Abstract
Seabirds represent a well documented biological transport pathway of nutrients from the ocean to the land by nesting in colonies and providing organic subsidies (feces, carcasses, dropped food) to these sites. We investigated whether seabirds that feed at different trophic levels vary in their potency as biovectors of metals, which can bioaccumulate through the marine foodweb. Our study site, located on a small island in Arctic Canada, contains the unique scenario of two nearby ponds, one of which receives inputs almost exclusively from upper trophic level piscivores (Arctic terns, Sterna paradisaea) and the other mainly from lower trophic level molluscivores (common eiders, Somateria mollissima). We used dated sediment cores to compare differences in diatoms, metal concentrations and also stable isotopes of nitrogen (δ15N), which reflect trophic position. We show that the seabirds carry species-specific mixtures of metals that are ultimately shunted to their nesting sites. For example, sediments from the tern-affected pond recorded the highest levels of δ15N and the greatest concentrations of metals that are known to bioaccumulate, including Hg and Cd. In contrast, the core from the eider-affected site registered lower δ15N values, but higher concentrations of Pb, Al, and Mn. These metals have been recorded at their greatest concentrations in eiders relative to other seabirds, including Arctic terns. These data indicate that metals may be used to track seabird population dynamics, and that some metal tracers may even be species-specific. The predominance of large seabird colonies on every continent suggests that similar processes are operating along coastlines worldwide.
Keywords: biological transport, paleolimnology, Arctic terns, common eiders, bioaccumulation
Seabirds often carry elevated contaminant loads as a result of biomagnification and bioaccumulation through the marine foodweb (1). As a result of their gregarious nature and propensity to form large breeding colonies, seabirds can create localized “hotspots” of contamination by shunting marine-derived contaminants from the ocean to the land via their guano and mortality (2). This biological transport pathway can lead to contaminant concentrations that far surpass those conducted by abiotic processes alone (e.g., winds, ocean currents) and, in some instances, reach toxic levels. For example, Brimble et al. (3) studied several ponds located near a large colony of northern fulmars (Fulmarus glacialis) on Devon Island, Nunavut, in High Arctic Canada, and recorded sedimentary metal concentrations that exceeded Canadian environmental guidelines for protecting wildlife. Likewise, in the Norwegian Arctic, Evenset et al. (4) recorded higher than background concentrations of persistent organic pollutants (POPs) in aquatic organisms from a seabird-affected lake, providing evidence that seabird-transported contaminants were entering local foodwebs. Similar results have been recorded for Antarctic penguin populations (5, 6).
The contaminant burden of seabirds is influenced by a variety of factors including physiology, metabolism, organism age, stage of annual cycle, and diet (7), with organisms that occupy the highest trophic levels generally containing the greatest concentrations. Evenset et al. (8) measured polychlorinated biphenyl (PCB) concentrations in guano samples from three seabird species representing a range of trophic levels, including little auks (Alle alle), black-legged kittiwakes (Rissa tridactyla), and glaucous gulls (Larus hyperboreus), and recorded order of magnitude increases with each trophic level increase. Mallory et al. (9) showed that top predators, such as glaucous gulls and northern fulmars, had higher hepatic concentrations of bioaccumulating metals, including As, Cd, and Hg, relative to common eiders (Somateria mollissima), which feed lower in the marine food chain. These data suggest that, depending on trophic position, seabirds will vary in their efficacy as contaminant biovectors.
Here, we explore whether seabird species that feed at different trophic levels differentially affect sedimentary metal burdens in Arctic ponds via biovector transport. We studied two nearby ponds (approximately 1 km apart; Fig. 1) in the Canadian High Arctic, one of which is affected primarily by Arctic terns (Sterna paradisaea), and the other mainly by common eiders. Arctic terns feed predominantly on nearshore fish, and thus occupy a higher trophic level than eiders, which feed mainly on marine benthic mollusks (10). The different positions of terns and eiders on the marine foodweb should result in different concentrations of bioaccumulating metals, and other contaminants, in the tissues and waste products of these seabirds. We tracked these differences by measuring metal concentrations in dated sediment cores from the tern- and eider-affected sites (hereafter referred to as Tern Pond and Eider Pond, respectively).
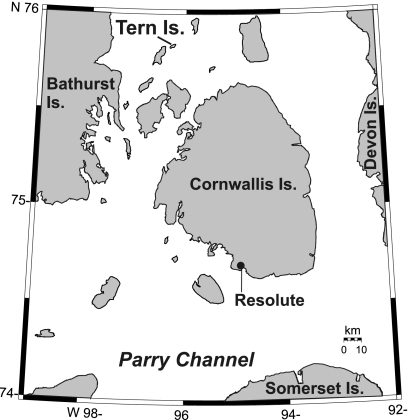
Fig. 1.
Map showing the location of Tern Island in the Canadian High Arctic.
The unique set of circumstances that led to the segregation of the tern and eider seabird colonies into separate drainage basins of the two study ponds has created a rare situation that allows for a quasi-experimental approach to paleolimnology. The closeness of the two ponds (only approximately 1 km apart) and near identical elevations (within approximately 15 m above sea level of each other) negates variability related to site-specific differences such as local climate effects, geology, and atmospheric deposition. This allowed us to make more definitive conclusions on metal contributions from different seabird species. In addition to the metal analyses, we measured the sediment cores for stable isotope ratios of nitrogen (δ15N), a proxy that can be used to track marine-derived nutrients and characterize trophic structure. We also ex-plored the influence of seabird-derived nutrient inputs on pond limnology and ecology by examining water chemistry and fossil diatom (Bacillariophyceae) assemblages.
Results and Discussion
Nutrient subsidies, in the form of seabird guano, delivered to the study ponds have resulted in production-related variables that are among the highest recorded in Arctic regions (Table 1; see Table S1 for full suite of limnological variables). The elevated total phosphorus unfiltered (TP) concentrations recorded in the study ponds are atypical (Table 1), especially in nutrient-poor Arctic regions where ultra-oligotrophic freshwaters are the norm. In fact, TP concentrations in the study ponds are several-fold greater than values typically recorded in High Arctic lakes and ponds (Table 2). The only TP concentrations recorded in the Arctic that are comparable to the Tern Island sites are from ponds that are similarly affected by ornithogenic inputs (11, 12), and sewage oxidation ponds used for facultative treatment of domestic human wastes (13, 14).
Table 1.
Selected physical, biological, and chemical variables from the Tern Island study ponds
| Variable | Tern | Eider |
| pH | 10.4 | 8.7 |
| Temp, °C | 9 | 6 |
| Cond, μS cm−1 | 366 | 206 |
| TN, mg L−1 | 4.82 | 0.845 |
| TP, μg L−1 | 97.3 | 33.3 |
| TP (filt.), μg L−1 | 57.3 | 18.8 |
| Chl a, μg L−1 | 1.7 | 0.6 |
| DIC, mg L−1 | 11.8 | 18.9 |
| DOC, mg L−1 | 39 | 8.6 |
| POC, mg L−1 | 2.52 | 0.792 |
| As, μg L−1 | 2.79 | 0.84 |
| Cd, μg L−1 | 0.046 | 0.012 |
TN, total nitrogen; TP (filt.), total phosphorus filtered; POC, particulate organic carbon. The full suite of water chemistry variables, including all metals is reported in Table S1. All water chemistry data are from one sampling event on July 25, 2008.
Table 2.
Summary statistics for TP, DOC, and lakewater pH for more than 400 lakes and ponds in the Canadian High Arctic
| Statistic | TP (n = 404), μg L−1 | DOC (n = 404), mg L−1 | pH (n = 407) |
| Mean | 17.9 | 4.84 | 8.1 |
| SD | 79.3 | 5.4 | 0.6 |
| Median | 8.6 | 3 | 8.2 |
| Mode | 3.3 | 1.8 | 8.3 |
| Maximum | 1520 | 40.1 | 9 |
| Minimum | 0.1 | 0.2 | 3.6 |
Data are from more than 400 lakes and ponds in the Canadian High Arctic collected by our laboratories (53). Note that some of our Arctic sites recorded erroneously high TP lakewater values as a result of resuspended particulate matter (54), which is not bioavailable for uptake by organisms. When these sites are removed, the mean TP concentration decreases to <10 μg L−1.
Lakewater pH, especially in the tern-affected site, is also high relative to seabird-free sites in the Canadian Arctic (Table 2). The elevated pH levels are a likely result of increased photosynthetic drawdown of limnetic CO2 (thereby increasing lakewater pH), driven by the elevated nutrient concentrations. Sedimentary δ13C values provide additional information on aquatic production. Increased delivery of nutrients from seabird inputs enhances algal productivity, which draws down the supply of limnetic dissolved inorganic carbon (DIC) and decreases algal discrimination in favor of 12C and ultimately results in an enrichment of δ13C values in the sediment (15). The mean δ13C value in the Tern Pond sediment core is −20.09 ± 1.48 (Table S2), whereas in the Eider Pond core it is −21.7 ± 0.76 (Table S3). Although these values are not as high as recorded in sediment from other seabird-affected ponds (16), they are higher than in Arctic lake sediments unaffected by marine nutrient inputs (17).
The seabird-derived nutrients have also boosted terrestrial production as evidenced by the abundant growth of grasses and mosses in the catchments of the study ponds (Fig. S1). Catchment vegetation can influence limnological variables, especially dissolved organic carbon (DOC), which increases in heavily vegetated catchments. DOC concentrations, especially in Tern Pond, are high for Arctic regions (Table 2), even in comparison with other seabird-affected sites (12).
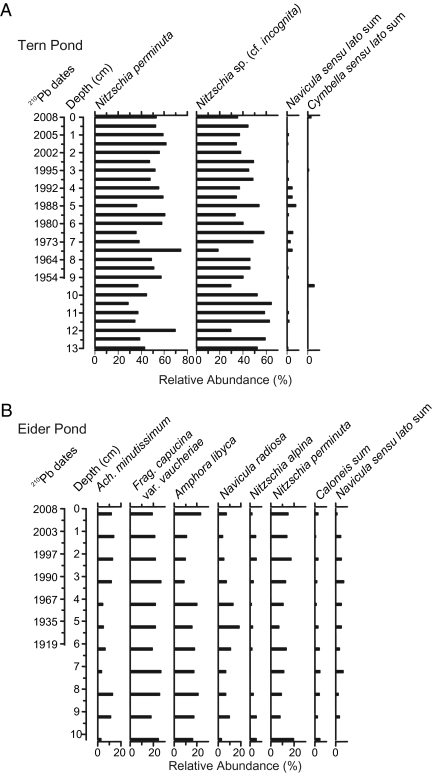
The fossil diatom assemblages recorded in the Tern Island study ponds have been documented elsewhere in the Canadian High Arctic (18), but rarely in such high relative abundances. In the sediment core from Tern Pond, Nitzschia perminuta typically represented more than 50% of the entire diatom assemblage, and was the only dominant species that was common to both study ponds (Fig. 2). Although this taxon is ubiquitous in Canadian High Arctic freshwaters (18), and thus its mere presence does not denote eutrophication, it typically does exceedingly well in sites with high nutrient levels and elevated lakewater pH. For example, N. perminuta is one of the most abundant species recorded in seabird-affected ponds at Cape Vera on Devon Island (12). Likewise, N. perminuta was the dominant taxon recorded in periphyton samples from Meretta Lake on Cornwallis Island, Nunavut; a lake that had received human sewage effluent for more than 40 years. When sewage inputs into Meretta Lake declined and nutrient levels decreased, so too did the relative abundances of N. perminuta (19). The greater relative abundances of N. perminuta in the Tern Pond core compared with the Eider Pond core (Fig. 2) probably reflect the higher nutrient concentrations in the tern-affected site (Table 1).
Fig. 2.
Fossil diatom profiles of the most common taxa from Tern Pond (A) and Eider Pond (B).
The sediment core from Eider Pond contains many diatom taxa, in high relative abundances, that are indicative of elevated nutrient and pH levels (Fig. 2). For example, Fragilaria capucina var. vaucheriae is known to be tolerant of organic pollution and domestic sewage (20). Achnanthidium minutissimum, although often considered a generalist, occurs more commonly in Arctic lakes and ponds with elevated nitrogen concentrations (21). Amphora libyca often occurs in sites with high pH and conductivity, whereas Navicula radiosa appears to increase with elevated nutrient concentrations (22). The presence of eutrophic indicator taxa in both study sites reflects the marine-derived nutrients transported by the seabirds and demonstrates the central role of seabirds in structuring freshwater diatom assemblages on Tern Island. Furthermore, the relatively complacent profiles of eutrophic assemblages documented in both study ponds suggest that seabirds have been present on Tern Island for at least the time period encompassed by the sediment cores (approximately 100 y or more).
The diatoms, together with the limnological data, demonstrate that seabirds have had a marked effect on the water quality and ecological characteristics of the study ponds. However, isotope data are central to demonstrating trophic level differences between the two seabird species and whether they are differentially affecting the sedimentary metal burdens of their respective ponds. Stable isotopes of nitrogen (δ15N) are commonly employed to examine food web dynamics and trophic structure in ecosystems, as δ15N is typically enriched by 3 to 5 per mil (‰) with each trophic level increase (23). Other research, including paleolimnological studies, has also used δ15N to track marine-derived nutrients, and ultimately populations of the animals that transport the nitrogen (24, 25), as δ15N is enriched in marine organisms relative to nitrogen from terrestrial and atmospheric sources (26).
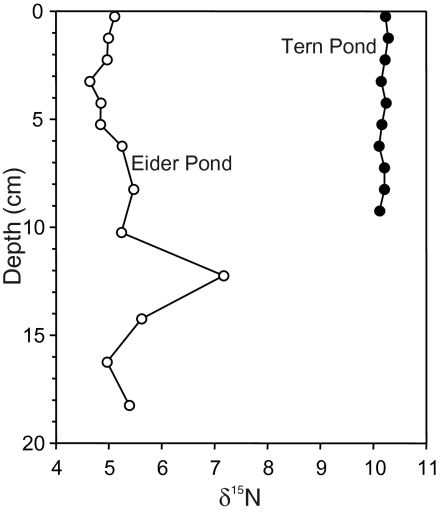
The δ15N values recorded in the Tern Island cores are higher than values commonly recorded in Arctic lakes with no marine influence, which typically range from 1 to 4‰ (Fig. 3; refs. 27 and 28). In fact, the only other sites with comparable δ15N values in Arctic freshwaters are from ponds that have received marine inputs from seabirds (29) and whale carcasses (27). The elevated δ15N values not only confirm the influx of marine-derived nutrients to the study ponds, but also appear to track differences in the trophic levels of the two seabirds. The Tern Pond core records δ15N values that are approximately 5‰ greater than in the Eider Pond core (Fig. 3), which is exactly what we would have predicted given the higher trophic level of Arctic terns relative to common eiders (23). Although there are no δ15N data for eider and tern feces, δ15N values recorded in early egg tissues taken from Tern Island show differences between the two species, with significantly (P < 0.05) greater δ15N values in terns (range, 13.9–17.3‰) relative to eiders (range, 13.3–14.9‰) (30). The higher δ15N values recorded in tern eggs is consistent with that species occupying a higher trophic position than eiders.
Fig. 3.
Sedimentary δ15N profiles from the Tern and Eider pond sediment cores.
The comparatively high δ15N values recorded in the sediment cores confirm the input of marine-derived nutrients and support inferences from the water chemistry and fossil diatom analyses that seabird inputs are structuring the limnological and biological characteristics of the ponds. Based on the relatively complacent fossil diatom profiles, we infer that seabirds have been present on Tern Island for at least the period encompassed by the sediment cores. This inference is strongly supported by the consistently high sedimentary δ15N values recorded in both ponds (Fig. 3). The use of δ15N profiles in pond sediment cores have been used to track shifts in seabird populations near Cape Vera on Devon Island, Nunavut (25), with increased δ15N values suggesting an increase in population, and vice versa. The relative stability and lack of any clear trends in the δ15N profiles from the study ponds suggests that seabird-derived N inputs (and presumably population numbers) on Tern Island have not fluctuated greatly during the past approximately 100 y.
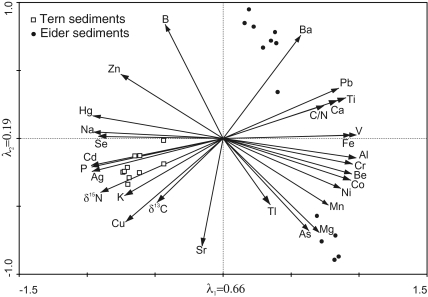
The successful application of δ15N in the study cores to track differences in seabird trophic structure suggests that there should be detectable differences in sedimentary metal concentrations, as many metals, whether from natural or anthropogenic sources, are known to bioaccumulate. In fact, the sediments from the two study ponds show a clear separation in the Principal Components Analysis (PCA) ordination, indicating distinct differences in metal composition between the two cores (Fig. 4). Variations in geology and atmospheric deposition are not likely responsible for the recorded differences in sedimentary metals because the two study sites are separated only by approximately 1 km and occupy similar geologic settings. Likewise, research from seabird-affected ponds on nearby Cape Vera (Devon Island, Nunavut), all of which occupy identical geological environments, has shown marked differences in sediment geochemistry depending on the degree of ornithogenic inputs (3, 12, 16, 29). Thus, we conclude that dietary and physiological differences between the common eiders and Arctic terns have led to variations in the quantity and type of metals that they accumulate and subsequently deposit.
Fig. 4.
PCA ordination showing patterns in metal concentrations between the two sediment cores collected from Tern and Eider Ponds.
Dietz et al. (31), Mallory et al. (10), and Campbell et al. (32) measured metal concentrations in tissues from several seabird species representing a range of trophic levels in Greenland and Canadian Arctic marine foodwebs. They recorded higher concentrations of bioaccumulating metals including total Hg, Cd, and As in top predators such as glaucous gulls and northern fulmars, as well as black guillemots (Cepphus grylle), the latter of which are analogous to Arctic terns in that they are primarily nearshore piscivores. This is consistent with our data, which show that the tern-affected sediments are characterized by higher concentrations of Hg and Cd, although As is greater in the Eider Pond sediments (Fig. 4).
Mallory et al. (10) recorded the highest concentrations of Pb, Mn, and Al in tissue samples from common eiders. Of particular note is that Pb was found in 75% of all eider livers, but not in any other birds. Accordingly, our data show that the higher Pb, Al, and Mn concentrations are associated with the eider-affected site (Fig. 4). The elevated levels of Pb, Mn, and Al in eiders relative to terns and other seabirds may be related to several factors, including greater concentrations of these trace elements at eider wintering sites or along migration routes. However, high concentrations of these same metals reported in marine waterfowl from other regions (33–35) suggest that dietary and/or physiological differences are the most likely mechanisms behind the differential accumulation.
The metal analyses on the Tern Island sediment cores reveal species-specific differences in metal biotransport, but they also provide information on which metals may be affecting pond biota, as sediments act as an important route of exposure for aquatic species. Cadmium is of particular interest as it has been shown to reach high concentrations in seabird-affected regions (3, 36), and has adverse biological effects on benthic organisms such as decreased invertebrate abundance, increased mortality, and behavioral changes (37). The surface sediment Cd concentrations recorded in our study ponds (0.71 and 4.4 μg g−1 for Eider and Tern ponds, respectively) are greater than the mean background concentration recorded in Canadian lake sediments of 0.32 μg g−1. In fact, both cores record Cd concentrations that exceed the Canadian sediment quality guidelines for the protection of aquatic life (38). In the case of Tern Pond, Cd concentrations exceed the “probable effects level” of 3.5 μg g−1, above which adverse biological effects frequently occur. The complete dataset of sedimentary metals, as well as stable isotopes of C and N, are reported for Tern and Eider Ponds in Tables S2 and S3, respectively.
The potential of biovectors to focus large quantities of metals to toxic concentrations necessitates their consideration in identifying environmental risks of bioaccumulating contaminants. At present, few contaminant models incorporate a biological transport component (2). The Arctic may be especially prone to biovector transport in part because of its relatively pristine ecosystems, but also because these regions host throngs of migratory animals every season, many of which form densely clustered populations, creating ideal situations for highly localized focusing of contaminants. Biological transport of metals and other contaminants is of global concern and seabirds are of particular importance as they are a dominant form of wildlife along coastlines worldwide. The nutrients deposited by seabird colonies create small oases of biological production near their nesting sites. The irony, of course, is that the contaminant hotspots created by seabird biotransport occur precisely where biological production is greatest.
Materials and Methods
The two study ponds are located less than 1 km from each other on Tern Island (unofficial name; 75°50′N, 96°20′W), a small (3 km2) island situated northwest of Cornwallis Island, Nunavut, Canada (Fig. 1). The nearest meteorological station at Resolute Bay (approximately 130 km away; Fig. 1) has recorded mean January and July temperatures of −32.4 °C and 4.3 °C, respectively, and mean annual precipitation of 150 mm (39). The catchments of both study ponds are uncharacteristically vegetated for the High Arctic (a result of the nutrient-rich guano inputs), with mosses and sedges surrounding their peripheries outwards to about 10 m. Snow covers the ground from October to May, during which time the ponds are frozen. The surrounding sea is ice-covered from October to July, except for small patches of open water (polynyas), which are important feeding areas for marine birds (40).
The pond that is influenced primarily by Arctic terns (Tern Pond) is located in the southern portion of the island and has approximate dimensions of 18 × 5 m, with a mean depth of approximately 30 cm (Fig. S1). Approximately 300 pairs of Arctic terns nest within the catchment of this pond, arriving in late June, and leaving with chicks in mid- to late August. Arctic terns are primarily piscivores, feeding on immature shoaling fish, but also feed on crustaceans, mollusks, and insects (41).
The pond that is influenced primarily by common eiders (Eider Pond) is located in the northern portion of the island. It is the larger of the two study ponds with approximate dimensions of 118 × 76 m, and a mean depth of approximately 60 cm (Fig. S1). A colony of approximately 50 to 100 eider hens nest within the catchment of this pond. Common eiders also arrive at the island in late June, and typically depart for the sea as soon as ducklings hatch, generally by early August. On the water, eiders habitually form dense groups, and many nonbreeding or failed-breeding birds also use the pond for bathing and social interactions during the breeding season. Common eiders occupy a lower position in the Arctic marine foodweb than Arctic terns, with a diet consisting predominantly of benthic mollusks, although crustaceans, echinoderms, and other marine invertebrates may also be consumed (42).
Sediment cores were recovered on July 25, 2008, from the centers of both study ponds by using 7.6-cm-diameter Lexan tubes that were pushed directly into the sediments. The core tubes were pushed until they met strong resistance, suggesting that in both study ponds the entire sedimentary record was retrieved. The cores were sectioned at 0.5 cm intervals on-site using a Glew (43) vertical extruder. Water samples (n = 1) were also taken from both study ponds following identical procedures to our laboratory’s previous Arctic limnological surveys originally outlined by Douglas and Smol (44). Water samples were sent to the National Laboratory for Environmental Testing in Burlington, ON, Canada, for analysis of major and minor ions, dissolved organic/inorganic carbon, metals, chlorophyll a, silica, and nutrients including phosphorus and nitrogen. Specific conductivity, temperature, and pH were measured on-site using a YSI model 33 conductivity meter, a thermometer, and a Hanna pHEP meter, respectively. Protocols for bottling, filtering and methods for chemical analyses followed Environment Canada (45, 46).
Sediment cores were dated using excess 210Pb activities (Fig. S2), as well as 137Cs, in an Ortec germanium crystal well detector following the procedures described in Schelske et al. (47) and Appleby (48). Core chronologies were developed using the constant rate of supply (CRS) model for Tern Pond and the constant initial concentration (CIC) model for Eider Pond.
Before metal analysis, sediment samples were pulverized with mortar and pestle and then subjected to an aqua regia digestion. ICP-MS was used to analyze 28 metals (limit of detection, μg/g): Ag (0.01), Al (1), As (0.4), Ba (0.01), Be (0.02), Bi (0.09), Ca (1), Cd (0.02), Co (0.01), Cr (0.5), Cu (0.1), Fe (0.5), K (1), Li (2), Mg (1), Mn (0.1), Mo (0.1), Ni (0.1), P (5), Pb (0.05), Sb (0.1), Se (0.7), Sn (0.5), Ti (0.2), Tl (0.02), U (0.002), V (0.1), and Zn (0.7). QA/QC was performed by running certified reference materials, internal standards, blanks, and duplicates with every batch of 20 samples.
Stable isotopes of nitrogen were analyzed at the University of Ottawa according to the methods described by Yamamuro and Kayanne (49). Diatom preparation for microscopic analysis followed standard procedures outlined by Battarbee et al. (50). In general, at least 300 diatom valves were counted for each interval, although a few intervals (n = 4 for Tern Pond; n = 2 for Eider Pond) contained low abundances such that only 50 to 100 valves could be identified and enumerated. Given the low diatom diversity in both cores (Fig. 2) these numbers still likely provided reliable counts. Ordination of sedimentary metals and stable isotopes of nitrogen and carbon by PCA was used to compare and contrast differences in ornithogenic inputs between the two study sites. PCA was conducted using Canoco version 4.5a (51). Diatom stratigraphies were created by using C2 (52).
Supplementary Material
Acknowledgments
We thank Kelly Boadway, Joshua Boadway, Karel Allard, Chris Grooms, and Kathryn McCleary for assisting in fieldwork. Bronwyn Keatley, two external reviewers, and the editor provided helpful comments on the manuscript. This project was funded by Natural Science and Engineering Research Council awards to J.P.S., M.S.V.D., and J.M.B. We are grateful to the Indian and Northern Affairs Canada, Natural Resources Canada (PCSP), and Environment Canada for financial and logistical support pertaining to fieldwork. This project is Polar Continental Shelf Program/Programme du Plateau Continental Polaire (PCSP/PPCP) no. 03709.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001333107/-/DCSupplemental.
References
- 1.Fisk AT, et al. Contaminant Levels, Trends and Effects in the Biological Environment – Canadian Arctic Contaminants Assessment Report II. Ottawa: Indian and Northern Affairs Canada; 2003. pp. 11–61. [Google Scholar]
- 2.Blais JM, et al. Biologically mediated transport of contaminants to aquatic systems. Environ Sci Technol. 2007;41:1075–1084. doi: 10.1021/es061314a. [DOI] [PubMed] [Google Scholar]
- 3.Brimble SK, et al. High Arctic ponds receiving biotransported nutrients from a nearby seabird colony are also subject to potentially toxic loadings of arsenic, cadmium, and zinc. Environ Toxicol Chem. 2009;28:2426–2433. doi: 10.1897/09-235.1. [DOI] [PubMed] [Google Scholar]
- 4.Evenset A, et al. A comparison of organic contaminants in two high Arctic lake ecosystems, Bjørnøya (Bear Island), Norway. Sci Total Environ. 2004;318:125–141. doi: 10.1016/S0048-9697(03)00365-6. [DOI] [PubMed] [Google Scholar]
- 5.Sun LG, Xie ZQ, Zhao JL. A 3,000-year record of penguin populations. Nature. 2000;407:858. doi: 10.1038/35038163. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Sun LA. A 1,800-year record of arsenic concentration in the penguin dropping sediment, Antarctica. Environ Geol. 2008;55:1055–1059. [Google Scholar]
- 7.Walsh PM. In: Heavy Metals in the Marine Environment. Furness RW, Rainbow PS, editors. Boca Raton, FL: CRC Press; 1990. pp. 183–204. [Google Scholar]
- 8.Evenset A, et al. Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems. Environ Sci Technol. 2007;41:1173–1179. doi: 10.1021/es0621142. [DOI] [PubMed] [Google Scholar]
- 9.Mallory ML, Wayland M, Braune B, Drouillard KG. Trace elements in marine birds, arctic hare and ringed seals breeding near Qikiqtarjuaq, Nunavut, Canada. Mar Pollut Bull. 2004;49:119–141. doi: 10.1016/j.marpolbul.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Mallory ML, Braune BM, Wayland M, Gilchrist HG, Dickson DL. Contaminants in common eiders (Somateria mollissima) of the Canadian Arctic. Environ Rev. 2004;12:197–218. [Google Scholar]
- 11.Mallory ML, Fontaine AJ, Smith PA, Wiebe Roberston MO, Gilchrist HG. Water chemistry of ponds on Southampton Island, Nunavut Canada: Effects of habitat and ornithogenic inputs. Arch Hydrobiol. 2006;166:411–432. [Google Scholar]
- 12.Keatley BE, Douglas MSV, Blais JM, Mallory ML, Smol JP. Impacts of seabird-derived nutrients on water quality and diatom assemblages from Cape Vera, Devon Island, Canadian High Arctic. Hydrobiologia. 2009;621:191–205. [Google Scholar]
- 13.Douglas MSV, Smol JP. Eutrophication and recovery in the High Arctic: Meretta Lake (Cornwallis Island, Nunavut, Canada) revisited. Hydrobiologia. 2000;431:193–204. [Google Scholar]
- 14.Michelutti N, Hermanson MH, Smol JP, Dillon PJ, Douglas MSV. Delayed response of diatom assemblage changes to sewage inputs in an Arctic lake. Aquatic Sci. 2007;69:523–533. [Google Scholar]
- 15.Brenner M, Whitmore TJ, Curtis JH, Hodell DA, Schelske CL. Stable isotope (δ13C and δ15N) signatures of sedimented organic matter as indicators of historic lake trophic state. J Paleolimnol. 1999;22:205–221. [Google Scholar]
- 16.Michelutti N, et al. Accelerated delivery of polychlorinated biphenyls (PCBs) in recent sediments near a large seabird colony in Arctic Canada. Environ Pollut. 2009;157:2769–2775. doi: 10.1016/j.envpol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Briner JP, et al. A multi-proxy lacustrine record of Holocene climate change on northeastern Baffin Island, Arctic Canada. Quatern Res. 2006;65:431–442. [Google Scholar]
- 18.Antoniades D, Hamilton PB, Douglas MSV, Smol JP. Diatoms of North America: The freshwater floras of Prince Patrick, Ellef Ringnes, and Northern Ellesmere Islands from the Canadian Arctic Archipelago. Iconographica Diatomologica. 2008;17:1–649. [Google Scholar]
- 19.Michelutti N, Douglas MSV, Smol JP. Tracking recent recovery from eutrophication in a high arctic lake (Meretta Lake, Cornwallis Island, Nunavut, Canada) using fossil diatom assemblages. J Paleolimnol. 2002;28:377–381. [Google Scholar]
- 20.Krammer K, Lange-Bertalot H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. Süßwasserflora von Mitteleuropa 2/3. Berlin: Spektrum Akademischer Verlag; 2000. [Google Scholar]
- 21.Lim DSS, Douglas MSV, Smol JP. Diatoms and their relationship to environmental variables from lakes and ponds on Bathurst Island, Nunavut, Canadian High Arctic. Hydrobiologia. 2001;450:215–230. [Google Scholar]
- 22.Håkansson H, Olsson S, Jiang H, Garbe-Schönberg CD. The sediment diatom association and chemistry of surface sediments of Lake Belauer See, Northern Germany. Diatom Res. 1998;13:63–91. [Google Scholar]
- 23.Hobson KA, et al. A stable isotope (δ13C, δ15N) model for the North Water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:5131–5150. [Google Scholar]
- 24.Finney BP, Gregory-Eaves I, Sweetman J, Douglas MSV, Smol JP. Impacts of climatic change and fishing on Pacific salmon abundance over the past 300 years. Science. 2000;290:795–799. doi: 10.1126/science.290.5492.795. [DOI] [PubMed] [Google Scholar]
- 25.Michelutti N, et al. Seabird-driven shifts in Arctic pond ecosystems. Proc Biol Sci. 2009;276:591–596. doi: 10.1098/rspb.2008.1103. 10.1098/rspb.2008.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckman AH, et al. Organochlorine contaminants in seven species of Arctic seabirds from northern Baffin Bay. Environ Pollut. 2004;128:327–338. doi: 10.1016/j.envpol.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Douglas MSV, Smol JP, Savelle JM, Blais JM. Prehistoric Inuit whalers affected Arctic freshwater ecosystems. Proc Natl Acad Sci USA. 2004;101:1613–1617. doi: 10.1073/pnas.0307570100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe AP, Cook CA, Hobbs WO. Are current rates of atmospheric nitrogen deposition influencing lakes in the eastern Canadian Arctic? Arct Antarct Alp Res. 2006;38:465–476. [Google Scholar]
- 29.Blais JM, et al. Arctic seabirds transport marine-derived contaminants. Science. 2005;309:445. doi: 10.1126/science.1112658. [DOI] [PubMed] [Google Scholar]
- 30.Akearok JA, Hebert CE, Braune BM, Mallory ML. Inter- and intraclutch variation in egg mercury levels in marine bird species from the Canadian Arctic. Sci Total Environ. 2010;408:836–840. doi: 10.1016/j.scitotenv.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Dietz R, Riget F, Johansen P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci Total Environ. 1996;186:67–93. doi: 10.1016/0048-9697(96)05086-3. [DOI] [PubMed] [Google Scholar]
- 32.Campbell LM, et al. Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay) Sci Total Environ. 2005;351-352:247–263. doi: 10.1016/j.scitotenv.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Henny CJ, Rudis DD, Roffe TJ, Robinson-Wilson E. Contaminants and sea ducks in Alaska and the circumpolar region. Environ Health Perspect. 1995;103(suppl 4):41–49. doi: 10.1289/ehp.103-1519270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franson JC, Hollmen T, Poppenga RH, Hario M, Kilpi M. Metals and trace elements in tissues of common eiders (Somateria mollissima) from the Finnish archipelago. Ornis Fenn. 2000;77:57–63. [Google Scholar]
- 35.Wayland M, et al. Trace elements in king eiders and common eiders in the Canadian arctic. Arch Environ Contam Toxicol. 2001;41:491–500. doi: 10.1007/s002440010276. [DOI] [PubMed] [Google Scholar]
- 36.Bargagli R, Sanchez-Hernandez JC, Martella L, Monaci F. Mercury, cadmium and lead accumulation in Antarctic mosses growing along nutrient and moisture gradients. Polar Biol. 1998;19:316–322. [Google Scholar]
- 37.Environment Canada . Canadian Sediment Quality Guidelines for Cadmium: Supporting Document. Ottawa: Environmental Conservation Service, Ecosystem Science Directorate, Science Policy and Environmental Quality Branch, Guidelines and Standards Division; 1997. [Google Scholar]
- 38.Canadian Council of Ministers of the Environment . Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Cadmium in Canadian Environmental Quality Guidelines, 1999. Winnipeg: Canadian Council of Ministers of the Environment; 1999. [Google Scholar]
- 39.Meteorological Service of Canada . National climate data and informational archive: Resolute CARS. Ottawa: Environment Canada; 2009. [Google Scholar]
- 40.Mallory ML, Fontaine AJ. Key marine habitat sites for migratory birds in Nunavut and the Northwest Territories. Canadian Wildlife Service Occasional Paper No. 109. Ottawa: Canadian Wildlife Service; 2004. [Google Scholar]
- 41.Hatch JJ. Arctic Tern (Sterna paradisaea) In: Poole A, editor. The Birds of North America Online. Ithaca, NY: Cornell Lab of Ornithology; 2002. [Google Scholar]
- 42.Goudie IR, Robertson GJ, Reed A. Common Eider (Somateria mollissima) In: Poole A, editor. The Birds of North America Online. Ithaca, NY: Cornell Lab of Ornithology; 2000. [Google Scholar]
- 43.Glew JR. A portable extruding device for close interval sectioning of unconsolidated core samples. J Paleolimnol. 1988;1:235–239. [Google Scholar]
- 44.Douglas MSV, Smol JP. Limnology of high arctic ponds Cape Herschel, Ellesmere Island, N.W.T.) Arch Hydrobiol. 1994;131:401–434. [Google Scholar]
- 45.Environment Canada . Manual of Analytical Methods: Major Ions and Nutrients. Vol. 1. Burlington, ON, Canada: National Laboratory for Environmental Testing, Canadian Centre for Inland Waters; 1994. [Google Scholar]
- 46.Environment Canada . Manual of Analytical Methods: Trace Metals. Vol. 2. Burlington, ON, Canada: National Laboratory for Environmental Testing, Canadian Centre for Inland Waters; 1994. [Google Scholar]
- 47.Schelske CL, Peplow A, Brenner M, Spencer CN. Low-background gamma counting: applications for 210Pb dating of sediments. J Paleolimnol. 1994;10:115–128. [Google Scholar]
- 48.Appleby PG. In: Tracking Environmental Changes Using Lake Sediments. Last WM, Smol JP, editors. Vol. 1. Dordrecht, The Netherlands: Kluwer; 2001. pp. 171–203. [Google Scholar]
- 49.Yamamuro M, Kayanne H. Rapid direct determination of organic-carbon and nitrogen in carbonate-bearing sediments with a Yanaco mt-5 chn analyzer. Limnol Oceanogr. 1995;40:1001–1005. [Google Scholar]
- 50.Battarbee RW, et al. In: Tracking Environmental Change Using Lake Sediments. Smol JP, Birks HJB, Last WM, editors. Vol. 3. Dordrecht, The Netherlands: Kluwer; 2001. pp. 155–202. [Google Scholar]
- 51.ter Braak CJF, Šmilauer P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5) Ithaca, NY: Microcomputer Power; 2002. [Google Scholar]
- 52.Juggins S. C2 Data Analysis, Version 1.3. Newcastle, UK: Univ of Newcastle; 2003. [Google Scholar]
- 53.Michelutti N, Douglas MSV, Smol JP. Evaluating diatom community composition in the absence of marked limnological gradients in the high Arctic: a surface sediment calibration set from Cornwallis Island (Nunavut, Canada) Polar Biol. 2007;30:1459–1473. [Google Scholar]
- 54.Antoniades D, Douglas MSV, Smol JP. Comparative physical and chemical limnology of two Canadian High Arctic regions: Alert (Ellesmere Island, NU) and Mould Bay (Prince Patrick Island, NWT) Arch Hydrobiol. 2003;158:485–516. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.