Abstract
The phagosomal lumen in macrophages is the site of numerous interacting chemistries that mediate microbial killing, macromolecular degradation, and antigen processing. Using a non-hypothesis-based screen to explore the interconnectivity of phagosomal functions, we found that NADPH oxidase (NOX2) negatively regulates levels of proteolysis within the maturing phagosome of macrophages. Unlike the NOX2 mechanism of proteolytic control reported in dendritic cells, this phenomenon in macrophages is independent of changes to lumenal pH and is also independent of hydrolase delivery to the phagosome. We found that NOX2 mediates the inhibition of phagosomal proteolysis in macrophages through reversible oxidative inactivation of local cysteine cathepsins. We also show that NOX2 activity significantly compromises the phagosome's ability to reduce disulfides. These findings indicate that NOX2 oxidatively inactivates cysteine cathepsins through sustained ablation of the reductive capacity of the phagosomal lumen. This constitutes a unique mechanism of spatiotemporal control of phagosomal chemistries through the modulation of the local redox environment. In addition, this work further implicates the microbicidal effector NOX2 as a global modulator of phagosomal physiologies, particularly of those pertinent to antigen processing.
Keywords: phagocytosis, cathepsin, disulfide reduction, antigen processing, lysosome
Unlike many specialized lineages of the mononuclear phagocyte system, tissue macrophages function in a diverse array of homeostatic and immune physiologies. Critical to many of these functions is the phagosome. Over the past decade, proteomic characterization of the phagosome in conjunction with biochemical analysis of phagosomal chemistries in reconstituted systems has given great insight into the function of this organelle (1, 2). More recently, measurement of phagosomal properties in live cells has enabled the in situ dissection of the complex cross-talk between spatiotemporally intimate phagosomal chemistries (3, 4). In particular, cross-talk influencing the control of phagosomal proteolysis has recently received much attention (4). It has become increasingly apparent that a tightly controlled, limited level of proteolysis within the endolysosomal system, as found in dendritic cells (DCs), is essential for efficient antigen processing (5, 6). Macrophages possess a reported 20- to 60-fold higher level of lysosomal proteolysis than DCs, which is implicated in limiting their efficiency as antigen-presenting cells (7–9). Nonetheless, macrophages are capable of productively presenting antigen to T cells and play an important role in the secondary immune response (10). This is presumably aided by the stringent control of the macrophage's lysosomal protease activities in its antigen-processing compartments.
Control of lysosomal proteases such as cathepsins occurs at several regulatory levels, including transcription, trafficking, prodomain removal, regulatory proteins (e.g. cystatins), and vacuolar pH (11). Modification of the redox environment has also been suggested as a conceivable control mechanism to regulate the cysteine cathepsins (such as B and L) that require a reducing environment for their activity (12). However, direct evidence for oxidative inactivation of these enzymes within the reducing environment of the endolysosomal continuum has been hitherto lacking (13, 14). Recently, a novel mechanism of phagosomal protease control in CD8+ DCs mediated through the modulation of phagosomal pH by NADPH oxidase (NOX2) has been described by Amigorena and colleagues (6, 15, 16). NOX2 is a multiprotein complex that is rapidly assembled on the early phagosomal membrane. Here, it oxidizes cytosolic NADPH to convert molecular oxygen to superoxide within the phagosomal lumen, and thus constitutes a major antimicrobial effector (17).
In this study, we identify a functional relationship between the NOX2-mediated oxidative burst and phagosomal proteolysis in macrophages. We show that the observed NOX2-mediated control of phagosomal proteolysis is brought about by the oxidative inactivation of cysteine cathepsins through prolonged modification of the lumenal redox environment, which also impacts the phagosome's ability to reduce disulfides.
Results and Discussion
A Bioactive Chemical-Based Screening Approach to Discover Interconnected Relationships Between Functional Parameters of the Phagosomal Lumen in Macrophages.
In a non-hypothesis-based study, we screened six functional parameters of the phagosomal lumen of bone marrow-derived murine macrophages (BMMØ) (acidification, bulk proteolysis, lipolysis, β-galactosidase activity, oxidative burst, and lysosomal contribution to the phagosome) against the 480-compound Harvard Institute of Chemistry and Cell Biology (ICCB) known bioactives library using real-time fluorometric techniques adapted to a microplate format (18, 19). Each phagosomal parameter was measured in duplicate or triplicate in real time following the phagocytosis of IgG-opsonized 3-μm experimental particles and analyzed with respect to dimethyl sulfoxide (DMSO) -treated controls (Fig. S1). Compounds were excluded if found to alter macrophage morphology, viability, or phagocytic index. Pattern analysis of compound-induced changes across all six phagosomal parameters revealed that many compounds which decreased the phagosomal oxidative burst also increased the rates of bulk proteolysis within the phagosome. These included compounds that directly inhibited NOX2 activity, such as diphenyleneiodonium (DPI), and compounds with known oxidative radical scavenging properties, such as quercetin and transresveratrol.
NOX2 Activity Decreases Rates of Phagosomal Proteolysis.
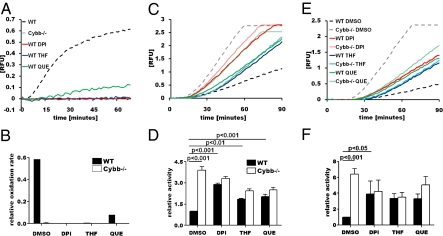
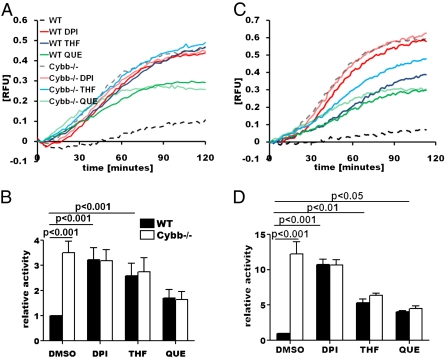
To validate the NOX2-proteolysis relationship identified in the primary screen, phagosomal oxidative burst and bulk phagosomal proteolysis of BMMØs derived from C57BL/6 (wild type; WT) and NOX2-deficient Cybb−/− mice were measured in the presence of DPI and a selection of known oxidative radical scavengers. Of these compounds, DPI, quercetin (hydroxyl radical scavenger), and 3,3′,4′-trihydroxyflavone (THF) (superoxide scavenger) were the most potent inhibitors of phagosomal radical-induced oxidation following phagocytosis of IgG-opsonized experimental particles (Fig. 1 A and B). Consistent with the findings from the preliminary screen, these compounds dramatically increased the rate of proteolysis of a particle-restricted albumin-based substrate following phagocytosis by resting WT BMMØs (Fig. 1 C and D), but did not increase rates of phagosomal proteolysis in Cybb−/− BMMØs. Moreover, the inherent rate of phagosomal proteolysis observed in Cybb−/− BMMØs was greater than 3-fold higher (3.9 ± 0.26) than that observed in WT BMMØs. Whole-cell lysates from WT and Cybb−/− BMMØs had equivalent levels of proteolytic activity, indicating that the observed inhibition of proteolysis in WT macrophages is locally effected (Fig. S2). Consistent with the induction of NOX2 following classical macrophage activation, IFN gamma (IFNγ) treatment exaggerated the disparity of phagosomal proteolysis between WT and Cybb−/− BMMØs (6.4 ± 0.69 -fold difference) (Fig. 1 E and F). Similar profiles were generated in IFNγ-activated macrophages that were deficient in inducible nitric oxide synthase (iNOS), indicating that the induction of iNOS does not contribute to the observed phenotype (Fig. S3). These findings demonstrate that the generation of oxidative radicals by NOX2 significantly decreases the local proteolytic efficiency of the maturing phagosome in macrophages.
Fig. 1.
Reactive oxygen species generation by phagosomal NOX2 decreases levels of phagosomal proteolysis in macrophages. Oxidative burst and bulk proteolytic activity within macrophage phagosomes were evaluated following phagocytosis of fluorescently labeled, IgG-coupled experimental particles. BMMØs were treated with 0.5 μM DPI, 10 μM THF, 25 μM quercetin (QUE), or DMSO alone for 1 h before phagocytic uptake. (A and B) Phagosomal oxidative burst was assessed by measurement of fluorescence liberated by oxidation of particle-associated H2HFF-OxyBURST substrate relative to calibration fluorescence in IFNγ-activated BMMØs. Phagosomal bulk proteolysis was assessed by measurement of fluorescence liberated through hydrolysis of particle-associated DQ-albumin relative to calibration fluorescence in resting (C and D) and IFNγ-activated (E and F) BMMØs. (A, C, and E) Real-time representative traces. Relative fluorescent units (RFU) are proportional to the degree of substrate oxidation/hydrolysis. (B, D, and F) Averaged rates/activities relative to DMSO-treated WT samples. Rates/activities were determined through calculation of the gradient of the linear portion of the real-time trace (as described by y = mx + c, where y = relative fluorescence, m = gradient, and x = time) relative to DMSO-treated WT samples. (D and F) Graphs represent averaged data from three independent experiments. Error bars denote SEM. P values were determined by one-way analysis of variance (ANOVA).
NOX2-Mediated Inhibition of Phagosomal Proteolysis in Macrophages Is Independent of Lumenal pH.
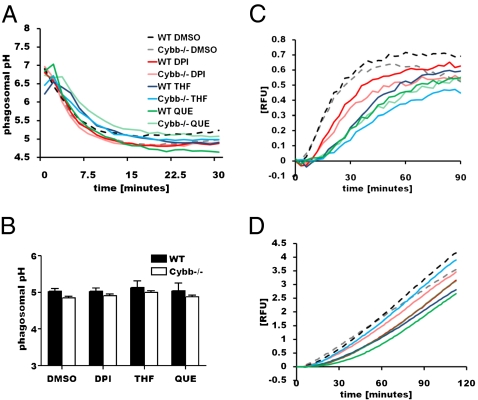
Amigorena and colleagues have previously reported that NOX2 activity in DCs leads to alkalinization of the phagosome which, in turn, decreases antigen destruction by the acidic lysosomal proteases (6, 16). To investigate whether NOX2-mediated perturbation of phagosomal acidification could account for the reduction in proteolytic efficiency in macrophages, we dynamically measured the phagosomal pH in the presence and absence of an oxidative burst. To achieve this, we followed the acidification of phagosomes containing an IgG-opsonized particle bearing a pH-sensitive fluorochrome in both WT and Cybb−/− BMMØs (19, 20). We found that rates and extents of phagosomal acidification were largely unaffected by the generation of an oxidative burst (Fig. 2A). These findings were consistent in macrophages classically activated with IFNγ (Fig. S4A). We did, how-ever, observe a small, but not statistically significant, difference in the final phagosomal pH of −0.18 (±0.12) and −0.13 (±0.14) between WT and Cybb−/− genotypes in resting and IFNγ-activated BMMØs, respectively (Fig. 2B and Fig. S4B). Although this modest alkalinization of the phagosome by NOX2 would unlikely effect a 3- to 6-fold decrease in proteolysis, we regressed the pH values against a pH/proteolysis curve generated in vitro using total lysosomal extract from BMMØs in buffers of known pH (Fig. S4C). Through regression analysis, we calculated that an alkalinization of 0.13–0.18 pH units, as mediated by NOX2, would theoretically increase the proteolytic efficiency of total lysosomal proteases by 18.2–28.4% (±35.5–37.0%). Together, these data suggest that phagosomal acidification is not significantly affected by NOX2 activity and is inconsistent with a mechanism of inhibition of phagosomal proteolysis in macrophages.
Fig. 2.
NOX2 activity does not affect phagosomal acidification, phagosome-lysosome communication, or phagosomal β-galactosidase activity in macrophages. (A and B) Phagosomal pH following phagocytosis was calculated using excitation ratio fluorometry of the pH-sensitive carboxyfluorescein on IgG-coupled beads followed by regression to a standard curve. (A) Representative acidification profiles in resting BMMØs. (B) Final lumenal pH at 30 min postinternalization in resting BMMØs from four independent experiments. Error bars represent SEM. (C) Profile of phagosome-lysosome communication in real time using FRET efficiency between a particle-restricted donor fluor and a fluid-phase lysosomal acceptor fluor. Relative fluorescent units (RFU) correlate to the concentration of lysosomal constituents within the phagosome at a given point in time. (D) Profile of β-galactoside hydrolysis in phagosomes in real time.
NOX2-Mediated Inhibition of Phagosomal Proteolysis Is Not Mediated by Changes to Phagosome-Lysosome Communication.
To determine whether the observed inhibition of proteolysis was mediated by a reduction of lysosomal hydrolase delivery to the maturing phagosome during NOX2 activity, we employed a fluorescence resonance energy transfer (FRET) -based assay to quantify phagosome-lysosome communication. This assay quantifies the accumulation of preformed lysosomal constituents within the maturing phagosome in real time by exploiting FRET between an acceptor fluor that has been chased into lysosomes and donor fluor restricted to an IgG-opsonized bead (19, 21). The profiles generated in WT and Cybb−/− BMMØs demonstrate that the rate and extent of lysosomal contribution to the phagosomal content is unchanged by NOX2 (Fig. 2C). Treatment with DPI and particularly quercetin and THF reduced the onset and rate of delivery of lysosomal constituents to the phagosome equally in WT and Cybb−/− BMMØs. The mechanisms by which quercetin and THF delay and reduce lysosomal contribution to the maturing phagosome are unknown. This observation, however, accounts for the incomplete restoration of phagosomal proteolysis by these compounds due to their “off-target” effects on phagosome-lysosome fusion (Fig. 1 D and F). To further rule out a possible NOX2-mediated reduction of lysosomal hydrolase delivery to the phagosome, we measured the acquisition of the lysosomally derived β-galactosidase activity to the maturing phagosome (Fig. 2D). Consistent with the FRET lysosomal contribution profiles, acquisition of β-galactosidase activity in the phagosome was unchanged by NOX2. Together, these observations indicate that the decreased proteolytic efficiency of the phagosome mediated by NOX2 is not a result of compromised phagosome-lysosome communication.
NOX2 Activity Inhibits the Catalytic Activities of Cysteine Cathepsins but Not Aspartic Cathepsins.
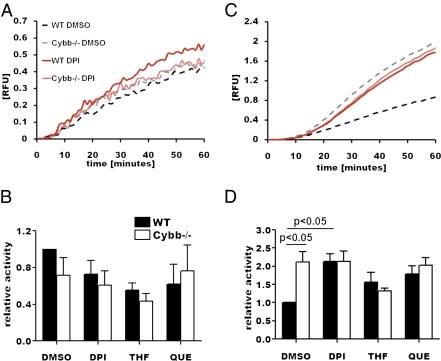
As NOX2 activity does not grossly disrupt lysosomal delivery to the phagosome, its effect on levels of phagosomal proteolysis may result from modulation of the catalytic activities of local proteases. If so, NOX2 activity would most likely disproportionally affect different classes of proteases. We thus evaluated the effect of NOX2 activity on the hydrolysis of fluorogenic substrates specific for cathepsin D/E (aspartic proteases) and cathepsin B/L (cysteine proteases) bound to IgG-opsonized beads following phagocytosis. The relative rates of hydrolysis of the cathepsin D/E substrate were statistically similar in the presence or absence of an oxidative burst (Fig. 3 A and B). In contrast, the rates of hydrolysis of the cathepsin B/L substrate were significantly reduced with NOX2 activity (Fig. 3 C and D). Consistent with the rates of bulk proteolysis, preactivation of the macrophages with IFNγ further reduced cysteine protease activity in WT, relative to Cybb−/− and NOX2-inhibited cells (Fig. S5 E and F). Of interest, quercetin and THF delayed and initially reduced cysteine cathepsin activity in a NOX2-independent fashion with respect to Cybb−/− samples (Fig. S5). This can be partially explained by their similar effect on lysosomal contribution to the maturing phagosome (Fig. 2 C and D), though was less apparent for the aspartic cathepsins. Western blot analysis of magnetically isolated phagosomes showed that cathepsins B and L were delivered to NOX2-proficient and NOX2-compromised phagosomes equally, demonstrating that NOX2 does not modulate the recruitment of these hydrolases (Fig. S6). Together, these data demonstrate that the oxidative burst in phagosomes affects the catalytic activity of phagosomally located cysteine cathepsins but not that of aspartic cathepsins.
Fig. 3.
NOX2 activity negatively regulates cysteine but not aspartic cathepsin activity in the phagosome. Relative activities of phagosomal proteases were evaluated using cathepsin D/E- and B/L-specific fluorogenic peptides bound to IgG-coupled experimental particles in the presence or absence of NOX2 activity. Phagosomal cathepsin D/E (aspartic cathepsins) (A and B) and cathepsin B/L (cysteine cathepsins) (C and D) activities in resting BMMØs. (A and C) Real-time representative traces. (B and D) Averaged rates between 15 and 40 min postinternalization, relative to DMSO-treated WT samples, from three independent experiments. Error bars represent SEM. P values were determined by ANOVA.
NOX2 Activity Decreases Proteolytic Efficiency of the Phagosome Through Reversible Oxidation of Cysteine Cathepsins.
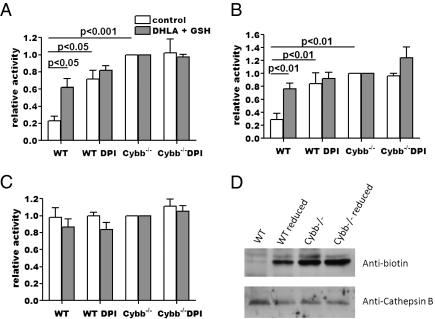
The active-site cysteine in the catalytic triad of cysteine proteases must be in its thiol form to enter the catalytic cycle (22). One conceivable mechanism of cysteine proteinase inhibition by NOX2 would be the reversible or irreversible oxidation of this residue, rendering the protease inactive. This could be mediated directly by NOX2-generated products, or indirectly through modulation of the local redox potential or reductive machinery of the phagosome. To evaluate the validity of such a mechanism, cysteine protease activities in isolated phagosomes were measured in vitro immediately after isolation or following reduction with dihydrolipoic acid (DHLA) and/or reduced glutathione (GSH). Both DHLA and GSH have been previously shown to reduce the active-site cysteine in cysteine cathepsins from several reversible sulfur oxidation products in a non-enzymatic fashion (12, 23, 24). Phagosomes were isolated from WT and Cybb−/− BMMØs 30 min after FcR-mediated particle uptake in the presence or absence of 0.5 μM DPI. Purified phagosomes were enumerated and standardized among samples and permeabilized, and relative peptidase activities were fluorometrically measured in vitro. Consistent with our previous findings, phagosomes isolated from untreated WT BMMØs degraded cathepsin B/L and cathepsin S substrates at significantly lower rates than those isolated from Cybb−/− BMMØs or DPI-treated BMMØs (Fig. 4 A and B). This further demonstrates that the NOX2-mediated decrease in proteolysis is independent of phagosomal pH and that it is also independent of NOX2-mediated modification of phagocytosed substrates. Additionally, as seen in phagosomes of live macrophages, the cathepsin D activities of the isolated phagosomes were similar across all samples (Fig. 4C). Treatment of the freshly isolated phagosomes with DHLA and/or GSH significantly increased the cysteine protease activities of phagosomes from WT BMMØs, relative to phagosomes isolated from Cybb−/− BMMØs (Fig. 4 A and B). This represented a 2.69 (±0.16) and a 2.64 (±0.18) -fold increase in the catalytic efficiency of WT phagosomes following reduction, relative to Cybb−/− phagosomes, for cathepsin B/L and cathepsin S, respectively. These data demonstrate that NOX2 mediates a sustained inactivation of the phagosomal cysteine cathepsins which is predominantly reversible by non-enzymatic reduction. This was further validated by reaction of a biotinylated fluoromethyl ketone-based irreversible inhibitor of cathepsin B with isolated phagosomes before and after reduction with DHLA and GSH (Fig. 4D). Consistent with the fluorometric analysis, Western blot analysis showed that DHLA and GSH significantly increased the proportion of active cathepsin B in WT phagosomes but had little effect on cathepsin B from Cybb−/− phagosomes. Total processed cathepsin B was similar between samples, ruling out any significant DHLA- or GSH-mediated enhancement of proteolytic processing of the enzyme. Together, these data strongly suggest that NOX2 activity inactivates cysteine cathepsins through reversible oxidative modification of the enzymes.
Fig. 4.
NOX2 inactivates phagosomal cysteine cathepsins via a reversible oxidative modification. Aspartic and cysteine cathepsin activities of phagosomes isolated from WT and Cybb−/− BMMØs ± 0.5 μM DPI were measured fluorometrically in vitro, with or without reduction by 1 μM DHLA and 30 mM GSH. (A–C) Relative activities were determined by the rate of increase in fluorescence of cathepsin-specific fluorogenic substrates at 37 °C, pH 5.5 and expressed relative to the corresponding Cybb−/− samples. (A) Cathepsin B (cysteine cathepsin); (B) cathepsin S (cysteine cathepsin); (C) cathepsin D/E (aspartic cathepsins). Graphs represent data from three independent experiments. Error bars denote SEM. P values were determined by ANOVA. (D) Relative proportions of active cathepsin B in phagosomes isolated from WT and Cybb−/− BMMØs were determined by reaction with the cathepsin B-specific biotinylated irreversible inhibitor biotin-FA-FMK with or without reduction by DHLA and GSH. Western blot images depict active (biotinylated) and total cathepsin B.
NOX2 Activity Depresses the Reductive Capacity of the Phagosome.
Whereas some sulfhydral proteases can function in a nonreducing environment, others, such as cathepsin B, spontaneously lose activity when removed from reducing environments through oxidation of their active-site cysteine (12, 25, 26). Because we found that the states of oxidative inactivation of cysteine cathepsins are largely reversible, we reasoned that NOX2 activity may act to indirectly inactivate cysteine cathepsins through perturbation of the reductive capacity of the phagosome necessary to maintain these proteases in their reduced, active state. To dynamically record the reductive capacity of the phagosome in live macrophages, we chemically modified a fluorogenic cystine-based reagent used by Cresswell and co-workers so it could be covalently coupled to albumin on an IgG-opsonized experimental particle (Fig. S7) (27). Within the phagosome, reduction of the particle-restricted cystine disulfide dequenches attached Bodipy FL fluorophores, liberating fluorescence relative to a calibration fluor. Hence, this assay reports directly on the capacity of the phagosomes to reduce mixed disulfides, such as those responsible for the reversible oxidative inactivation of cysteine cathepsins. After validation of this assay in vitro, we recorded the rates of disulfide reduction within phagosomes in WT and Cybb−/− BMMØs with and without treatment with DPI, THF, or quercetin. The resulting traces revealed a pattern of phagosomal disulfide reduction that begins within 10 min of phagosomal formation and continues beyond 2 h, at which point the assay becomes substrate-limited (Fig. 5A and Fig. S7B). Strikingly, in the absence of NOX2 activity, rates of disulfide reduction were significantly enhanced, as illustrated by Cybb−/− BMMØs displaying a 3.5-fold (±0.46) increase over WT BMMØs (Fig. 5 A and B). Consistent with a NOX2-mediated inhibition of phagosomal reductive capacity, activation with IFNγ completely abolished the ability of the early phagosome to reduce disulfides in WT BMMØs, whereas Cybb−/− BMMØs maintained robust reductive capacities (Fig. 5 C and D). Similarly, phagosomes with an intact oxidative burst were unable to efficiently dissociate the disulfide-linked heavy and light chains of IgG in IFNγ-activated BMMØs (Fig. S8). Both quercetin and THF partially restored the reductive capacity of the phagosome in WT BMMØs but paradoxically decreased the rates of reduction in Cybb−/− BMMØs. This NOX2-independent effect of these compounds could contribute to the NOX2-independent delay in cysteine cathepsin activity in the presence of quercetin and THF (Fig. S5). Together, these data demonstrate that the reductive capacity of the phagosome is compromised by NOX2 activity, which is consistent with a mechanism of reversible oxidative inactivation of phagosomal cysteine cathepsins.
Fig. 5.
NOX2 activity diminishes the reductive capacity of the phagosome. Phagosomal reductive capacity was assessed by measurement of fluorescence liberated through reduction of a modified fluorogenic cystine-based reagent covalently bound to IgG-coupled experimental particles in resting (A and B) and IFNγ-activated (C and D) BMMØs. (A and C) Real-time representative traces. (B and D) Averaged rates relative to DMSO-treated WT samples from three independent experiments. Error bars denote SEM. P values were determined by ANOVA.
NOX2 Activity Has a Sustained Effect on the Reductive and Proteolytic Capacities of the Phagosome.
A surprising feature of these functional relationships is that NOX2 activity has a sustained effect on the reductive and proteolytic capacities of the phagosome. Correlation of the timing of the oxidative burst, with respect to that of the relative rates of reduction within the phagosome, reveals that the NOX2-associated decrease in reductive capacity extends past the cessation of the oxidative burst (Fig. S9). Although there is a gradual increase in the rates of disulfide reduction after the conclusion of the burst (40 min), the more mature phagosome never attains a reductive capacity that is equivalent to NOX2-deficient phagosomes over the period recorded (2 h) (Fig. 5C). We reason that this could be due to a NOX2-mediated depletion of reductive equivalents in the early phagosome, which leaves a local “sink” of reductive potential energy at the conclusion of the burst. This sink would delay re-establishment of the reductive environment. Alternatively, similarly to that reported with DCs, a small proportion of NOX2 complexes may remain associated, and minimally active, in the mature phagosome of macrophages. A low level of association of NOX2 complexes with the mature phagosome may have negligible microbicidal effect but may have sufficient local activity to inhibit the re-establishment of a reductive lumenal environment. Either proposed mechanism could account for the sustainment of NOX2-mediated inhibition of disulfide reduction after the oxidative burst, which in turn would prolong oxidative inhibition of phagosomal proteolysis into the mature phagosome as observed (Fig. 1E). These findings implicate NOX2 in the modification of two functional parameters of the lumenal biology of the phagosome beyond its reported temporal association with the vacuole.
Concluding Remarks.
NOX2 is emerging as a global modulator of phagosomal physiology beyond its microbicidal function (6, 28). Here, through a non-hypothesis-based screen to identify phagosomal functional relationships, we found that NOX2 activity controls the level of phagosomal proteolysis in macrophages. We show that this is independent of lumenal pH and hydrolase recruitment to the phagosome, and that it specifically affects the activities of local cysteine proteinases. Finally, we demonstrate that this constitutes an additional level of control over phagosomal proteolysis which is mediated through the reversible oxidative inactivation of cysteine cathepsins by NOX2-dependent modulation of the redox potential of the phagosome.
In addition to NOX2, the redox control of phagosomal proteolysis highlights the importance of the reductive machinery of the endolysosomal system. γ-IFN-inducible thioreductase (GILT) has been shown to catalyze disulfide reduction of exogenous protein in the phagosomal lumen and to perform numerous biologically relevant functions in innate immunity and antigen presentation (26, 29–31). Although specific endogenous substrates of GILT have not been fully elucidated, it is conceivable that GILT contributes to the re-reduction of oxidized forms of cysteine cathepsins, which would restore their activity following an oxidative burst. This potential pathway would constitute an opposing reductive loop for the redox control of phagosomal machinery, and is supported by the mirrored up-regulation of GILT and NOX2 following macrophage exposure to IFNγ (32).
NOX2’s impact on the activities of certain protease subsets and on cargo disulfide reduction within the maturing phagosome of macrophages is likely to have significant functional implications for the efficiency and pattern of antigen processing within this compartment. With respect to levels of phagosomal proteolysis, based on several reports outlining its inverse relationship with the efficiency of antigen processing, NOX2-mediated diminution of overall rates of proteolysis in macrophages would likely enhance the generation and preservation of antigenic peptides (5, 7). Moreover, because many proteases prefer specific cleavage sites, NOX2-mediated oxidative inactivation of cysteine proteases, but not aspar-tic proteases, would change the pattern of proteolysis of a given antigen during, and following, an oxidative burst. With respect to NOX2-mediated inhibition of disulfide reduction, NOX2 activity would limit the intraphagosomal denaturation of antigens containing disulfide bonds. This could potentially “hide” vast stretches of an antigen's polypeptide, as well as limit the presentation of specific oligopeptides that contain a disulfide linkage in the native protein (30, 33). Together, NOX2’s control over proteolysis and disulfide reduction is anticipated to not only affect the efficiency of antigen processing in the macrophage but also the repertoire of the antigenic peptides generated and their order of immunodominance.
Materials and Methods
Mice and Cells.
C57BL/6 mice were purchased from Charles River Laboratories. Cybb−/− and iNOS−/− mice were purchased from Jackson Laboratories. BMMØs were derived from bone marrow as previously described (19).
Live-Cell Fluorometric Phagosomal Analysis.
Fluorescently labeled, IgG-coupled 3-μm silica particles were prepared as detailed (18, 20, 21) and used for phagosomal lumenal characterization in live BMMØs as previously described (19–21). Further details on specific fluorometric assays may be found in SI Materials and Methods.
Phagosome Isolation and in Vitro Determination of Cathepsin Activities.
Phagosomal isolation for detection and activity measurement of specific cathepsins by Western blotting and fluorometry was achieved through magnetic-assisted isolation of phagosomes as previously described (34).
Further details of materials and methods are supplied in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Yan Shi, Dr. Amy Warren, and Dr. Arvi Rauk, University of Calgary, for critical reading of the manuscript. This work was supported by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and Alberta Innovates.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914867107/-/DCSupplemental.
References
- 1.Desjardins M, Griffiths G. Phagocytosis: Latex leads the way. Curr Opin Cell Biol. 2003;15:498–503. doi: 10.1016/s0955-0674(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 2.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: Aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: Dynamic assays of phagosome function. Nat Rev Immunol. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 5.Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med. 2006;203:2049–2055. doi: 10.1084/jem.20052442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 8.Lennon-Duménil AM, et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. 2002;196:529–540. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Swanson J. The endocytic activity of dendritic cells. J Exp Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unanue ER. Perspective on antigen processing and presentation. Immunol Rev. 2002;185:86–102. doi: 10.1034/j.1600-065x.2002.18510.x. [DOI] [PubMed] [Google Scholar]
- 11.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood TD. Redox control of protein degradation. Antioxid Redox Signal. 2000;2:851–878. doi: 10.1089/ars.2000.2.4-851. [DOI] [PubMed] [Google Scholar]
- 13.Pillay CS, Elliott E, Dennison C. Endolysosomal proteolysis and its regulation. Biochem J. 2002;363:417–429. doi: 10.1042/0264-6021:3630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox D, Mason RW. Inhibition of cysteine proteinases in lysosomes and whole cells. Biochem J. 1992;285:495–502. doi: 10.1042/bj2850495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savina A, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Mantegazza AR, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanderVen BC, Yates RM, Russell DG. Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic. 2009;10:372–378. doi: 10.1111/j.1600-0854.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6:413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Yates RM, Russell DG. Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol. 2008;445:311–325. doi: 10.1007/978-1-59745-157-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yates RM, Hermetter A, Russell DG. Recording phagosome maturation through the real-time, spectrofluorometric measurement of hydrolytic activities. Methods Mol Biol. 2009;531:157–171. doi: 10.1007/978-1-59745-396-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschke H, Barrett A, Rawlings N. In: Lysosomal Cysteine Proteases. Sheterline P, editor. New York: Oxford Univ Press; 1998. [Google Scholar]
- 23.Lockwood TD. Cathepsin B responsiveness to glutathione and lipoic acid redox. Antioxid Redox Signal. 2002;4:681–691. doi: 10.1089/15230860260220193. [DOI] [PubMed] [Google Scholar]
- 24.Biaglow JE, et al. A method for measuring disulfide reduction by cultured mammalian cells: Relative contributions of glutathione-dependent and glutathione-independent mechanisms. Anal Biochem. 2000;281:77–86. doi: 10.1006/abio.2000.4533. [DOI] [PubMed] [Google Scholar]
- 25.Jordans S, et al. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009;10:23. doi: 10.1186/1471-2091-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maric M, et al. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 27.Lackman RL, Jamieson AM, Griffith JM, Geuze H, Cresswell P. Innate immune recognition triggers secretion of lysosomal enzymes by macrophages. Traffic. 2007;8:1179–1189. doi: 10.1111/j.1600-0854.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 29.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: Characterization of a γ-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci USA. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hastings KT, Lackman RL, Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol. 2006;177:8569–8577. doi: 10.4049/jimmunol.177.12.8569. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trost M, et al. The phagosomal proteome in interferon-γ-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Collins DS, Unanue ER, Harding CV. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991;147:4054–4059. [PubMed] [Google Scholar]
- 34.Ullrich HJ, Beatty WL, Russell DG. Direct delivery of procathepsin D to phagosomes: Implications for phagosome biogenesis and parasitism by Mycobacterium. Eur J Cell Biol. 1999;78:739–748. doi: 10.1016/S0171-9335(99)80042-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.