Abstract
Grb2 is an adaptor molecule that mediates Ras-MAPK activation induced by various receptors. Here we show that conditional ablation of Grb2 in thymocytes severely impairs both thymic positive and negative selections. Strikingly, the mutation attenuates T-cell antigen receptor (TCR) proximal signaling, including tyrosine phosphorylation of multiple signaling proteins and Ca2+ influx. The defective TCR signaling can be attributed to a marked impairment in Lck activation. Ectopic expression of a mutant Grb2 composed of the central SH2 and the C-terminal SH3 domains in Grb2−/− thymocytes fully restores thymocyte development. Thus, Grb2 plays a pivotal role in both thymic positive and negative selection. It amplifies TCR signaling at the top end of the tyrosine phosphorylation cascade via a scaffolding function.
Keywords: signal transduction, T-cell development, Grb2, tyrosine phosphorylation
T lymphopoiesis is a sequential developmental process regulated by multiple environmental signals. The final outcome is the generation of different lineages of T cells with a diverse antigen receptor repertoire (1–4). Diversification of the T-cell antigen receptor (TCR) is initially generated through successive rearrangements of the V-(D)-J genes in CD4 and CD8 double-negative (DN) T precursors. Successful rearrangement of the TCR β gene allows DN T cells to express pre-TCR and to develop into CD4 and CD8 double-positive (DP) cells, at which stage the TCR repertoire is further shaped by thymic selection. Cells expressing a TCR with too strong or too weak an affinity/avidity for MHC-peptide complexes will die via apoptosis, the processes termed negative selection or neglect. In contrast, cells bearing a TCR with moderate avidity toward MHC-peptide complexes will survive the selection (positive selection) and become mature CD4+ or CD8+ single positive (SP) thymocytes (5–8).
The importance of TCR signaling in thymic positive and negative selection has been demonstrated in a variety of experimental models. However, it remains to be determined precisely how TCR signaling is initiated and propagated intracellularly during thymic selection. The first step in initiating the TCR signaling cascade is thought to be the phosphorylation of tyrosine residues in immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3ζ chain by the Src family tyrosine kinase Lck (9–11). Phosphorylated ITAMs of the CD3ζ chain then recruit tyrosine kinase Zap70 to the TCR complexes, where it can be activated by autophosphorylation. Upon activation, Zap70 phosphorylates the membrane anchor protein linker for activation of T cells (LAT), which in turn assembles signaling complexes containing multisignaling molecules, including Grb2, SLP-76, Vav, Gads, PLCγ-1, and Itk. The coordination between these signaling components in the complexes determines multiple downstream cellular responses, including Ca2+ mobilization, cytoskeleton reorganization, and activation of nuclear transcription factors, which eventually define various T-cell development programs (9, 12–14).
Grb2 is a positive regulator of Ras signaling downstream of many growth factor receptors, and plays an important function in embryogenesis and malignant transformation (15, 16). It contains one central SH2 domain flanked by two SH3 domains. The latter can associate with Sos, a GDP/GTP exchange factor (GEF) for Ras. Grb2 is constitutively expressed in T cells and associates with LAT or the CD3 complex via Shc upon TCR stimulation (9). These observations suggest that Grb2 plays an important role in TCR signaling. A recent experiment has shown that haploid germline deficiency of Grb2 impairs thymic negative selection but not positive selection (17). The haploid mutation of Grb2 does not affect the activation of MAPK Erk1/2 upon TCR stimulation, but instead weakens TCR-induced p38 activation. These results are consistent with a recent finding that TCR induces Ras-Erk1/2 activation mainly through Ras-GRP but not Grb2 (18). To investigate the precise function of Grb2 in T-cell biology, we have generated T-cell–specific Grb2 deficient mice and analyzed TCR signaling and T-cell development and function in these mice. Our results demonstrate that Grb2 is not only required for thymic negative selection but is also critical for thymic positive selection. Furthermore, we report that Grb2 functions at the top end of the TCR-induced tyrosine kinase cascade, filling a unique role that amplifies Lck signaling during thymocyte development.
Results
Impaired Thymocyte Development in Grb2−/−(T) Mice.
We generated grb2 gene floxed (Grb2f) mice by gene targeting (Fig. S1A). Grb2f/f mice were born at a Mendelian ratio and appeared to be normal and fertile. To inactivate Grb2 specifically in T-lineage cells, we bred Grb2f/f mice to Lck-cre transgenic (Tg) mice that express a cre transgene starting from DN3 thymocytes (19). Deletion of the grb2 gene and ablation of Grb2 protein in total thymocytes or purified thymocyte subsets were confirmed by Southern blot and Western blot analysis, respectively (Fig. S1 B and C). The remaining trace amount of Grb2 in the mutant thymocytes detected by the Western blot could be due to a few leaking cells without the deletion or a long half-life of Grb2 protein (Fig. S1C). For simplicity, we hereafter refer to the Grb2f/f Lck-cre Tg mutation and mice as the Grb2−/−(T) mutation and mice, respectively.
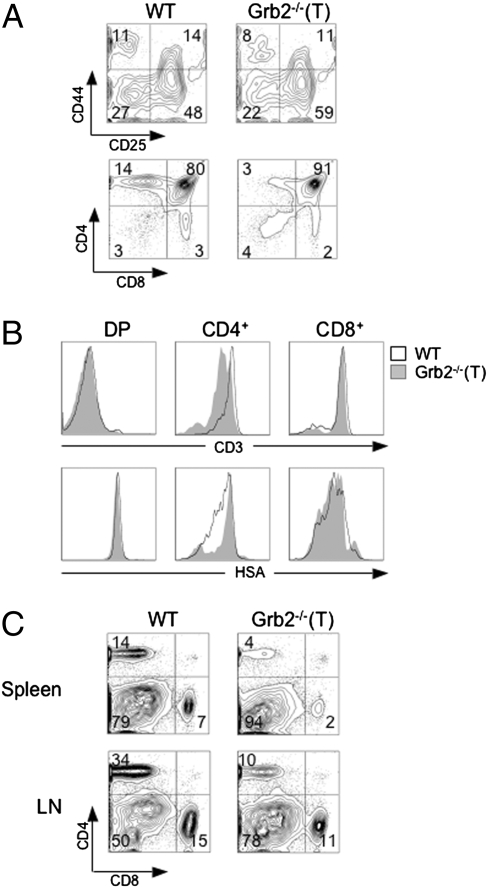
To determine whether the ablation of Grb2 influences the development of T cells, we examined the cellularity of T-lineage cells in the thymus, spleen, and lymph nodes of Grb2−/−(T) mice by flow cytometry. The total number of thymocytes in Grb2−/−(T) mice was slightly reduced as compared with wild-type (WT) littermates, suggesting that the Grb2−/−(T) mutation altered thymocyte development (Fig. 1A and Table S1). The mutation had a moderate impact on the development of DP thymocytes, because the total numbers of DP T cells were 30% less in the mutant than in control mice (Fig. 1A and Table S1). However, the mutant mice possessed markedly reduced numbers of CD4+ (P < 0.01) and moderately reduced numbers of CD8+ SP T cells (P < 0.01) as compared with WT mice (Fig. 1A and Table S1). The Grb2−/−(T) CD4+, but not CD8+ SP T cells, failed to up-regulate the cell surface CD3 and to down-modulate HSA (Fig. 1B), suggesting that the mutation blocked the maturation of thymocytes from the DP to the SP stage. The defective development of CD4+ and CD8+ T cells was also reflected in peripheral mature T cells, as the total numbers of mutant CD4+ and CD8+ T cells were dramatically reduced in both the spleen (CD4+ T cells, P < 0.01, and CD8+ T cells, P < 0.01) and lymph nodes (CD4+ and CD8+ T cells, P < 0.01) (Fig. 1C and Table S1). These peripheral CD4+ and CD8+ T cells were Grb2−/−(T) T cells as evidenced by a Western blot analysis (Fig. S2). These results indicate that Grb2 plays an important role in the development and maturation of both CD4+ and CD8+ T cells.
Fig. 1.
Impaired thymocyte development in Grb2−/−(T) mice. (A) Cellularity and CD4 and CD8 expression of Grb2−/−(T) thymocytes. Total thymocytes stained with anti-CD4 and anti-CD8 (Lower), or gated DN (CD4− CD8− B220− Gr1− CD11b−) thymocytes stained with anti-CD44 and anti-CD25 (Upper). Samples are from 8-week-old WT and Grb2−/−(T) mice. Total numbers of T cells and thymocyte subsets are given in Table S1. Results represent more than seven Grb2−/−(T) mice and WT mice (P < 0.01). (B) Cell surface expression of CD3 and HSA on thymocyte subsets. Shown are histograms of CD3 or HSA expression on gated DP, CD4+ and CD8+ SP cells. (C) Anti-CD4 and anti-CD8 staining of T cells from the spleen and lymph nodes (LN) of WT and Grb2−/−(T) mice. Results are representative of more than seven Grb2−/−(T) mice and WT controls (P < 0.01; Table S1).
Impairment of Thymic Positive Selection in Grb2−/−(T) Mice.
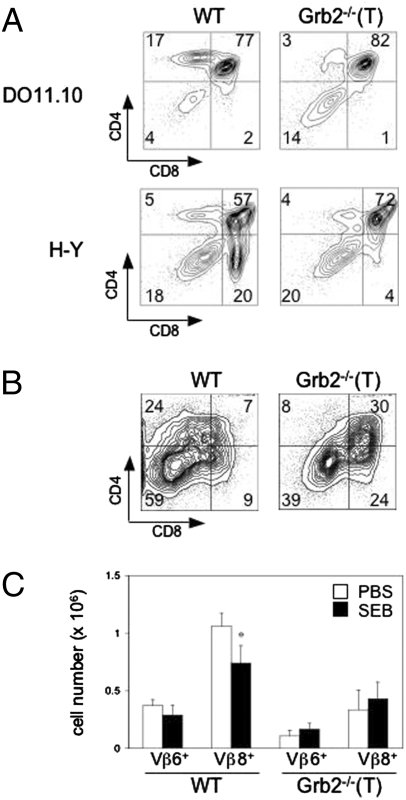
Maturation of DP thymocytes into CD4+ and CD8+ SP T cells involves both thymic positive and negative selection. To test whether the Grb2−/−(T) mutation affects thymic positive selection of CD4+ and CD8+ T cells, we crossed Grb2−/−(T) mice with DO11.10 TCR and H-Y TCR Tg mice, respectively (20). DO11.10 TCR Tg mice express a transgenic TCR that specifically recognizes a chicken ovalbumin (OVA) peptide presented by the MHC-II, I-Ad molecules. During development, WT thymocytes expressing the DO11.10-transgenic TCR are positively selected in the presence of I-Ad. H-Y TCR Tg mice express a TCR specific to the male antigen H-Y in the context of H-2b (21). Positive selection of CD8+ thymocytes can be tested in female H-Y TCR Tg mice because female mice do not express the H-Y antigen. The Grb2−/−(T) mutation did not have an apparent effect on the development of DP thymocytes in either DO11.10 or female H-Y TCR Tg mice (Fig. 2A). However, whereas ∼20% of total thymocytes in WT DO11.10 TCR Tg mice were CD4+ SP cells, these CD4+ cells were virtually absent (less than 3%) in Grb2−/−(T) thymi, indicating that positive selection of CD4+-lineage T cells was severely impaired in the absence of Grb2. The Grb2−/−(T) mutation also affected the positive selection of CD8+ SP T cells, as evidenced by a marked reduction of CD8+ SP thymocytes in female Grb2−/−(T) H-Y Tg mice as compared with WT controls (4% vs. 20%). From these results, we conclude that Grb2-mediated signaling is essential for the positive selection of both CD4+ and CD8+-lineage T cells.
Fig. 2.
Impaired thymic positive and negative selection in Grb2−/−(T) mice. (A) Impaired thymic positive selection. Thymocytes were from Grb2−/−(T) DO11.10 Tg mice or female Grb2−/−(T) H-Y Tg mice stained with anti-CD4 and anti-CD8. Results are representative of more than three independent experiments. (B) Impaired thymic negative selection. Shown are thymocytes from Grb2−/−(T) H-Y Tg male mice. (C) SEB-mediated deletion of thymocytes. On 48 h post-SEB treatment, thymocytes from WT or Grb2−/−(T) mice injected intraperitoneally with SEB were analyzed for Vβ6, Vβ8, CD4, and CD8 expression. Total numbers of Vβ6+ or Vβ8+ thymocytes of indicated genotypes are shown as bars. Data represent more than five mice in each group.
Attenuation of Thymic Negative Selection by the Grb2−/−(T) Mutation.
To test whether the Grb2−/−(T) mutation altered thymic negative selection, we examined CD8+ T cell development in male H-Y TCR Tg mice (21). In WT male H-Y TCR Tg mice, thymocytes expressing a H-Y TCR were negatively selected, leading to depletion of both DP and CD8+ SP thymocytes (Fig. 2B). However, in male Grb2−/−(T) H-Y Tg mice the depletion of both DP and CD8+ SP thymocytes was significantly attenuated compared with WT controls (30% vs. 7% for DP cells and 24% vs. 9% for CD8+ SP cells, respectively) (Fig. 2B). The majority of CD8+ SP thymocytes in the mutant mice were the H-Y idiotype positive (T3.70+) cells, indicating that these cells were H-Y antigen-specific T cells that survived the negative selection.
Negative selection of thymocytes can also be tested using superantigen SEB-mediated depletion of TCR Vβ8+ thymocytes, as TCR Vβ8 specifically interacts with SEB (22, 23). We found that SEB treatment significantly reduced the number of Vβ8+ SP thymocytes (P < 0.005) in WT mice; however, SEB treatment failed to cause any measurable change of Vβ8+ thymocytes in Grb2−/−(T) mice (Fig. 2C). The total numbers of Vβ6+ thymocytes, which did not bind to SEB, were not affected in WT or Grb2−/−(T) mice, indicating that the depletion of Vβ8+ thymocytes in SEB treated mice is not a result of a systemic activation of mature T cells. Thus, we conclude that thymic negative selection is not enhanced but instead attenuated in Grb2−/−(T) mice.
Alteration of Multiple TCR-Signaling Pathways in Grb2−/−(T) Thymocytes.
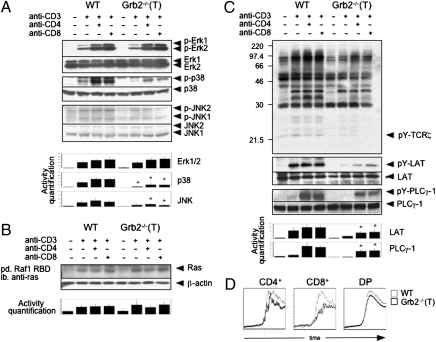
Upon TCR triggering, Grb2 forms a complex with Sos, a Ras GEF, and is recruited to the LAT/TCR complex (9). This has been previously interpreted as evidence that Grb2 mediates TCR-induced activation of the Ras-Erk kinase pathway in T cells. Although recent data show that Ras-GRP is a major player in TCR-induced Ras-Erk kinase activation, involvement of Grb2 in this regulation has not yet been completely excluded. Hence, we examined whether the activation of Erk1/2 was altered in Grb2−/−(T) DP thymocytes. To avoid the potential bias caused by the presence of different numbers of CD4+ SP thymocytes in WT and Grb2−/−(T) thymi (Fig. 2B), we used purified (more than 95%) DP thymocytes for the assay. We found that the activation of Erk1/2 in the mutant thymocytes was not significantly different from that in WT cells when cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD4 (Fig. 3A). Consistent with this result, the level of TCR-induced Ras activation, as measured by a Ras pull-down assay using a recombinant Ras-binding domain (RBD) of Raf as bait, was also comparable in WT and Grb2−/−(T) thymocytes after various periods of TCR stimulation (Fig. 3B and Fig. S3). These results indicate that Grb2 is dispensable in TCR-induced Ras-Erk kinase activation in thymocytes.
Fig. 3.
TCR signaling in Grb2−/−(T) thymocytes. (A) Western blot analysis of MAPK activation in Grb2−/−(T) thymocytes. DP thymocytes were stimulated with anti-CD3ε alone or anti-CD3ε and anti-CD4 or anti-CD8 for 2 min. Activities of Erk1/2, p38, and JNK 1/2 were detected by antibodies against the active forms of Erk1/2 (p-Erk1/2), p38 (p-p38), and JNK1/2 (p-JNK1/2), respectively. Protein loadings were quantified by anti-Erk1/2, anti-p38, and anti-JNK1/2. MAPK activities were quantified by densitometry of each band, normalized to protein loading, and shown as bars. Results represent three independent experiments (*P < 0.01). (B) Active form Ras pull-down assay. Cells were stimulated with anti-CD3ε and anti-CD4 for 2 min (short period of stimulation depicted in Fig. S3). Ras protein was quantified using an anti-Ras. (C) Tyrosine phosphorylation of total proteins and individual TCR-proximal signaling components. Thymocytes from Grb2−/−(T) and WT mice were stimulated with anti-CD3ε alone, or anti-CD3ε together with anti-CD4 or anti-CD8. The levels of tyrosine phosphorylation were determined using an anti-phosphotyrosine antibody (4G10). The amounts of signals on the blots were quantified using a densitometer. Activities of LAT or PLCγ-1 were quantified and shown as bars. Results represent three independent experiments (*P < 0.05). (D) Calcium mobilization in Grb2−/−(T) thymocytes. Gray and black lines represent Ca2+ mobilization in WT and Grb2−/−(T) T cells, respectively.
Interestingly, our results showed that the Grb2−/−(T) mutation markedly attenuated TCR-induced p38 activation (P < 0.01) and significantly weakened JNK activation in thymocytes (P < 0.01) (Fig. 3A), suggesting that the TCR signaling pathway leading to p38 and JNK activation was impaired. To determine the point at which the TCR signaling cascade was affected by the Grb2−/−(T) mutation, we examined various TCR-proximal signaling events after TCR crosslinking. Surprisingly, we found that the Grb2−/−(T) mutation resulted in much weaker tyrosine phosphorylation of multiple proteins such as TCRζ chain, LAT, and PLCγ-1 after TCR stimulation (P < 0.05) (Fig. 3C). Consistent with the lower PLCγ-1 phosphorylation, Ca2+ mobilization was also attenuated in the mutant thymocytes (Fig. 3D). Based on these results, we conclude that Grb2 positively controls multiple TCR signaling pathways in thymocytes. Because tyrosine phosphorylation of the TCRζ chain and LAT was markedly attenuated in Grb2−/−(T) thymocytes, we predict that Grb2 exerts a positive regulatory function at the proximal end of the TCR-induced tyrosine phosphorylation cascade.
Attenuation of Lck Activation in Grb2−/−(T) Thymocytes.
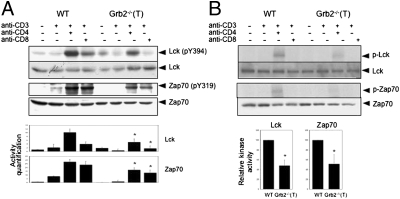
Tyrosine phosphorylation of the TCRζ chain by Lck is one of the earliest events in TCR signaling (9). We therefore tested the hypothesis that the Grb2−/−(T) mutation attenuates the function of tyrosine kinases such as Lck and Zap70. We found that the Grb2−/−(T) thymocytes (P < 0.05) exhibited a significantly lower level of Zap70 activity (Zap70 (pY319) and autophosphorylation than did WT thymocytes after anti-CD3 and anti-CD4 stimulation (Fig. 4 A and B). Activation of Lck was also markedly diminished in the mutant cells (P < 0.05) as compared with WT cells under the same stimulation conditions. Taken together, our data demonstrate that ablation of Grb2 in thymocytes results in the loss of proper Lck activation amplified by TCR and CD4 costimulation. Because Lck activation is the first event that occurs in the TCR signaling cascade, we propose that the attenuated Lck activation is responsible for the impairment of the multiple TCR downstream signaling pathways in Grb2−/−(T) thymocytes.
Fig. 4.
Attenuation of TCR-induced Zap70 and Lck activation in Grb2−/−(T) thymocytes. Purified DP thymocytes from WT and Grb2−/−(T) mice were incubated at 37 °C for 4–6 h and then stimulated with anti-CD3ε alone or anti-CD3ε plus anti-CD4 or anti-CD8. Lck and Zap70 activities in cell lysates were determined by Western blot analysis using either anti-active form of Lck [Lck (pY394)) or Zap70 (Zap70 (pY319)] (A), or by an in vitro autophosphorylation assay (B). Shown are representative results of more than three independent experiments. Amounts of signals were quantified by densitometry and normalized to protein loading. (*P < 0.05).
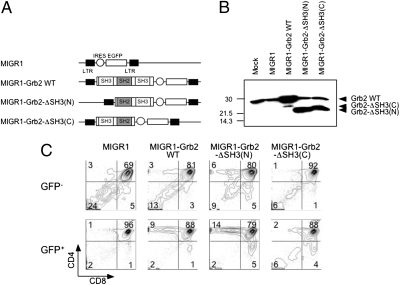
Dependence of Thymocyte Development on the C-Terminal SH3 Domain of Grb2.
Grb2 is a scaffold protein that functions by mediating protein–protein interactions. However, our coimmunoprecipitation assay could not prove any meaningful association between Grb2 and Lck, suggesting that Grb2 interacts with other molecule(s) that regulate thymocyte development and Lck activity. To determine which domain of Grb2 is critical for thymocyte development, we generated GFP-based bicistronic retroviral vectors expressing a WT Grb2 or one of the Grb2 mutants that deleted either the C-terminal [Grb2-ΔSH3(C)] or the N-terminal SH3 domain [Grb2-ΔSH3(N)] (Fig. 5 A and B). We introduced these Grb2 mutants into Grb2−/−(T) bone marrow (BM) stem cells by retrovirus-mediated gene transduction, transplanted the retrovirus infected BM stem cells into lethally irradiated Rag2−/− recipient mice to generate BM chimeras, and then analyzed T-cell development in the BM chimeric mice by flow cytometry. As expected, we found that expression of a WT Grb2 in Grb2−/−(T) thymocytes fully restored the development of donor-derived CD4+ T cells (Fig. 5B). In contrast, whereas expression of the Grb2-ΔSH3(N) mutant rescued the development of CD4+ T cells as efficiently as did the WT Grb2, expression of Grb2-ΔSH3(C) failed to correct the defective development of Grb2−/−(T) thymocytes (Fig. 5C). Thus, these results demonstrate that development of CD4+ T cells depends on the SH2 and the C-terminal, but not the N-terminal, SH3 domain of Grb2.
Fig. 5.
A critical role of the C-terminal SH3 domain of Grb2 for thymocyte development. (A) GFP-based bicistronic retroviral vectors expressing a WT or mutant form of Grb2. Grb2-ΔSH3(N), mutant Grb2 lacking the N-terminal SH3; Grb2-ΔSH3(C), mutant Grb2 lacking the C-terminal SH3 domain. (B) Western blot showing the expression of WT and mutants Grb2 in 293T cells. (C) Development of WT or mutant Grb2-expressing thymocytes in BM chimeric mice. Grb2−/−(T) BM cells were infected with GFP-based bicistronic retroviral vector expressing either a WT or a mutant Grb2 and transplanted into irradiated Rag2−/− mice. Development of Grb2 transgene-expressing thymocytes was analyzed by flow cytometry. Shown are FACS analysis of gated GFP− [Grb2−/−(T)] and GFP+ (Grb2 transgene-expressing) thymocytes. Transgenes used for BM reconstitution are indicated at the top of each plot. All experiments shown were performed at least twice with comparable results.
Discussion
The role of Grb2 in T-cell development was first described in heterozygous Grb2-deficient mice, in which the haploid germline deficiency of Grb2 weakened the negative, but not positive, selection of thymocytes (17). This observation has been taken as evidence that Grb2 differentially regulates thymic positive and negative selection. In the present study, we have characterized the function of Grb2 in TCR signaling and thymocyte development using T-cell–specific Grb2−/−(T) mice. Although we could not conclude whether Grb2 plays any role in the development of DN thymocytes, because of a partial deletion of the grb2 gene in our system at this stage of thymocyte development (Fig. S4), our data reveal that the Grb2−/−(T) mutation imposes a moderate effect on the development of DP thymocytes. In contrast to the previous finding, we demonstrate that ablation of Grb2 in thymocytes results in a severe impairment of both thymic positive and negative selection. As a consequence, peripheral CD4+ and CD8+ T cells are dramatically reduced in Grb2−/−(T) mice. Given that both thymic positive and negative selection are impaired in Grb2−/−(T) mice, we conclude that Grb2 plays a key role in the control of both thymic positive and negative selection.
Previous studies using in vitro cell lines and germline Grb2−/− mice revealed an important role of Grb2 in growth factor receptor–mediated activation of Ras-Erk kinase (15, 16). The contribution of Grb2 to receptor signaling can be attributed to the direct association of Grb2 with Sos and to the recruitment of Sos to the activated receptors by Grb2, where Sos could activate Ras through its GDP-GTP exchange activity. In T cells, Grb2 also associates with Sos and is recruited to cell membranes via the phosphotyrosine residues of LAT or the TCR complex upon TCR stimulation (9, 12). These findings have led to an assumption that Grb2 may be responsible for TCR-induced Ras-Erk kinase activation in T cells (9). A recent study further shows that Sos regulates Ras activation in an “on or off” manner possibly via Grb2, whereas Ras-GRP may gradually tune Ras activity in cultured T cells, suggesting that Ras activation can be “digital or analog” (24). However, our experiments demonstrate that the Grb2−/−(T) mutation does not affect the activation of Ras-Erks, but does affect the activation of JNK and p38 kinases in thymocytes. This result is mostly in agreement with previous findings on Grb2 haploid germline mutant T cells, except with no major change in TCR-induced Ras activity (17). It is possible that Sos contributes to Ras activation in thymocytes through other adaptors than Grb2. It has been shown that ablation of Ras-GRP is sufficient to block Ras activation induced by PMA/bryostatin 1 in thymocytes or TCR-induced proliferation of thymocytes (18). These results also argue against a major role of Grb2 and suggest that Ras-GRP is a major player in TCR-induced Ras-Erk kinase activation in thymocytes. It is interesting to note that although Ras activation is normal, several signaling events, including tyrosine phosphorylation of Lck, Zap70, and PLCγ-1 that act at the upstream of Ras-GRP, are attenuated in Grb2−/−(T) cells. It remains to be determined whether these results imply that in our assay system the attenuated Lck, Zap70, and PLCγ-1 signals are already above the threshold required for Ras-GRP activation.
Our data show that the Grb2−/−(T) mutation attenuates tyrosine phosphorylation of a broad spectrum of signaling components downstream of the TCR. This finding indicates that Grb2 is a positive regulator of multiple TCR proximal signaling pathways. Because Lck activity is significantly attenuated in Grb2−/−(T) DP thymocytes, we propose that Grb2 controls the earliest TCR signaling event, in particular, the activation of tyrosine kinase Lck. Our results show that Lck activity in Grb2−/−(T) thymocytes is not completely eliminated when cells are stimulated with anti-CD3 but, rather, significantly weakened when cells are triggered by anti-CD3 and anti-CD4. This finding reveals that Grb2 is an amplifier of Lck signaling upon TCR and CD4 costimulation and acts at the top of the TCR-induced tyrosine phosphorylation cascade. Recently, it has been observed that Grb2 may help oligomerization of LAT into macromolecular cluster (25). In addition, it has been reported that Grb2 negatively regulates BCR-induced Ca2+ signaling in chicken B cells, and this regulation is dependent on the association between Grb2 and NTAL/LAB as well as the recruitment of Grb2 to the lipid rafts (26). Thus, it is conceivable that, by interacting with different signaling partners, Grb2 may exert very different regulatory function in different receptor signaling.
At present, it is not clear how Grb2 positively regulates Lck signaling. Accumulating data indicate that Lck activity is negatively regulated by tyrosine kinase Csk, which phosphorylates the inhibitory tyrosine (Y505) on Lck (27). Membrane phosphatase CD45 may reverse this inhibition by dephosphorylating the phosphotyrosine Y505 of Lck. In resting T cells, Csk is localized in membrane lipid rafts, where it associates with the adaptor PAG/Cbp (28, 29). In contrast, CD45 is distributed outside the lipid rafts. It is believed that Lck is shuttled between lipid rafts and nonlipid rafts, with its active form located in the lipid rafts, whereas the inactive version of Lck predominantly stays outside these domains. Thus, the opposing function of CD45 and Csk, together with the trafficking of Lck between lipid rafts and nonraft membrane domains, provides an equilibrium pool of signal-competent Lck, which would be available to phosphorylate the TCR complex and Zap70 when the TCR is triggered. As Grb2 is not directly associated with Lck, it is possible that Grb2 regulates Lck activity by affecting the trafficking of Lck, CD45, and Csk in different membrane locations. Consistent with this assumption, our experiments show that Grb2 becomes associated with the membrane rafts in activated T cells. It is therefore important to determine whether the association of Csk, CD45, and Lck with membrane rafts is altered in Grb2−/−(T) thymocytes. Alternatively, Grb2 may interact with a novel regulator that positively regulates Lck activity. Because the expression of a mutant Grb2 composed of the central SH2 domain and the C-terminal SH3 domain is sufficient to restore normal development of Grb2−/−(T) thymocytes, this putative regulator could contain phosphotyrosine residues and/or proline-rich motifs that interact respectively with the SH2 and the C-terminal SH3 domains of Grb2. Identification of the molecules that interact with the SH2 and the C-terminal SH3 domains of Grb2 will help to clarify this issue.
Materials and Methods
Mice.
grb2 Gene floxed mice were generated according to a previous protocol (30). Briefly, we generated a gene targeting construct using a 9.7-kb BamHI-SalI fragment of genomic DNA containing exons 3, 4, and 5 of grb2 gene (Fig. S1). Homologous recombinants were identified by Southern blot hybridization (Fig. S1). To generate grb2 floxed allele, one targeted ES cell clone was transiently transfected with a Cre-expression vector to delete the neor-gene cassette according to a published protocol (30). To produce Grb2−/− mice, we bred grb2 floxed mice to Lck-Cre Tg mice (19). Grb2−/−(T) mice used in this study were of mixed 129 and C57BL/6 background. Rag2−/− and H-Y TCR Tg mice were from Taconic, and DO11.10 TCR Tg mice were from Jackson Laboratory. All animals were housed in specific pathogen-free barrier facilities at the National Institutes of Health or Columbia University in accordance with institution-approved protocols.
In Vivo Treatment with Superantigen.
Six-week-old mice were intraperitoneally injected with PBS or 20 μg of Staphylococcal enterotoxin B (SEB; Sigma). Thymocytes were analyzed by flow cytometry for cell surface makers including CD4, CD8, Vβ6, and Vβ8 48 h after SEB treatment.
Flow Cytometry and Antibodies.
All mice used for studies on thymocyte development were 6–8 weeks old. For cell surface staining, 106 cells were stained with fluorescent-coupled antibodies in 100 μL PBS buffer containing 1% BSA and 0.01% sodium azide for 20 min. The following antibodies were used for staining: anti-CD3ε (145-2C11), anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-CD24 (J1d), and anti-H-Y TCR idiotype (T3.70), anti-Vβ6 (RR4-7) and anti-Vβ8 (F23.1) (BD Pharmingen). Anti-DO11.10 TCR idiotype (KJ1-26) was from Caltac. Data were collected on either FACS Caliber or LSR II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Western Blotting and Immunoprecipitation.
DP thymocytes were purified by an enrichment protocol using magnetic cell sorting (MACS) (Miltenyi Biotec). To activate TCR signaling, we incubated 107 thymocytes with biotinylated anti-CD3ε (145-2C11) alone, or with anti-CD3ε + anti-CD4 (GK1.5) or anti-CD8 (53-6.7) (each 5 μg/mL) on ice for 15 min. Cells were then resuspended in 500 μL prewarmed (37 °C) PBS buffer containing streptavidin (10 μg/mL) for 2 min to cross-link the TCR. After cross-linking, cell pellets were lysed in RIPA buffer. Immunoprecipitation and Western blot hybridization were performed according to a standard protocol (31). The following antibodies were used for Western blot hybridization: anti-phosphotyrosine (4G10, Upstate biotech); anti-phospho-Zap70 (pY319) (Santa Crutz Biotech); anti-phospho-Lck (pY394) (a gift from Dr. A. Shaw); anti-phospho-Erk (E-4) and JNK (G-7) (Santa Crutz Biotechnology); anti-phospho-p38 (Biosource); anti-Grb2 (C-23, C-7), anti-actin (11C), anti-Erk1 (K-23), anti-Erk2 (C-14), anti-JNK1/2 (FL), anti-P38 (N-20), anti-PLCγ−1 (53), anti-Lck (2102), and anti-Zap70 (LR) (Santa Crutz Biotechnology); rabbit anti-LAT polyclonal antibody (gift from L. Samelson). Autophosphorylation assay for Lck and Zap70 was conducted according to a previous protocol (32). To measure MAPK activities, the densitometric readings of bands on films were evaluated using an imaging densitometer (Bio-Rad).
Statistical Analysis.
The significance between two series of data were determined by a paired two-tailed Student's t test. Values of P < 0.05 were accepted as statistically significant.
Intracellular Ca2+ Influx and Ras Pull-Down Experiment.
Thymocytes (107) were loaded with Indo-I (10 μg/mL, Molecular Probes) at 37 °C for 30 min and then surface-stained for CD4 and CD8 at room temperature for 15 min. To determine Ca2+ influx, cells were resuspended in prewarmed (37 °C) HBSS buffer containing 1% BSA, stimulated with biotinylated anti-CD3ε (10 μg/mL) followed by streptavidin cross-linking, and analyzed on FACS Vantage. The active Ras pull-down assay was carried out using recombinant human Ras binding domain (RBD) of Raf-1 according to the instructions of the manufacturer (Upstate Biotech). In brief, 107 thymocytes were stimulated with anti-CD3ε (5 μg/mL) at 37 °C for 0.5, 1, or 2 min. The active form of Ras in cell lysate was precipitated by adding recombinant Raf-1 RBD protein and incubating at 4 °C for 45 min. Ras proteins precipitated by recombinant Raf-1 RBD was quantified by Western blot hybridization using anti-Ras (RAS10).
Retroviral Infection and Bone Marrow Chimeras.
To generate WT and mutant Grb2 expression vectors, we cloned cDNA encoding a WT Grb2, Grb2-ΔSH3(N), or Grb2-ΔSH3(C) into a GFP-based bicistronic retroviral vector (MIGR1) (33). To generate bone marrow (BM) chimera, we intraperitoneally injected 5′-fluorouracil (5 mg/mouse) into 8-week-old Grb2−/−(T) mice. Five days later, BM cells were collected and cultured for 48 h in the presence of IL-3 (20 ng/mL), IL-6 (50 ng/mL), and SCF (50 ng/mL) (R&D). Retrovirus was prepared according to a published protocol (33). Cultured BM stem cells were infected with virus by low-speed centrifugation. Two days after infection, 106 BM cells were transferred i.v.into lethally irradiated (900 rads) Rag2−/− mice. One month later, chimeric mice were examined for development of transgene-expressing (GFP+) thymocytes by flow cytometry.
Supplementary Material
Acknowledgments
We thank Drs. Larry Samelson for anti-LAT antibody and discussion, A. S. Shaw for anti-phospho-Lck (pY394), J. R. Hayman for Grb2 genomic DNA clone, A. M. Pendergast for Grb2 cDNA, Junji Takeda for Lck-Cre Tg mice, O. Jovanovic for proofreading the manuscript, and other members of our laboratory for discussion. H.G. is an Irene Diamond Associate Professor. This study is supported in part by the National Institutes of Health Intramural Research Programs and by the Irene Diamond Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0905039107/-/DCSupplemental.
References
- 1.Benoist C, Mathis D. Positive selection of T cells: Fastidious or promiscuous? Curr Opin Immunol. 1997;9:245–249. doi: 10.1016/s0952-7915(97)80143-4. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 3.Kisielow P, von Boehmer H. Development and selection of T cells: Facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 4.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 5.Ashton-Rickardt PG, Van Kaer L, Schumacher TN, Ploegh HL, Tonegawa S. Repertoire-determining role of peptide in the positive selection of CD8+ T cells. Immunol Rev. 1993;135:157–182. doi: 10.1111/j.1600-065x.1993.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 7.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 8.Sebzda E, et al. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 10.Samelson LE, Donovan JA, Isakov N, Ota Y, Wange RL. Signal transduction mediated by the T-cell antigen receptor. Ann N Y Acad Sci. 1995;766:157–172. doi: 10.1111/j.1749-6632.1995.tb26659.x. [DOI] [PubMed] [Google Scholar]
- 11.Weil R, Veillette A. Signal transduction by the lymphocyte-specific tyrosine protein kinase p56lck. Curr Top Microbiol Immunol. 1996;205:63–87. doi: 10.1007/978-3-642-79798-9_4. [DOI] [PubMed] [Google Scholar]
- 12.Samelson LE. Adaptor proteins and T-cell antigen receptor signaling. Prog Biophys Mol Biol. 1999;71:393–403. doi: 10.1016/s0079-6107(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 13.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 14.Myung PS, Boerthe NJ, Koretzky GA. Adapter proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 2000;12:256–266. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 15.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 17.Gong Q, et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 18.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 19.Takahama Y, et al. Functional competence of T cells in the absence of glycosylphosphatidylinositol-anchored proteins caused by T cell-specific disruption of the Pig-a gene. Eur J Immunol. 1998;28:2159–2166. doi: 10.1002/(SICI)1521-4141(199807)28:07<2159::AID-IMMU2159>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 21.Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 22.White J, et al. The V beta-specific superantigen staphylococcal enterotoxin B: Stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson EJ, Kingston R, Owen JJ. Newly generated thymocytes are not refractory to deletion when the alpha/beta component of the T cell receptor is engaged by the superantigen staphylococcal enterotoxin B. Eur J Immunol. 1990;20:2517–2520. doi: 10.1002/eji.1830201125. [DOI] [PubMed] [Google Scholar]
- 24.Das J, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houtman JC, et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 26.Stork B, et al. Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity. 2004;21:681–691. doi: 10.1016/j.immuni.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Chow LM, Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- 28.Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 29.Leo A, Schraven B. Adapters in lymphocyte signalling. Curr Opin Immunol. 2001;13:307–316. doi: 10.1016/s0952-7915(00)00220-x. [DOI] [PubMed] [Google Scholar]
- 30.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 31.Naramura M, et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 32.Ashe JM, Wiest DL, Abe R, Singer A. ZAP-70 protein promotes tyrosine phosphorylation of T cell receptor signaling motifs (ITAMs) in immature CD4(+)8(+) thymocytes with limiting p56(lck) J Exp Med. 1999;189:1163–1168. doi: 10.1084/jem.189.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou YR, et al. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.