Abstract
Mohawk (Mkx) is a member of the Three Amino acid Loop Extension superclass of atypical homeobox genes that is expressed in developing tendons. To investigate the in vivo functions of Mkx, we generated Mkx−/− mice. These mice had hypoplastic tendons throughout the body. Despite the reduction in tendon mass, the cell number in tail tendon fiber bundles was similar between wild-type and Mkx−/− mice. We also observed small collagen fibril diameters and a down-regulation of type I collagen in Mkx−/− tendons. These data indicate that Mkx plays a critical role in tendon differentiation by regulating type I collagen production in tendon cells.
Tendons are dense, fibrous connective tissues that connect muscle to bone, transmitting the forces that allow for body movement (1). Tendon damage from overuse or degeneration due to aging is a common clinical problem because damaged tendon tissue heals very slowly and rarely recovers completely (2). The establishment of new therapies, such as regenerative medicine, for injured tendons has been delayed by a limited understanding of tendon biology (1, 3).
Tendons are composed primarily of collagen fibrils that cross-link to each other to form fibers (4). A small number of tendon cells reside between parallel chains of these fibrils and synthesize the specific ECM that contains collagens and proteoglycans (4, 5). The elasticity of tendons is provided by the large amount of collagen, predominantly type I collagen and small amounts of other collagens, including types III, IV, V, and VI (4, 6–9). The proteoglycans found in tendons, including decorin, fibromodulin, biglycan, and lumican, act to lubricate and organize collagen fiber bundles (4, 5). Targeted disruption of these proteoglycans in mice leads to abnormal collagen fibrils in tendons (3, 10–13). Tendon disruptions have also been described in patients with defects in collagen production, such as Ehlers-Danlos Syndrome, in which the type I collagen gene is mutated (14). These studies indicate that the ability of tendon cells to produce ECM is important for tendon formation.
Recently, it was reported that Scleraxis (Scx), a basic helix–loop–helix (bHLH) transcription factor expressed in the tendon progenitors and cells of all tendon tissues (15, 16), is essential for tendon differentiation. Scx knockout mice show severe disruption of force-transmitting tendons, although ligaments, which are tissues connecting bone to bone that closely resemble tendons in their components, and short-range anchoring tendons are not affected (17). It was also reported that Scx positively regulates the expression of type I collagen, a main ECM component of tendons (18). However, the type I collagen does not completely disappear from the tendons of Scx knockout mice (17), suggesting the presence of other regulatory factors for type I collagen. The tendon differentiation mechanisms remain largely unknown, with Scx being the only known transcription factor regulating tendon differentiation.
Mohawk (Mkx; also known as Irxl1) is the sole member of a newly characterized class within the Three Amino acid Loop Extension (TALE) superclass of atypical homeobox genes (19). These transcription factors are essential for a large set of developmental processes, including cell proliferation, differentiation, and positional specification (20–24). Initial characterization of mouse Mkx revealed a dynamic transcription pattern restricted to progenitors of tendon, skeletal muscle, and cartilage, as well as the sex chords of the male gonad and the ureteric bud tip of the metanephrogenic kidney (19, 25, 26). We previously identified Mkx as a transcription factor expressed in developing tendons by constructing a whole-mount in situ hybridization database, termed “EMBRYS” (http://embrys.jp/embrys/html/MainMenu.html), which contains expression data of 1,520 transcription factors and cofactors expressed in E9.5, E10.5, and E11.5 mouse embryos (27).
To investigate the in vivo functions of Mkx, we generated Mkx knockout mice. Here, we show that Mkx null mice have hypoplastic tendons throughout their body. Although the size of tendons is drastically reduced in Mkx null mice, the cell number in tail tendon fiber bundles shows no significant difference between wild-type and Mkx null mice. We also observed abnormal collagen fibrils in Mkx null tendons. Furthermore, we show that the expression of type I collagen, which is a major tendon ECM component, is down-regulated in the Achilles tendon of Mkx null mice. These data indicate that Mkx is a transcription factor controlling tendon differentiation by regulating type I collagen production in tendon cells.
Results
Generation of Mkx Mutant Mice.
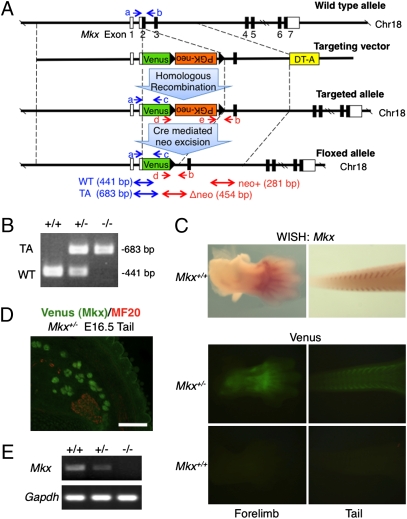
We inactivated the Mkx gene by homologous recombination in ES cells using a targeting vector to replace the Mkx gene from the start of translation to the end of exon 2 with the Venus gene and PGK-neomycin-resistance (PGK-neo) cassette (Fig. 1A). We predicted that the Venus gene, an improved GFP gene (28), would be expressed in Mkx positive cells in heterozygous mice generated with the targeting construct. We identified two correctly targeted ES cell clones by Southern blot analysis (Fig. S1 A and B). These two clones were microinjected into 8-cell stage embryos and germ-line transmission in the resulting chimeric mice was confirmed by Southern blotting and genomic PCR (Fig. 1B and Fig. S1C). Mkx+/− embryos showed robust Venus expression that recapitulated Mkx expression faithfully at E13.5 forelimb and tail (Fig. 1C). Also, these Venus knockin heterozygous mice showed tendon-specific Venus expression in the tail of E16.5 embryos (Fig. 1D). In addition to embryos, we investigated Venus expression in adult mice. Because Mkx expression in tendons of adult mice had not been determined, we analyzed expression of Mkx in tendon tissues of C57BL/6 adult mice by RT-PCR and found that Mkx was strongly expressed (Fig. S2A). Venus was specifically expressed in Achilles, tail, and trunk tendons of 6-week-old Mkx heterozygous mice (Fig. S2 B–M). These data indicate that this mutant mouse is useful for tendon biological analysis and expression analysis of Mkx. To confirm inactivation of the Mkx gene, we performed RT-PCR analysis of Mkx mutant mice. The analysis of adult Achilles tendon RNA showed a complete absence of Mkx expression in Mkx−/− mice (Fig. 1E). Genotyping of 206 newborn offspring derived from heterozygous–heterozygous crosses revealed a normal Mendelian ratio of genotypes (Table S1). Heterozygous and homozygous mutant mice were viable and fertile, and weight measurements did not show differences compared with wild-type mice (Fig. S3).
Fig. 1.
Generation of Mkx mutant mice. (A) Diagram of the Mkx targeting construct. Blue and red arrows (a–e) show genomic PCR primers for genotyping. White box, UTR; Black box, coding region; DT-A, diphtheria toxin A; WT, wild-type allele; TA, targeted allele. (B) Genomic PCR of wild-type and Mkx mutant mice for genotyping using primers a, b and c. (C) Whole-mount in situ hybridization of Mkx (Upper) and whole-mount visualization of Venus signals (Lower) in E13.5 forelimb and tail of wild-type or Mkx mutant embryos. (D) Immunohistochemistry for anti-myosin heavy chain (MF20; red) and visualization of Venus in cryosection of E16.5 tail of a Venus knockin Mkx heterozygous embryo. (Scale bar, 100 μm.) (E) RT-PCR analysis for Mkx and Gapdh of Achilles tendon in wild-type and Mkx mutant mice.
Tendon Defects Are Observed in Mkx Null Mice.
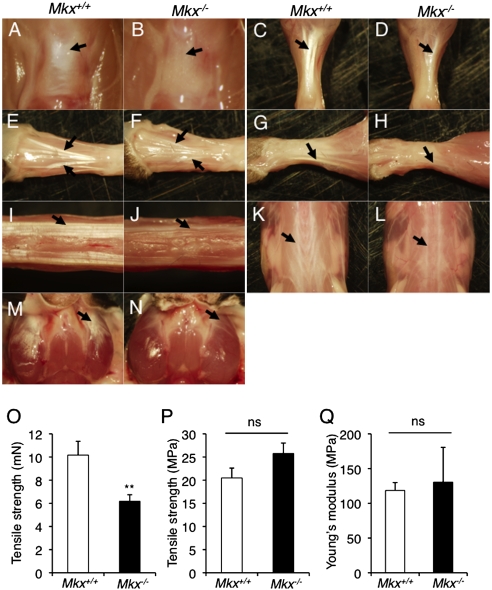
To investigate the function of Mkx in tendon formation, we analyzed tendons of Mkx null mice. Tendons (patellar, Achilles, and tail tendons, dorsal extensor tendons of the forelimb and hindlimb, tendons of the trunk, and platysma tendons) of 3-month-old Mkx null mice were hypoplastic and pale white in color compared with those of wild-type mice (Fig. 2 A–N). This phenotype was also observed in Mkx null mice, in which a neo cassette was excised by Cre recombination (Fig. S4); however, heterozygous mice with or without a neo cassette were not affected (Fig. S2 B–M). To examine the mechanical properties of Mkx null tendons, we performed a tensile test. These experiments showed a diminution of tensile strength in Mkx null Achilles tendons (Fig. 2O), indicating functional depression. However, tensile strength per unit area and Young's modulus, which is a measure of the stiffness of an isotropic elastic material indicating elasticity per unit area, did not show significant change between wild-type and Mkx null mice (Fig. 2 P and Q). This suggests that the diminution of tensile strength in Mkx null tendons is due to reduced tendon mass.
Fig. 2.
Tendon defects are observed in Mkx null mice. (A–N) The appearance of the patellar tendon (A and B; black arrow), Achilles tendon (C and D; black arrow), hindlimb tendons (E and F), forelimb tendons (G and H), tail tendons (I and J), back tendons (K and L; black arrow) and platysma tendon (M and N; black arrow) in 3-month-old wild-type and Mkx null mice. (O and P) Absolute value of tensile strength (O) and tensile strength per unit area (P) of Achilles tendons in wild-type and Mkx null mice. Error bars, SEM (n = 7). ns, no significance. (Q) Young's modulus of Achilles tendons in wild-type and Mkx null mice. Error bars, SEM (n = 7). ns, no significance.
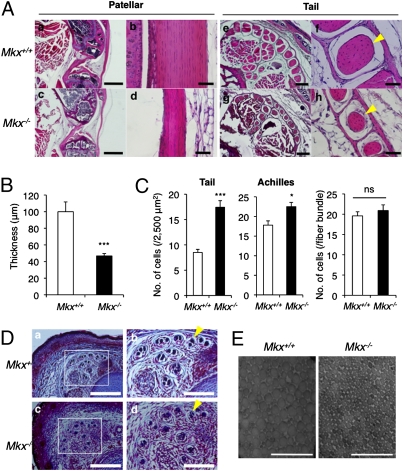
We performed histological analyses to further confirm the tendon defects observed in Mkx null mice. H&E staining of patellar tendons from 3-month-old Mkx null mice revealed that Mkx null patellar tendons were thinner than wild-type tendons (Fig. 3A a–d). Seven-day-old Mkx null mice also had thin patellar tendons, which were approximately half the thickness of the tendons from wild-type mice (Fig. 3B). However, the cruciate ligament, which closely resembles tendons with regard to its components, was not affected in Mkx knockout mice (Fig. S5). Tail tendons in Mkx null mice also show small tendon fiber bundles (Fig. 3A e–h; yellow arrowheads). However, Mkx null tail tendons had high tendon cell density and the cell number of tail tendon fiber bundles was not significantly different between wild-type and Mkx null mice (Fig. 3C). The enhanced cell density was also observed in the Achilles tendon of knockout mice (Fig. 3C). This suggests that Mkx null tendon cells are not completely functional.
Fig. 3.
Tendon mass is decreased in Mkx null mice. (A) H&E staining of the patellar (a–d) and tail (e–h) tendons in 3-month-old wild-type and Mkx null mice. Yellow arrowheads indicate a fiber bundle in the tail tendon. [Scale bars: (a and c) 1 mm; (b and d) 50 μm; (e and g) 200 μm; (f and h) 50 μm.] (B) Patellar tendon thickness of 7-day-old wild-type and Mkx null mice. Error bars, SEM (n = 8). (C) Cell density of tail or Achilles tendons (Left) and cell number in a tail tendon fiber bundle (Right) in 3-month-old wild-type and Mkx null mice. Error bars, SEM (Left, n = 8 ~10; Right, n = 14). ns, no significance. (D) Azan staining of E18.5 embryonic tails in wild-type and Mkx null mice. Yellow arrowheads indicate a fiber bundle in the tail tendon. [Scale bars: (Left) 500 μm; (Right) 200 μm.] (E) Transmission electron microscopic view of collagen fibrils in the Achilles tendon of wild-type and Mkx null mice. (Scale bars, 200 nm.)
To determine whether tendon defects in Mkx knockout mice are also observed in embryonic stages, we performed azan staining of tail tendons in Mkx null embryos at E18.5. The size of the tail tendons in Mkx null embryos was not affected, but we observed a low density of aniline blue staining (Fig. 3D; yellow arrowheads), which detects collagen fibers. To analyze the collagen fibrils of Mkx null tendons in detail, we performed an ultrastructural analysis of Achilles tendons using electron microscopy. Collagen fibril diameters in the Mkx null mice were uniformly smaller than those of wild-type mice (Fig. 3E). These data suggest a reduction of collagens in Mkx null tendons and a critical role for Mkx in tendon differentiation in vivo.
Mkx Null Tendon Cells Reduced Type I Collagen Production.
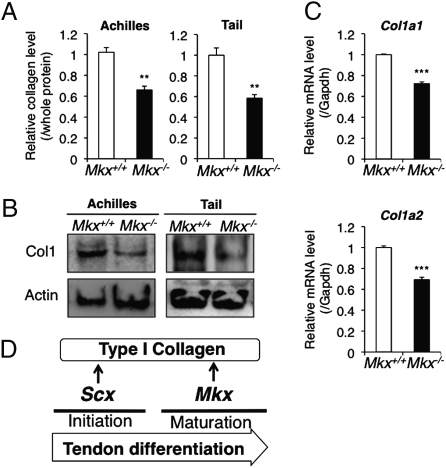
Despite tendon mass reduction in Mkx null mice, tendon cell number showed no significant changes between wild-type and Mkx null mice (Fig. 3C). This suggests that Mkx null tendon cells have a reduced ability to synthesize ECM or enhance ECM catabolic activity. In addition, histological analysis by azan staining of E18.5 Mkx null tail tendons (Fig. 3D) and ultrastructural analysis of Achilles tendons (Fig. 3E) suggested a reduction of collagens in Mkx null tendons. To investigate the production of collagens by Mkx null tendon cells, we first measured the amount of soluble collagens in Mkx null tendons by Sircol Soluble Collagen Assay. This experiment indicated that total soluble collagens were decreased in Achilles and tail tendons of Mkx null mice compared with those from wild-type mice (Fig. 4A). Furthermore, we found a reduction in the protein level of type I collagen, which is a main component of tendon ECM, in Achilles and tail tendons of Mkx knockout mice by Western blotting (Fig. 4B). We also found that the mRNA levels of Col1a1 and Col1a2, which encode the type I collagen, were decreased in Achilles tendons of Mkx null mice through quantitative real-time PCR (Fig. 4C). However, the expression of the transcription factor Scx, which is a positive regulator of type I collagen, was not reduced and rather increased in these tendons (Fig. S6), indicating that the decrease of collagen I is not due to the down-regulation of Scx. We also investigated the expression of other tendon component genes such as elastin (Eln), fibrillin1 (Fbn1), Col3a1, Col6a1, TenascinC (Tnc), and proteoglycans in mutant Achilles tendon by real time PCR analysis. This experiment indicated that decorin (Dcn), which is a proteoglycan regulating collagen fiber formation, was down-regulated (Fig. S6). These data reveal that Mkx plays an important role in regulating the expression of type I collagen and its associate molecules in tendon cells.
Fig. 4.
Type I collagen productivity is decreased in Mkx null tendon cells. (A) Soluble collagen measurement in whole soluble protein of Achilles or tail tendons of 8-week-old wild-type or Mkx null mice. Error bars, SEM (n = 3). (B) Western blot analysis of type I collagen (Col1) and β-actin (actin) in Achilles or tail tendons of 8-week-old wild-type or Mkx null mice. (C) Real-time PCR analysis for Col1a1 and Col1a2 in Achilles tendons of 8-week-old wild-type or Mkx null mice. Error bars, SEM (n = 3). (D) Proposed tendon differentiation network.
Discussion
We investigated the in vivo functions of Mkx, the homeobox gene expressed in developing tendons, by analyzing the tendons of Mkx knockout mice. We examined two major tendons, Achilles and tail tendon, whose developmental origins are known to be different. Achilles tendon in the limbs is induced in mesenchyme directly and the tail tendon in the trunk is derived from syndetome (15, 29). As a result, cell density (Fig. 3C), collagen quantity (Fig. 4A) and type I collagen expressions (Fig. 4B) were reduced in both Achilles tendon and tail tendon of Mkx null mice. This indicates that Mkx may play a role in various tendons, regardless of the developmental origins, although such factors are unknown.
The bHLH transcription factor Scx is also known to be a positive regulator of tendon differentiation via its role in promoting type I collagen expression (17, 18). However, phenotypes of both null mutants are different. Although Scx null mutants exhibit a loss of segments or complete tendons (17), Mkx null mice have reduced tendon mass without a decrease in the number of tendon cells. These data suggest that Scx is essential for the initiation of tendon differentiation, whereas Mkx plays a critical role in tendon maturation (Fig. 4D). Regardless of the high expression of Scx in Mkx null mice (Fig. S6), type I collagen gene expression is decreased (Fig. 4C), indicating the existence of other positive regulators of type I collagen that should be regulated by Mkx. Recent work indicates that Mkx functions as a transcriptional repressor by recruiting the Sin3A/histone deacetylase corepressor complex (30), therefore Mkx may act as a repressor for negative regulatory factors of type I collagen.
It has been reported that the Tgfb2, Tgfb3 double knockout mice or Tgfbr2 knockout mice show loss of most tendons, and TGF-β led to an early induction of a tendon master gene Scx expression (31). This indicates that TGF-β–Scx pathway regulates the initial differentiation of tendon. Here, we show that Mkx plays a critical role at the tendon maturation stage. It would be of interest to examine whether TGF-β may also be critical for late tendon differentiation via Mkx regulation.
To get more insight into the function of Mkx on the collagen network development, we examined a set of other tendon component genes’ expressions and observed that decorin was also decreased in Mkx null mice tendons (Fig. S6). Reduced decorin, known to be a regulator of collagen assembly, expression may provide an explanation for the collagen fibril size reduction phenotype in Mkx null mice. Although we focused on tendon development, Mkx may also play a role in tendon homeostasis in adults. In this regard, it would be interesting to examine the potential function of Mkx in aging tendons.
These findings will serve as a basis for understanding molecular mechanisms of tendon differentiation and may provide a therapeutic target for tendon injuries and tendon related diseases, such as Ehlers-Danlos Syndrome.
Materials and Methods
Generation of Mkx Mutant Mice.
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the National Institute for Child Health and Development. A vector was constructed to replace the endogenous Mkx locus with the Venus gene and PGK-neo cassette by homologous recombination in ES cells (Fig. 1A). 5′ and 3′ sequences flanking the endogenous Mkx locus were amplified by PCR from a C57BL/6 genomic BAC clone (BACPAC Resource Center). These homology arms were cloned into a vector incorporating both a neomycin resistance cassette for positive selection and a diphtheria toxin (DT-A) gene for negative selection. The targeting vector was linearized and electroporated into TT2F ES cells. Recombinant ES clones were isolated after culture in medium containing G418 antibiotic and screened for proper integration by Southern blotting with the 5′probe, 3′probe and neo cassette sequence (Fig. S1 A and B). Two clones exhibited proper integration, which was validated through genomic sequencing, and were chosen for microinjection into eight-cell stage embryos. The resulting chimeric offspring were crossed to C57BL/6 mice and germ-line transmission was confirmed by Southern blotting (Fig. S1C) and PCR (Fig. 1B). The floxed PGK-neo cassette was removed by crossing with Meox-Cre transgenic mice (purchased from The Jackson Laboratory) (32) and Cre-mediated neo excision was analyzed by genomic PCR. PCR primer sequences for genotyping are shown in Table S2.
Whole-Mount Visualization of Fluorescent Signals and in Situ Hybridization.
For whole-mount visualization of fluorescent signals, embryos were observed directly and tissues were skinned before observation. Whole-mount in situ hybridization for Mkx was performed as described previously (33). The details of Mkx probe synthesis for whole-mount in situ hybridization can be obtained on the “EMBRYS” web site (http://embrys.jp/embrys/html/MainMenu.html).
Histological Analysis and Immunohistochemistry.
Mkx null mice and wild-type littermates were obtained from an intercross of Mkx+/− maintained on a C57BL/6 background. For histological analysis, patellar and tail tendons were harvested from embryos or adult mice and fixed with 4% paraformaldehyde in PBS at 4 °C overnight. Tissues were dehydrated, embedded in paraffin, and sectioned, and each section was stained with H&E or azocarmine-aniline blue (azan). For immunohistochemistry, tails from E16.5 embryos were dissected and fixed with 4% paraformaldehyde in PBS at 4 °C for 2 h. The tissues were embedded in O.C.T. compound (Sakura Finetek) and frozen rapidly in liquid nitrogen. Specimens were sectioned at 10 μm. Cryosections were air-dried and blocked with Blocking One (Nacalai Tesque) for 1 h. The sections were then incubated with anti-myosin heavy chain antibody (MF20; DSHB) at 4 °C overnight, rinsed, and incubated for 1 h with Alexa 594 (Molecular Probes). These experiments were performed with at least three independent samples to confirm reproducibility.
Tensile Testing.
To evaluate the mechanical properties of the Achilles tendon, we used the entire tendon unit (from the myotendinous junction to the calcaneal tuberosity). A uniaxial materials testing system (Autograph AGS-G; Shimadzu Corp. Ltd.) was used to determine tensile properties with a 500 N load cell, as described previously (34) with some modifications. To facilitate gripping during testing, the proximal end of the Achilles tendon and foot of the mouse were fixed in custom-made clamps and the specimens were pulled at a constant strain rate of 0.5 mm/sec. All samples broke within the gauge length. The initial length and cross-sectional area of each specimen were measured using digital calipers and by microscopy of H&E stained sections, respectively. Force data were collected in Trapezium (Shimadzu Corp. Ltd.) software at a frequency of 50 Hz. For each specimen, a stress–strain curve was created from the load–displacement curve and Young's modulus was calculated from each stress–strain curve using the cross-sectional area.
Counting Cell Numbers of Tendon.
The paraffin-sections or cryosections of tail or Achilles tendons from 3-month-old Mkx null or wild-type mice were prepared and stained with H&E or DAPI as described in Histological Analysis and Immunohistochemistry. The hematoxylin or DAPI stained nuclei were counted in each 50 μm × 50 μm area or tail tendon fiber bundle. At least eight areas or bundles were counted in three or four of Mkx null or wild-type mice.
Western Blot Analysis and Collagen Measurements.
Total soluble protein was obtained from Achilles tendon and tail tendon by homogenization in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% DOC, 0.1% SDS, 1% Nonidet P-40; pH 8.0) and used for Western blot analysis and collagen measurements. For Western blotting, total soluble protein was separated by SDS/PAGE followed by a semidry transfer to PVDF. Membranes were blocked for 30 min with Blocking One (Nacalai Tesque), incubated with anti-collagen I antibody (ab292; Abcam) or anti-β-actin antibody (A5316; SIGMA) at 4 °C overnight, rinsed, and then incubated for 1 h with HRP-conjugated anti-rabbit IgG (IgG) antibody (A6154; SIGMA) or HRP-conjugated anti-mouse IgG antibody (A2304; SIGMA). The blot was then developed with Chemi-Lumi One (Nacalai Tesque). Total soluble collagen measurements of homogenate in Achilles and tail tendons were performed using the Sircol Soluble Collagen Assay (Biocolor) according to the manufacturer's instructions. Total soluble protein was measured using the DC Protein Assay (Bio-Rad) and used the data for normalization. These experiments have been repeated at least three times to confirm reproducibility.
Electron Microscopy.
Small pieces (approximately 1 mm3) were excised from Achilles tendons of wild-type and Mkx null mice, and fixed with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer overnight. The samples were then washed in 0.1 M cacodylate buffer and rinsed with physiological saline. They were dehydrated through an ethanol series, embedded in epoxy resin, and cut into ultrathin (approximately 100 nm) sections. Sections were mounted on copper grids, contrasted with aqueous uranyl acetate and lead citrate and examined by transmission electron microscope (H-7100; Hitachi).
RNA Isolation, RT-PCR, and Quantitative Real-Time PCR.
Total RNA was isolated from Achilles tendons, tail tendons and femoral muscles using ISOGEN (Nippongene), and reverse transcribed using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare). RT-PCR was performed with Go-Taq polymerase (Promega). Quantitative real-time RT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems). The expression of Gapdh was used as a control for mRNA expression. Gene expression changes were quantified using the delta-delta CT method. This experiment was performed with three independent samples and confirmed reproducibility. Primer sequences for RT-PCR and real-time PCR are described in Table S2.
Statistical Analysis.
The two-tailed independent Student's t-test was used to calculate all P values. Asterisks in figures indicate differences with statistical significance as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank K. Sakuma, R. Higashiyama, A. Sawada, S. Ito, M. Horiuchi, J. Hasegawa, and T. Kotani for help with generation of Mkx mutant mice; M. Narasaki for technical assistance of ultrastructural analysis; and C. Shukunami, Y. Sugimoto, and Y. Nishizaki for beneficial advice. This project was supported by Grants from the Genome Network Project (Ministry of Education, Culture, Sports, Science and Technology) and partially from Solution-Oriented Research for Science and Technology (Japan Science and Technology Agency) and Grant ID05-24 from the National Institute of Biomedical Innovation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000525107/-/DCSupplemental.
References
- 1.Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: Collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Maffulli N. Biology of tendon injury: Healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 3.Bi Y, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JH, Halper J. Tendon proteoglycans: Biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 6.Williams IF, McCullagh KG, Silver IA. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12:211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]
- 7.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 10.Danielson KG, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jepsen KJ, et al. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 12.Ameye L, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarti S, et al. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao JR, Bristow J. The Ehlers-Danlos syndrome: On beyond collagens. J Clin Invest. 2001;107:1063–1069. doi: 10.1172/JCI12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweitzer R, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 16.Cserjesi P, et al. Scleraxis: A basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 17.Murchison ND, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 18.Léjard V, et al. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DM, et al. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev Dyn. 2006;235:792–801. doi: 10.1002/dvdy.20671. [DOI] [PubMed] [Google Scholar]
- 20.Selleri L, et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128:3543–3557. doi: 10.1242/dev.128.18.3543. [DOI] [PubMed] [Google Scholar]
- 21.Brendolan A, et al. A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development. 2005;132:3113–3126. doi: 10.1242/dev.01884. [DOI] [PubMed] [Google Scholar]
- 22.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 23.van Tuyl M, et al. Iroquois genes influence proximo-distal morphogenesis during rat lung development. Am J Physiol Lung Cell Mol Physiol. 2006;290:L777–L789. doi: 10.1152/ajplung.00293.2005. [DOI] [PubMed] [Google Scholar]
- 24.diIorio P, Alexa K, Choe SK, Etheridge L, Sagerström CG. TALE-family homeodomain proteins regulate endodermal sonic hedgehog expression and pattern the anterior endoderm. Dev Biol. 2007;304:221–231. doi: 10.1016/j.ydbio.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Liu W, Maltby KM, Lan Y, Jiang R. Identification and developmental expression analysis of a novel homeobox gene closely linked to the mouse Twirler mutation. Gene Expr Patterns. 2006;6:632–636. doi: 10.1016/j.modgep.2005.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi JK, Bruneau BG. Irxl1, a divergent Iroquois homeobox family transcription factor gene. Gene Expr Patterns. 2007;7:51–56. doi: 10.1016/j.modgep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama S, et al. A systems approach reveals that the myogenesis genome network is regulated by the transcriptional repressor RP58. Dev Cell. 2009;17:836–848. doi: 10.1016/j.devcel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai T, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 29.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DM, Beres BJ, Wilson-Rawls J, Rawls A. The homeobox gene Mohawk represses transcription by recruiting the sin3A/HDAC co-repressor complex. Dev Dyn. 2009;238:572–580. doi: 10.1002/dvdy.21873. [DOI] [PubMed] [Google Scholar]
- 31.Pryce BA, et al. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama S, et al. Dynamic gene expression of Lin-28 during embryonic development in mouse and chicken. Gene Expr Patterns. 2008;8:155–160. doi: 10.1016/j.gep.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentleman E, et al. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials. 2003;24:3805–3813. doi: 10.1016/s0142-9612(03)00206-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.