by manganese in vitro is consistent with stabilization of the protein by the metal in vivo.
Fig. 5.
Effect of manganese on the absorption spectra of heme bound to purified recombinant Irr. (A) Shows 8 μM Irr and 4 μM heme, in the presence of 100 μM dithionite as a reductant, and the absorption spectrum taken between 380 and 480 nm. The sample was then titrated with 2, 4, 6, 8, 10, 20 μM MnCl2, and the spectrum was taken after each addition. The maximum peak was observed with no metal, and the minimum absorption at 20 μM MnCl2. (B). The experiment was carried out as described in A, using either no metal (none), 20 μM ZnCl2 (Zn), or 20 μM FeSO4 (Fe).
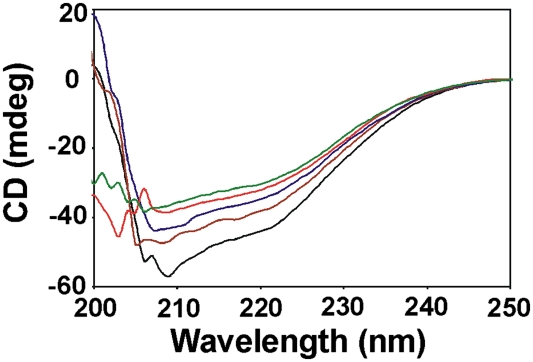
The Irr protein contains α-helical structure in the absence of added metal as determined by circular dichroism (CD) spectrometry (Fig. 6). Addition of MnCl2 altered this secondary structure, as seen by the loss of spectral features at 207 and 222 nm. Thus, manganese binds Irr and alters its overall structure, and correlates with a decreased ability to bind heme.
Fig. 6.
Effect of manganese on the secondary structure of purified recombinant Irr. Far UV CD spectra of 8 μM Irr were recorded in the absence (black) and presence of increasing concentrations of manganese (brown, blue, red, and green, representing 8, 16, 40, and 80 μM MnCl2, respectively).
Effect of Manganese on Irr Degradation by Exogenously Added Heme.
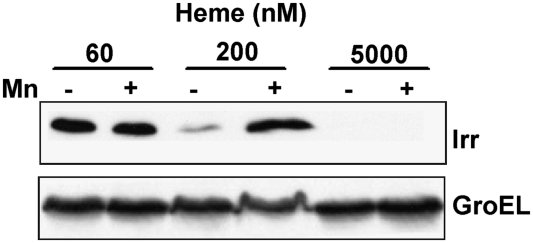
The heme biosynthesis mutant ΔhemAH is a heme auxotroph, and 60 nM of exogenously added heme is sufficient to support growth, but is not sufficient to allow Irr degradation (19) (Figs. 4 and 7). However, cells of the mutant strain grown in 200 nM heme acquire manganese responsiveness, with low levels observed in manganese-deficient cells (Fig. 7). Thus, manganese inhibits heme-dependent Irr degradation. Cells grown in 5,000 nM heme did not accumulate Irr in the absence or presence of manganese (Fig.7), showing that manganese cannot inhibit degradation at a higher heme concentration. These findings suggest that the effectiveness of manganese on preventing Irr degradation depends on the heme concentration. Because the heme concentration available to Irr is, in turn, dependent on the iron concentration, these data are consistent with iron-dependent degradation of Irr in manganese-replete cells.
Fig. 7.
Effect of manganese on Irr levels in the presence of exogenously added heme. Cells of the heme-deficient mutant strain ΔhemAH were grown under different concentrations of heme, in the presence (+) or absence (–) of manganese. Irr and GroEL proteins were measured in cells by immunoblotting as described in Fig. 3.
Discussion
In the present study, we found that manganese controls iron homeostasis in B. japonicum, and provide evidence for the molecular basis of metabolic integration of these metals. Collectively, the data support the view that manganese binds to Irr, the global iron regulator in B. japonicum, and stabilizes the protein under iron limitation by inhibiting binding of heme to it (Fig. S3). Under manganese limitation, heme binds to Irr and promotes its degradation, leading to changes in the expression of Irr-regulated genes. Thus, under iron limitation, where Irr accumulates because of lower heme availability, manganese limitation lowers the threshold of heme that can trigger Irr degradation.
The effectiveness of manganese in controlling the cellular Irr level depends on the amount of heme available to Irr, which likely allows iron-dependent degradation of Irr even under manganese-replete conditions. Under a very low-heme condition, such as that of a heme synthesis mutant supplemented with the minimum amount of exogenous heme to support growth, Irr is stable and independent of the manganese status (Figs. 4 and 7). This result is expected because there is insufficient heme to degrade Irr, and thus manganese is not necessary to stabilize the protein. At a high-heme concentration, such as that of a heme-synthesis mutant supplemented with 5 μM hemin, Irr does not accumulate even under high-manganese conditions (Fig. 7), presumably because the heme content is sufficiently high to out-compete manganese. In wild-type cells, the amount of heme available to Irr is directly related to the iron substrate for heme biosynthesis (22). Thus, when iron is replete, there is likely to be sufficiently high heme to mitigate the stabilizing effect of manganese. This finding is consistent with the observed very low Irr level in iron replete cells of the wild type, regardless of the manganese status (Fig. 4). Under low-iron conditions, heme synthesis is attenuated and insufficient to degrade Irr in manganese-replete cells, but is sufficient to promote Irr degradation when the manganese level is low. Thus, manganese exerts an effect only under iron-limited conditions.
The Mn2+ transporter MntH is required for growth of iron-deficient cells in E. coli, which has been interpreted to mean that manganese can substitute for iron in activating mononuclear enzymes (6). In that case, iron deficiency creates a need for manganese import to compensate for the lack of iron. The current work also shows a requirement for mntH in low iron of B. japonicum if manganese is also deficient (Fig. 1), but the reasons for this appears to be different from that proposed in E. coli. In B. japonicum, manganese is the regulatory metal that caused a change in iron metabolism that resulted in cellular iron deficiency. These observations do not suggest substitution of one metal for the other, but rather a mechanism for decreasing the iron content when manganese is limiting.
Manganese has been shown to protect cells against oxidative stress, whereas iron can promote it by generation of reactive oxygen species (26). The basis of manganese protection is not completely understood, but the substitution of manganese for iron likely mitigates iron-dependent hydroxyl radical formation (6). It is plausible that B. japonicum responds to manganese limitation by attenuating the cellular iron content to avoid oxidative stress. The fact that a B. japonicum mntH mutant has growth phenotypes in the absence of applied stress, whereas an E. coli mntH strain does not (6, 24), suggests that B. japonicum is more dependent on manganese. Perhaps B. japonicum is exposed to more oxidative stress than E. coli, either because of its environment or because it generates more endogenous reactive oxygen species. In that case, B. japonicum would be less tolerant of iron when manganese is limiting. Irr positively controls iron transport genes (14, 15, 17), and an irr mutant is defective in iron uptake (18). Thus, diminution of Irr activity under manganese limitation will decrease iron acquisition.
The notion that B. japonicum may be more adapted to manganese is also suggested by the fact that the transcriptional regulator Fur responds to both Mn2+ and Fe2+ in vitro (27), but is specific for manganese in vivo (24). Thus, the concentration of manganese accessible to play a regulatory function may be greater in this organism. Divalent iron and manganese share similar sizes and coordination geometries, thus a regulatory protein that can respond to both metals must be able to discriminate between them. Irr, a Fur family protein, responds to iron through the status of heme biosynthesis rather than direct binding of the metal (22). This sensing mechanism may circumvent the problem of manganese competing with a regulatory iron-binding site because the dissimilar structures of heme and manganese can be readily discriminated.
In a previous study, we found that a B. japonicum mntH mutant does not grow in low-manganese media, even with an iron supplement when glycerol was used as a carbon source (24). The present work showed growth of the mntH strain in manganese-deficient media supplemented with iron containing pyruvate instead of glycerol as the carbon source. Although we have not explored an explanation for this, the addition of pyruvate bypasses the need for pyruvate kinase in feeding carbon into the TCA cycle, and pyruvate kinase has been shown to be a manganese-dependent enzyme in several organisms (28). Alternatively, pyruvate can scavenge hydrogen peroxide (29), which may make manganese import dispensible. Further studies should resolve this issue.
Materials and Methods
Strains and Media.
B. japonicum USDA110 was the parent strain used in the present study.
Strain ΔmntHΩΔ is a mutant derivative of the parent strain containing a DNA cassette encoding spectinomycin and streptomycin replacing the mntH gene (24). Strain ΔhemAH is a double mutant defective in the hemA and hemH genes encoding heme biosynthesis enzymes (19). B. japonicum strains were routinely grown at 29 °C in GSY medium (30). Strain ΔmntH was grown in the presence of 25 μg/mL streptomycin and100 μg/mL of spectinomycin and strain ΔhemAH was grown with 100 μg/mL spectinomycin and 50 μg/mL kanamycin. Furthermore, the hemAH double mutant was maintained in medium supplemented with 15-μM hemin to fulfill its heme auxotrophy. For experiments with low-heme conditions, the medium was supplemented with 0.06 μM heme, which allows growth comparable to the wild type (19).
For low-iron or -manganese conditions, modified GSY medium was used, which contains 0.5 g/L yeast extract instead of 1 g/L with no exogenous iron source. The actual iron and manganese concentrations in this medium were 0.3 and 0.4 μM, respectively, as determined with a PerkinElmer Model 1100B atomic absorption spectrometer. In the present study, iron limitation was determined by the accumulation of Irr. Manganese limitation was determined by the induction of the mntH gene. High-iron and -manganese media were modified GSY supplemented with 20 μM FeCl3 or 50 μM MnCl2, respectively. Low Zn and Co conditions were achieved by omitting the respective metals from the modified GSY medium.
Bacterial Growth Studies.
The parent and mntH strains were grown in modified GSY medium with various combinations of iron and manganese concentrations, and with pyruvate instead of glycerol as the added carbon source. Growth rates were analyzed by measuring the optical density of cells at 540 nm until reaching stationary phase.
Determination of Intracellular Iron Content.
One-hundred-fifty milliliters of culture were grown under low-iron conditions in the presence or absence of 50 μM MnCl2 to early log phase (OD540 0.2) and harvested by centrifugation at 8,000 × g at 4 °C for 15 min. The cell pellet was prepared for and run on atomic absorption spectroscopy to determine the intracellular iron content as previously described (15). Protein concentrations were determined using the BCA assay (Sigma) using BSA as a standard.
Analysis of mRNA.
Transcript levels of selected genes were determined by qPCR, as described previously (15). Relative starting quantities of the mRNAs for the genes of interest and gapA were calculated from the corresponding standard curves. Quantity of the interested genes was normalized to the quantity of gapA for each respective condition. The results were expressed as average of triplicate samples ± SD.
In Vivo Cross-Linking, Co-IP, and Quantitative Analysis of Irr-Bound DNA.
Cross-linking of DNA to protein in cells and co-IP using anti-Irr antibodies were carried out as described previously (16). Immunoprecipitated DNA was analyzed by qPCR using primers that delimit the promoter regions of interest. Relative starting quantities were normalized to the mock samples and the values obtained were considered directly proportional to the Irr occupancy of the promoters of the respective genes. The cycle threshold values for input DNA ranged from 13.3 to 14.2. These low cycle threshold values over a narrow range show normalized input DNA and that the immunoprecipitated DNA was not limited by the input DNA.
Immunoblot Analysis.
Irr and GroEL levels were measured in cells grown under varying conditions of metal or heme. Cell harvesting, protein quantification, and immunoblotting were carried out as described previously (21).
Heme-Binding Experiments.
Binding of ferrous heme to purified recombinant Irr was determined by recording the absorption spectrum of 4 μM hemin and 8 μM Irr in PBS (pH 7.4), in the presence of 100 μM dithionite to reduce ferric heme to ferrous heme. The binding reaction was titrated with either MnCl2, ZnCl2, or FeSO4 at concentrations indicated in the text. All spectra were recorded between 380 and 480 nm on a SpectraMax MS spectrophotometer (Molecular Devices).
Circular Dichroism.
For CD, 8 μM Irr in PBS (pH 7.4) was titrated with increasing concentrations of MnCl2 in a fluorimeter cell (Starna Cells, Inc.) with a 1-cm path length at 25 °C. Far UV spectra (190–280 nm) were collected for the protein alone and after each titration with metal with a Jasco J-715 CD spectrometer equipped with a thermostatic water bath. Data were processed using the Jasco software and exported to Microsoft Office Excel 2003.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 GM067966 (to M.R.O.) and National Institutes of Health Training Grant T32 AI707614 (to T.H.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002342107/-/DCSupplemental.
References
- 1.Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 2.Au C, et al. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLoS ONE. 2009;4:e7792. doi: 10.1371/journal.pone.0007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makui H, et al. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 4.Garrick MD, et al. DMT1: A mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- 5.Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 6.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+) J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 11.Mongkolsuk S, Helmann JD. Regulation of inducible peroxide stress responses. Mol Microbiol. 2002;45:9–15. doi: 10.1046/j.1365-2958.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 12.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 13.Small SK, Puri S, O'Brian MR. Heme-dependent metalloregulation by the iron response regulator (Irr) protein in Rhizobium and other Alpha-proteobacteria. Biometals. 2009;22:89–97. doi: 10.1007/s10534-008-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph G, et al. The Iron control element, acting in positive and negative control of iron-regulated Bradyrhizobium japonicum genes, is a target for the Irr protein. J Bacteriol. 2006;188:733–744. doi: 10.1128/JB.188.2.733-744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, et al. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Mol Microbiol. 2006;60:427–437. doi: 10.1111/j.1365-2958.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangwan I, Small SK, O'Brian MR. The Bradyrhizobium japonicum Irr protein is a transcriptional repressor with high-affinity DNA-binding activity. J Bacteriol. 2008;190:5172–5177. doi: 10.1128/JB.00495-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small SK, Puri S, Sangwan I, O'Brian MR. Positive control of ferric siderophore receptor gene expression by the Irr protein in Bradyrhizobium japonicum. J Bacteriol. 2009;191:1361–1368. doi: 10.1128/JB.01571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamza I, Chauhan S, Hassett R, O'Brian MR. The bacterial Irr protein is required for coordination of heme biosynthesis with iron availability. J Biol Chem. 1998;273:21669–21674. doi: 10.1074/jbc.273.34.21669. [DOI] [PubMed] [Google Scholar]
- 19.Qi Z, Hamza I, O'Brian MR. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Irr) protein. Proc Natl Acad Sci USA. 1999;96:13056–13061. doi: 10.1073/pnas.96.23.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Ishimori K, O'Brian MR. Two heme binding sites are involved in the regulated degradation of the bacterial iron response regulator (Irr) protein. J Biol Chem. 2005;280:7671–7676. doi: 10.1074/jbc.M411664200. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Panek HR, O'Brian MR. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol Microbiol. 2006;60:209–218. doi: 10.1111/j.1365-2958.2006.05087.x. [DOI] [PubMed] [Google Scholar]
- 22.Qi Z, O'Brian MR. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Mol Cell. 2002;9:155–162. doi: 10.1016/s1097-2765(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 23.Escolar L, Pérez-Martín J, de Lorenzo V. Opening the iron box: Transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohle TH, O'Brian MR. The mntH gene encodes the major Mn(2+) transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol Microbiol. 2009;72:399–409. doi: 10.1111/j.1365-2958.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acuña G, Ebeling S, Hennecke H. Cloning, sequencing, and mutational analysis of the Bradyrhizobium japonicum fumC-like gene: evidence for the existence of two different fumarases. J Gen Microbiol. 1991;137:991–1000. doi: 10.1099/00221287-137-4-991. [DOI] [PubMed] [Google Scholar]
- 26.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman YE, O'Brian MR. The ferric uptake regulator (Fur) protein from Bradyrhizobium japonicum is an iron-responsive transcriptional repressor in vitro. J Biol Chem. 2004;279:32100–32105. doi: 10.1074/jbc.M404924200. [DOI] [PubMed] [Google Scholar]
- 28.Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 29.Nath KA, et al. alpha-Ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione-induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:C227–C236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 30.Frustaci JM, Sangwan I, O'Brian MR. Aerobic growth and respiration of a δ-aminolevulinic acid synthase (hemA) mutant of Bradyrhizobium japonicum. J Bacteriol. 1991;173:1145–1150. doi: 10.1128/jb.173.3.1145-1150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.