Abstract
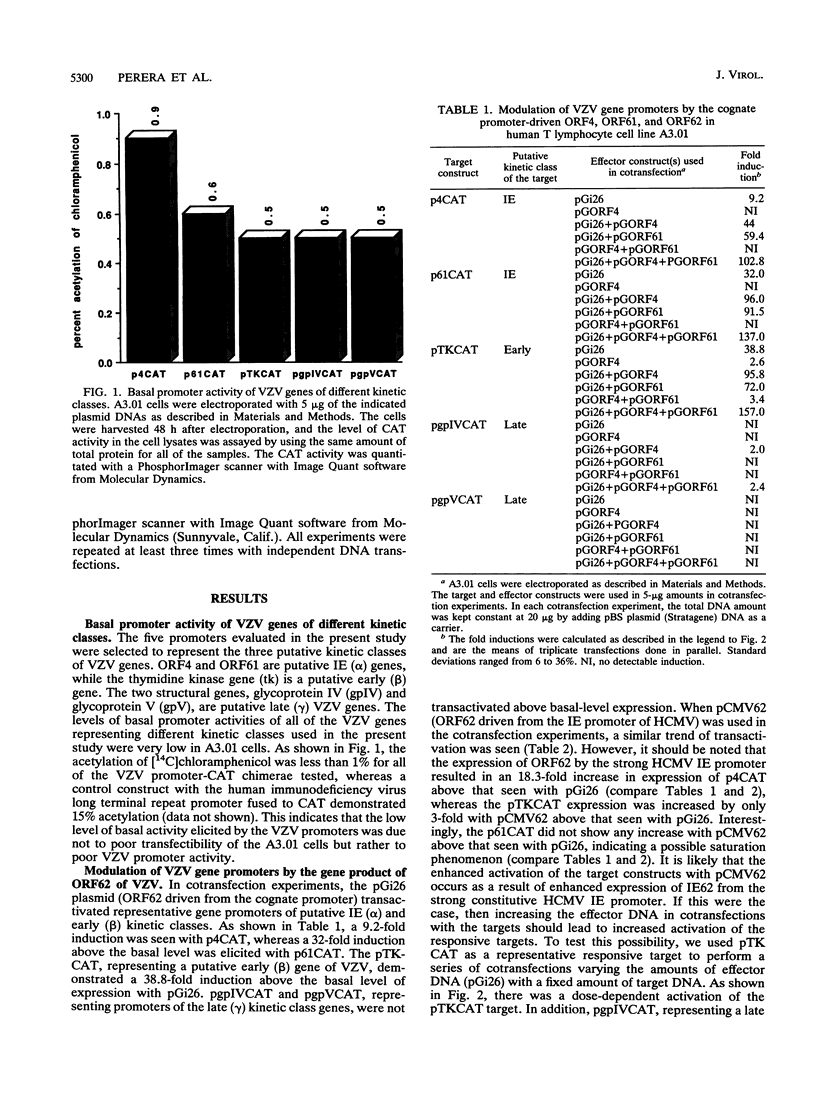
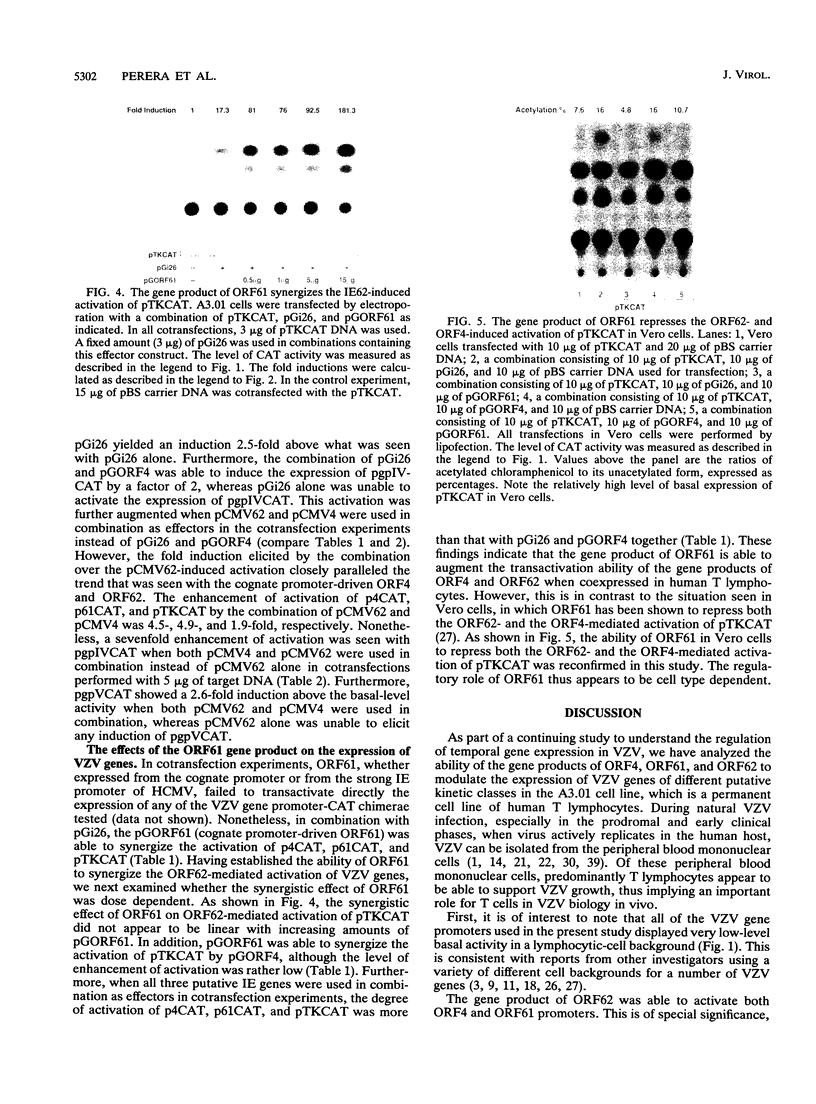
Varicella-zoster virus (VZV), a neurotropic alphaherpesvirus, is the etiologic agent of chicken pox and shingles (zoster) in humans. Using an in vitro transient expression assay, we have evaluated the ability of the putative immediate early VZV genes, ORF4, ORF61, and ORF62 (the analogs of the herpes simplex virus alpha 27, alpha 0, and alpha 4 genes, respectively), to modulate the expression of VZV genes of different putative kinetic classes in a human T lymphocyte cell line. These cells are of the type in which VZV can be readily detected in the viremic phase of human infection. We present evidence to indicate that, in this system, the gene product of ORF62 (IE62) is a major regulatory protein in VZV and is capable of activating VZV genes of all putative kinetic classes. In addition, we demonstrate that the gene product of ORF4 and, unlike the apparent situation in Vero cells, the gene product of ORF61 may play an accessory regulatory role in synergizing the activation of VZV genes induced by the gene product of ORF62 in human T lymphocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Itakura N., Kajita Y., Suga S., Yoshikawa T., Yazaki T., Ozaki T., Yamanishi K., Takahashi M. Severity of viremia and clinical findings in children with varicella. J Infect Dis. 1990 Jun;161(6):1095–1098. doi: 10.1093/infdis/161.6.1095. [DOI] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G. F., Mahalingam R., Wellish M., Gilden D. H. Trans-activation of viral tk promoters by proteins encoded by varicella zoster virus open reading frames 61 and 62. Virus Res. 1990 Jan;15(1):57–68. doi: 10.1016/0168-1702(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Varicella-zoster virus. The Fourteenth Fleming lecture. J Gen Virol. 1991 Mar;72(Pt 3):475–486. doi: 10.1099/0022-1317-72-3-475. [DOI] [PubMed] [Google Scholar]
- Disney G. H., Everett R. D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990 Nov;71(Pt 11):2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- Disney G. H., McKee T. A., Preston C. M., Everett R. D. The product of varicella-zoster virus gene 62 autoregulates its own promoter. J Gen Virol. 1990 Dec;71(Pt 12):2999–3003. doi: 10.1099/0022-1317-71-12-2999. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci U S A. 1982 Jan;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D. H., Hayward A. R., Krupp J., Hunter-Laszlo M., Huff J. C., Vafai A. Varicella-zoster virus infection of human mononuclear cells. Virus Res. 1987 Apr;7(2):117–129. doi: 10.1016/0168-1702(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Inchauspe G., Ostrove J. M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989 Dec;173(2):710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Kinchington P. R., Hougland J. K., Arvin A. M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992 Jan;66(1):359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropchak C. M., Graham G., Palmer J., Winsberg M., Ting S. F., Wallace M., Prober C. G., Arvin A. M. Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis. 1991 May;163(5):1016–1022. doi: 10.1093/infdis/163.5.1016. [DOI] [PubMed] [Google Scholar]
- Koropchak C. M., Solem S. M., Diaz P. S., Arvin A. M. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989 May;63(5):2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P., Kinchington P. R., Ruyechan W. T., Hay J. A detailed analysis of transcripts mapping to varicella zoster virus gene 14 (glycoprotein V). Virology. 1991 Oct;184(2):625–635. doi: 10.1016/0042-6822(91)90432-b. [DOI] [PubMed] [Google Scholar]
- Maguire H. F., Hyman R. W. Polyadenylated, cytoplasmic transcripts of varicella-zoster virus. Intervirology. 1986;26(4):181–191. doi: 10.1159/000149700. [DOI] [PubMed] [Google Scholar]
- McKee T. A., Disney G. H., Everett R. D., Preston C. M. Control of expression of the varicella-zoster virus major immediate early gene. J Gen Virol. 1990 Apr;71(Pt 4):897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- Nagpal S., Ostrove J. M. Characterization of a potent varicella-zoster virus-encoded trans-repressor. J Virol. 1991 Oct;65(10):5289–5296. doi: 10.1128/jvi.65.10.5289-5296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrove J. M. Molecular biology of varicella zoster virus. Adv Virus Res. 1990;38:45–98. doi: 10.1016/s0065-3527(08)60859-3. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Reinhold W., Fan C. M., Zorn S., Hay J., Straus S. E. Transcription mapping of the varicella-zoster virus genome. J Virol. 1985 Nov;56(2):600–606. doi: 10.1128/jvi.56.2.600-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Ichikawa T., Matsui Y., Kondo H., Nagai T., Asano Y., Yamanishi K., Takahashi M. Lymphocyte-associated viremia in varicella. J Med Virol. 1986 Jul;19(3):249–253. doi: 10.1002/jmv.1890190307. [DOI] [PubMed] [Google Scholar]
- Pellett P. E., McKnight J. L., Jenkins F. J., Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Samuel J. E., Holmes R. K., O'Brien A. D. Identification of three amino acid residues in the B subunit of Shiga toxin and Shiga-like toxin type II that are essential for holotoxin activity. J Bacteriol. 1991 Feb;173(3):1151–1160. doi: 10.1128/jb.173.3.1151-1160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Reinhold W. C., Straus S. E., Ostrove J. M. Directionality and further mapping of varicella zoster virus transcripts. Virus Res. 1988 Feb;9(2-3):249–261. doi: 10.1016/0168-1702(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Hyman R. W. The immediate early proteins of varicella-zoster virus. Virology. 1987 Feb;156(2):423–426. doi: 10.1016/0042-6822(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsover A., Leventon-Kriss S., Langer A., Smetana Z., Zaizov R., Potaznick D., Cohen I. J., Gotlieb-Stematsky T. Detection of varicella-zoster virus in lymphocytes by DNA hybridization. J Med Virol. 1987 Jan;21(1):57–66. doi: 10.1002/jmv.1890210108. [DOI] [PubMed] [Google Scholar]







