Summary
Background
Experimental evidence suggests that xanthine oxidase inhibitors can reduce myocardial oxygen consumption for a particular stroke volume. If such an effect also occurs in man, this class of inhibitors could become a new treatment for ischaemia in patients with angina pectoris. We ascertained whether high-dose allopurinol prolongs exercise capability in patients with chronic stable angina.
Methods
65 patients (aged 18–85 years) with angiographically documented coronary artery disease, a positive exercise tolerance test, and stable chronic angina pectoris (for at least 2 months) were recruited into a double-blind, randomised, placebo-controlled, crossover study in a hospital and two infirmaries in the UK. We used computer-generated randomisation to assign patients to allopurinol (600 mg per day) or placebo for 6 weeks before crossover. Our primary endpoint was the time to ST depression, and the secondary endpoints were total exercise time and time to chest pain. We did a completed case analysis. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN 82040078.
Findings
In the first treatment period, 31 patients were allocated to allopurinol and 28 were analysed, and 34 were allocated to placebo and 32 were analysed. In the second period, all 60 patients were analysed. Allopurinol increased the median time to ST depression to 298 s (IQR 211–408) from a baseline of 232 s (182–380), and placebo increased it to 249 s (200–375; p=0·0002). The point estimate (absolute difference between allopurinol and placebo) was 43 s (95% CI 31–58). Allopurinol increased median total exercise time to 393 s (IQR 280–519) from a baseline of 301 s (251–447), and placebo increased it to 307 s (232–430; p=0·0003); the point estimate was 58 s (95% CI 45–77). Allopurinol increased the time to chest pain from a baseline of 234 s (IQR 189–382) to 304 s (222–421), and placebo increased it to 272 s (200–380; p=0·001); the point estimate was 38 s (95% CI 17–55). No adverse effects of treatment were reported.
Interpretation
Allopurinol seems to be a useful, inexpensive, well tolerated, and safe anti-ischaemic drug for patients with angina.
Funding
British Heart Foundation.
Introduction
Allopurinol has been shown to improve mechano-energetic uncoupling in the myocardium during heart failure,1–3 which means that it decreases myocardial oxygen demand per unit of cardiac output. The mechanism probably involves an effect on myocardial energetics.4,5 Whatever the precise mechanism, the process whereby allopurinol reduces myocardial oxygen consumption has so far only been shown in heart failure and almost exclusively in experimental heart failure.1–5 However, a large group of patients who might benefit from a drug that decreases oxygen consumption are those with angina pectoris, but there are no studies (clinical or experimental) in which this possibility has been investigated. We therefore set out to investigate whether allopurinol prolongs exercise in patients with chronic stable angina pectoris.
Methods
Study overview
The randomised, double-blind, placebo-controlled, crossover trial of allopurinol in patients with angina pectoris was done at Ninewells Hospital, Perth Royal Infirmary, and Arbroath Infirmary (all in UK). It was approved by the Fife, Forth Valley and Tayside Research Ethics Committee, and was done in accordance with the Declaration of Helsinki. Participants provided signed, written informed consent.
Study protocol
Individuals (aged 18–85 years) were recruited from outpatients at two Tayside Hospitals. They were eligible if they had angiographically documented coronary artery disease, a positive exercise tolerance test (ETT), and a history of symptoms of chronic, stable, effort-induced angina for at least 2 months. All concomitant antianginal drugs were allowed and continued unchanged during the study.
Exclusion criteria were inability of participant to do ETT because of back or leg problems (n=24), myocardial infarction or acute coronary syndrome for at least 2 months, coronary revascularisation (percutaneous or coronary artery bypass graft) within the previous 6 months, left ventricular ejection fraction of less than 45% (n=7), estimated glomerular filtration rate of less than 45 mL per min or creatinine concentration greater than 180 mmol/mL (n=5), substantial valvular disease (n=1), had gout or was already taking allopurinol, atrial arrhythmias or electrocardiogram (ECG) abnormalities interfering with ST-segment interpretation, previous ventricular arrhythmias on ETT (n=2), or severe hepatic disease or taking warfarin (n=6), azathioprine (n=1), or 6-mercaptopurine.
After an initial history and examination, participants underwent an ETT according to the full Bruce protocol. During each ETT, a 12-lead ECG was recorded continuously, and printed every 30 s and at the point of 1 mm ST depression. A second ETT was done within 14 days. Eligible participants had to manifest ischaemia (ST depression ≥1 mm compared with resting ECG) on both visits with a between-visit difference in time to ST depression of less than 15%. Otherwise, a third ETT was done and there had to be a difference of less than 15% between the second and third tests. The last baseline ETT before any treatment was given was used in the analysis. All ETTs were supervised by AN and a research nurse; both were unaware of the treatment allocation.
Randomisation and masking
Eligible participants were randomly assigned to allopurinol or matching placebo for 6 weeks. The dose of oral allopurinol was 100 mg once a day during the first week, 300 mg once a day in the second week, and 300 mg twice a day during the rest of the treatment. Participants were then crossed over, without any washout, to the other treatment for a further 6 weeks. Allopurinol 600 mg per day was chosen because we previously showed that it improved endothelial function and oxidative stress much more than did 300 mg per day.6 A random allocation sequence was computer-generated elsewhere. The individual who prepared the blinded numbered containers of drugs was not otherwise connected to the study, and the individuals doing the study and the patients had no access to the allocation sequence until the end, when final results were analysed. Allocation was done by trial staff unaware of what treatment A or B was. All tablets were identical in size, colour, and taste. Overall, masking was judged to be good. No stratification was done. Patients were allowed to continue with all their antianginal drugs, which remained unchanged throughout. At the end of each treatment, patients were clinically assessed and underwent a further ETT. Thus, the baseline ETT used in the analysis was done before drug or placebo was given, and no further drug-free ETT was done between the first and second treatments. The primary endpoint was the time to ST depression, and the secondary endpoints were total exercise time and time to symptoms (chest pain). In total, there were usually four visits—ie, two predrug baseline visits and one visit at the end of every treatment. Blood was monitored at visits 1, 3, and 4 for full blood count, urea electrolytes, and liver function.
The time to ST depression was analysed by two independent observers (AN and DSCA) who were unaware of the treatments; their results were virtually identical (Pearson r=0·9975, 95% CI 0·997–0·998). Bland Altman analysis bias was −0·95 s (SD 10·7; 95% CI −22 to 20). Blood was taken at baseline and at the end of every treatment phase for measurement of concentrations of brain natriuretic peptide and C-reactive protein. Adverse events were assessed by symptom enquiry at every visit and by monitoring routine blood samples. Patients also kept diaries to record anginal episodes and their intake of glyceryl trinitrate.
Statistical analysis
Results were presented as mean (SD) when parametric tests were used, and as median (IQR) when non-parametric tests were used. For non-parametric data, a Mann-Whitney U test was used. We also used the multilevel model as recommended by Mills and colleagues7 for analysing crossover studies. This model was used specifically to allow assessment of whether there were any effects with time or any carryover effect from one treatment phase to another. We also used the multilevel model of Mills and colleagues7 to assess treatment effect for the parametric data: these results were double checked by use of a paired t test after the exclusion of carryover effects.8 Point estimates (absolute difference in time [s] between allopurinol and placebo) and their 95% CIs for the three main treatment effects were calculated as recommended by Hollander and Wolfe.9
Power calculations were based originally on an expected average improvement of 50 s (SD 90) in the time to ST depression, as from previous reports.10–15 We only needed 34 individuals in a crossover study to have 90% power at p<0·05, but the reported above mean improvement varied greatly from one study to another such that we decided prospectively to increase our numbers and overall recruit 60 individuals who completed the study.
This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN 82040078.
Role of funding source
The funder had no other role in the trial whatsoever, except for supplying finance and peer reviewing the original grant application. AN had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author had the final responsibilty to submit this report for publication.
Results
Figure 1 shows the trial profile; and table 1 shows the baseline characteristics of the participants. Patients were recruited from January, 2007, to July, 2008, with follow-up during the 3 months thereafter. Five withdrew before completion of the study. Of the remaining 60 patients, 28 began with allopurinol and 32 with placebo. The patients who withdrew were all excluded from analysis because none of them completed their first course of treatment, and they only had baseline ETT—ie, we did a completed case analysis rather than an intention-to-treat analysis because there were very few dropouts and their reasons for dropping out were unrelated to baseline values or to their response. Noteworthy is that no adverse effects of treatment were reported.
Figure 1.
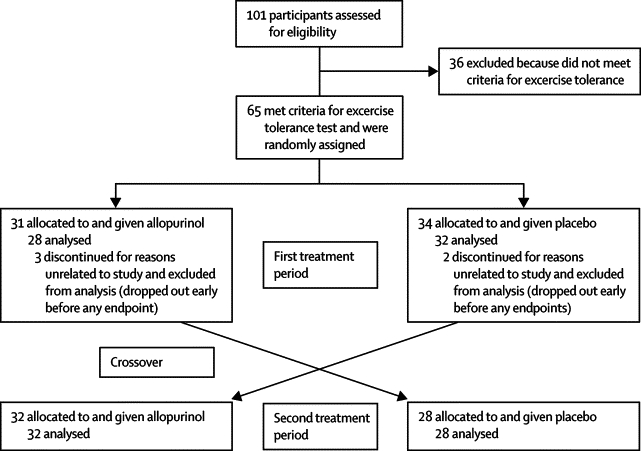
Trial profile
Table 1.
Baseline characteristics of participants
| All (n=60) | Placebo first (n=32) | Allopurinol first (n=28) | Dropouts (n=5) | |||
|---|---|---|---|---|---|---|
| Age (years; mean, SD) | 64·6 (9·3) | 64·0 (8·9) | 65·2 (9·6) | 66·6 (7·7) | ||
| Men | 50 (83%) | 28 (88%) | 22 (79%) | 3 (60%) | ||
| Women | 10 (17%) | 4 (13%) | 6 (21%) | 2 (40%) | ||
| Angina Canadian Cardiovascular Society stage16 | ||||||
| I | 9 (15%) | 4 (13%) | 5 (18%) | 0 | ||
| II | 42 (70%) | 23 (72%) | 19 (68%) | 3 (60%) | ||
| III | 9 (15%) | 5 (16%) | 4 (14%) | 2 (40%) | ||
| Number of vessels with coronary artery disease | ||||||
| 1 | 10 (17%) | 4 (13%) | 6 (21%) | 1 (20%) | ||
| 2 | 24 (40%) | 14 (44%) | 10 (36%) | 2 (40%) | ||
| 3 | 26 (43%) | 14 (44%) | 12 (43%) | 2 (40%) | ||
| Left ventricle systolic function | ||||||
| Normal | 51 (85%) | 26 (81%) | 25 (89%) | 4 (80%) | ||
| Mild impairment | 9 (15%) | 6 (19%) | 3 (11%) | 1 (20%) | ||
| Renal function | ||||||
| Normal | 55 (92%) | 28 (88%) | 27 (96%) | 4 (80%) | ||
| Mild impairment | 5 (8%) | 4 (13%) | 1 (4%) | 1 (20%) | ||
| Medical history | ||||||
| Hypertension | 27 (45%) | 13(41%) | 14 (50%) | 2 (40%) | ||
| Diabetes mellitus | 7 (12%) | 4 (13%) | 3 (11%) | 1 (20%) | ||
| Hypercholesterolaemia | 26 (43%) | 15 (47%) | 11 (39%) | 2 (40%) | ||
| Peripheral vascular disease | 1 (2%) | 1 (3%) | 0 | 0 | ||
| Cerebral ischaemic attack or transient ischaemic attack | 4 (7%) | 3 (9%) | 1 (4%) | 1 (20%) | ||
| Myocardial infarction | 12 (20%) | 7 (22%) | 5 (18%) | 0 | ||
| Percutaneous coronary intervention | 7 (12%) | 3 (9%) | 4 (14%) | 0 | ||
| Coronary artery bypass graft | 7 (12%) | 4 (13%) | 3 (11%) | 0 | ||
| Smoking status | ||||||
| Current smoker | 5 (8%) | 4 (13%) | 1 (4%) | 0 | ||
| Ex-smoker | 31 (52%) | 14 (44%) | 17 (61%) | 2 (40%) | ||
| Non-smoker | 24 (40%) | 14 (44%) | 10 (36%) | 3 (60%) | ||
| Drugs | ||||||
| Aspirin | 60 (100%) | 32 (100%) | 28 (100%) | 4 (80%) | ||
| β blocker | 52 (87%) | 27 (84%) | 25 (89%) | 4 (80%) | ||
| Oral nitrate | 29 (48%) | 16 (50%) | 13 (46%) | 4 (80%) | ||
| Calcium antagonists | 13 (22%) | 7 (22%) | 6 (21%) | 0 | ||
| Nicorandil | 13 (22%) | 8 (25%) | 5 (18%) | 1 (20%) | ||
| Angiotensin-converting-enzyme inhibitor | 28 (47%) | 17 (53%) | 11 (39%) | 2 (40%) | ||
| Angiotensin-receptor blocker | 6 (10%) | 2 (6%) | 4 (14%) | 1 (20%) | ||
| Statin | 58 (97%) | 31 (97%) | 27 (96%) | 3 (60%) | ||
Data are number (%), unless otherwise indicated. Percentages might not add up to 100% because of rounding.
Routine haematology (full blood count) and biochemistry (urea, electrolytes, liver function tests) showed that only alanine transaminase activity increased slightly in patients given allopurinol (from baseline mean 73 U/L [SD 18] to 76 U/L [19]). Allopurinol significantly lowered concentrations of serum uric acid compared with placebo (baseline 0·36 mmol/L [0·06]); placebo 0·36 mmol/L [0·08]; allopurinol 0·14 [0·05] mmol/L; paired t test p<0·0001). These results were kept masked from the investigators to maintain treatment blinding. Differences in concentrations of haemoglobin, serum urea, creatinine, and total cholesterol were not significant (table 2).
Table 2.
Haematology and biochemistry results
| Baseline | Placebo | Allopurinol | |
|---|---|---|---|
| Haemoglobin (g/L) | 13·8 (1·3) | 13·6 (1·1) | 13·5 (1·4) |
| White blood cells (×109 per L) | 6·7 (1·6) | 6·8 (1·6) | 6·6 (1·4) |
| Platelets (×109 per L) | 224·4 (60·3) | 219·6 (58·6) | 218·4 (73·1) |
| Sodium (mmol/L) | 140·5 (2·7) | 140·4 (2·6) | 140·4 (2·6) |
| Potassium (mmol/L) | 4·3 (0·3) | 4·3 (0·3) | 4·3 (0·3) |
| Urea (mmol/L) | 6·4 (1·5) | 6·9 (1·8) | 6·5 (1·7) |
| Creatinine (μmol/L) | 84·9 (16·1) | 85·0 (17·3) | 82·9 (15·9) |
| Estimated glomerular filtration rate (mL per min) | 59·6 (1·7) | 59·1 (3·2) | 59·4 (2·4) |
Data are mean (SD).
Exercise tests were stopped according to the policies of our local National Health Service—ie, most ETTs were stopped because of exhaustion or limiting angina, although they were stopped in seven participants with ST depression of greater than 2 mm in association with angina. Table 3, figure 2, figure 3, and figure 4 show that allopurinol significantly increased total exercise time, time to ST depression, and time to symptoms. Compared with baseline, 35 (58%) patients given placebo improved in their time to ST depression versus 51 (85%) given allopurinol. More patients taking allopurinol than those taking placebo showed an improvement in total exercise time (47 [78%] vs 27 [45%]), and time to chest pain (45 [75%] vs 33 [55%]). Treatment did not alter the reason for ending ETT. There was no significant correlation between baseline urate and the effect of allopurinol on any exercise variable.
Table 3.
Effect of allopurinol on total exercise time, time to ST depression, and time to symptoms
| Baseline | Placebo | Allopurinol | Point estimate*(95% CI) | Mann-Whitney p value* | |
|---|---|---|---|---|---|
| Total exercise time (s) | 301 (251–447) | 307 (232–430) | 393 (280–519) | 58 (45–77) | 0·0003 |
| Time to ST depression (s) | 232 (182–380) | 249 (200–375) | 298 (211–408) | 43 (31–58) | 0·0002 |
| Time to symptoms (s) | 234 (189–382) | 272 (200–380) | 304 (222–421) | 38 (17–55) | 0·001 |
Data are median (IQR), unless otherwise indicated.
For difference between allopurinol and placebo.
Figure 2.
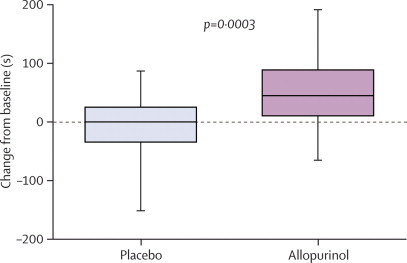
Change in total exercise time from baseline
Data are median (IQR).
Figure 3.
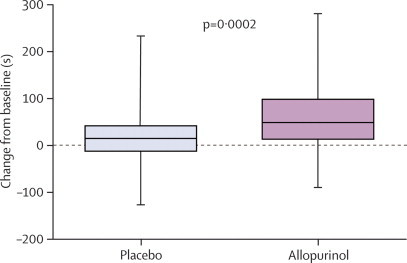
Change in time to ST depression from baseline
Data are median (IQR).
Figure 4.
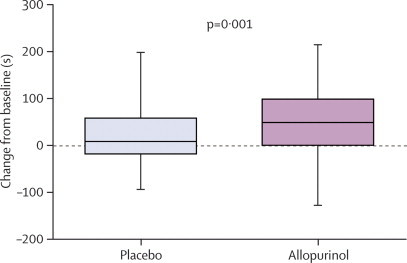
Change in time to chest pain symptoms from baseline
Data are median (IQR).
For all three main exercise variables, there was no effect with time, or any carryover effect between treatments (multilevel model). The p value for treatment-carryover effect was 0·80 for time to ST depression, 0·91 for total exercise time, and 0·60 for time to chest pain. The p value for time effect was 0·74 for time to ST depression, 0·39 for total exercise time, and 0·25 for time to chest pain. For missing data for the five participants who dropped out, we reanalysed the data by inserting the overall mean, with little difference to any endpoint.
Haemodynamic data were normal (table 4). The change from baseline in the maximum heart rate and rate pressure product during exercise was significantly higher with allopurinol than with placebo when analysed with the multilevel model (paired t test). At the end of the first stage of the Bruce protocol, the change in systolic blood pressure from baseline was significantly lower with allopurinol than with placebo (multilevel model analysis and paired t test). The diastolic blood pressure during exercise was significantly lower in the presence of allopurinol. A similar analysis for the end of the second stage of the Bruce protocol was not done because less than half of patients reached the end of stage two. The number of patients who completed stage two was 28 at baseline, 21 assigned to placebo, and 36 assigned to allopurinol. No significant treatment-order effects were noted; time effects were significant for rate pressure product at the end of stage 1 (p=0·02) and for maximum diastolic blood pressure (p=0·002).
Table 4.
Haemodynamic responses during exercise testing
| Baseline | Placebo | Allopurinol | p value* | |
|---|---|---|---|---|
| Heart rate (beats per min) | ||||
| Baseline | 62·3 (10·3) | 61·3 (9·2) | 63·8 (8·6) | 0·025 |
| Stage 1 | 95·2 (13·7) | 94·3 (13·3) | 95·6 (13·5) | 0·154 |
| Peak exercise | 113·6 (15·3) | 112·4 (15·6) | 118·5 (15·2) | 0·0006 |
| Systolic blood pressure (mm Hg) | ||||
| Baseline | 126·8 (16·6) | 124·3 (13·7) | 123·7 (16·2) | 0·755 |
| Stage 1 | 141·6 (21·0) | 140·0 (16·1) | 135·5 (19·3) | 0·042 |
| Peak exercise | 159·3 (22·6) | 155·1 (18·4) | 158·7 (22·4) | 0·116 |
| Diastolic blood pressure (mm Hg) | ||||
| Baseline | 72·8 (8·6) | 72·9 (7·7) | 72·2 (9·9) | 0·577 |
| Stage 1 | 72·9 (10·6) | 74·8 (8·6) | 71·7 (10·1) | 0·008 |
| Peak exercise | 76·1 (12·7) | 78·5 (10·2) | 75·4 (11·9) | 0·015 |
| Rate pressure product (beats per min×mm Hg) | ||||
| Baseline | 7897 (1709) | 7607 (1471) | 7910 (1577) | 0·123 |
| Stage 1 | 13 349 (2997) | 13 114 (2617) | 12 756 (2798) | 0·174 |
| Peak exercise | 18 210 (4104) | 17 484 (3655) | 18 842 (3791) | 0·001 |
Data are mean (SD).
For difference between allopurinol and placebo.
Allopurinol reduced concentrations of brain natriuretic peptide (from baseline median 84·3 pg/mL [IQR 44·8–186·0] to 65·6 pg/mL [37·0–122·7]) compared with placebo (80·4 pg/mL [40·1–132·8]; p=0·045). There was no significant change in concentrations of C-reactive protein from baseline (1·49 mg/L [0·48–2·88]) with allopurinol (1·47 mg/L [0·46–2·71]) or placebo (141 mg/L [0·63–2·78]; p=0·757).
43 participants completed and returned their angina diary cards (table 5). Patients were chosen because they had reproducible ST depression during ETT and not because they were symptomatic with episodes of angina. Allopurinol non-significantly halved the median number of angina episodes per week. Analysis of only the 26 individuals who had one or more episodes per week showed that allopurinol reduced the number of anginal episodes, and use of glyceryl trinitrate (table 5).
Table 5.
Angina episodes
| Placebo | Allopurinol | p value* | |
|---|---|---|---|
| All responders (n=43) | |||
| Angina episodes per week | 1·0 (0–2·5) | 0·5 (0–1·5) | 0·153 |
| Glyceryl trinitrate (tablets per week) | 0·2 (0–2·0) | 0·2 (0–1·2) | 0·157 |
| Responders with one or more angina per week (n=26) | |||
| Angina episodes per week | 2·3 (1·5–4·4) | 1·3(0·5–2·3) | 0·053 |
| Glyceryl trinitrate use (tablets per week) | 1·9 (0·9–3·4) | 0·5 (0–2·0) | 0·064 |
Data are median (IQR).
For difference between allopurinol and placebo.
Discussion
High-dose allopurinol significantly prolonged the time to ST depression, the total exercise time, and the time to angina in patients with chronic stable angina during a standard exercise test, suggesting that endogenous xanthine oxidase activity contributes somehow to exercise-induced myocardial ischaemia. These results also show that high-dose allopurinol prolongs exercise in stable angina pectoris.
The magnitude of the anti-ischaemic effect with allopurinol seems similar to that noted with other antianginal drugs—eg, the absolute increase in median time to ST depression with allopurinol was 43 s (19% increase). Data reported with other antianginal drugs were 36 s (13%) with amlodipine,10,11 60 s (11%) with nitrates,12 12–47 s (4–14%) with phosphodiesterase inhibitors,13,14 46 s (13·5%) with ivabradine,17 and about 50 s (15%) with atenolol and ranolazine.15
What is the mechanism of the anti-ischaemic effect of allopurinol? The prolonged exercise time and increased rate pressure product contradict any effect of allopurinol on reducing cardiac work in the way that β blockers and ivabradine do. Allopurinol can reduce myocardial oxygen consumption for a particular stroke volume.1,3,4 This effect might be related to reduction in oxidative stress because xanthine oxidase is known to use molecular oxygen to produce oxidative stress, and hence blocking the enzyme might prevent oxygen wastage and thereby increase the supply of molecular oxygen in ischaemic tissue.6,18,19 Additionally, oxidative stress could directly cause an anti-ischaemic effect.20 Substrates for ATP, such as AMP, are broken down ultimately by xanthine oxidase, and, as a result, inhibition of this enzyme augments high-energy phosphates, such as ATP, which should supply essential energy to tissues that are depleted of energy by ischaemia.19,21 Therefore, allopurinol could be a pill that increases oxygen and energy (ATP) within ischaemic tissue.
Other mechanisms could also contribute. For example, allopurinol improves peripheral endothelial function,6,22–28 which has two consequences. First, it might be matched by an improvement in coronary endothelial function, meaning that coronary microvascular flow might improve.29 Second, it is matched by a reduction in augmentation index, which means the left ventricular afterload is reduced.30,31 Offloading the heart is a common mechanism of action for other antianginal drugs.
Thus, the anti-ischaemic effect of allopurinol might be due to its effects on oxygen and energy from ATP, but this effect could be supplemented with improved coronary blood flow and reduced left ventricular afterload. Furthermore, the effect of allopurinol on endothelial or vascular function, and its effects on oxygen and energy could be interlinked since increased oxygen consumption seems to occur especially when xanthine oxide is upregulated and nitric oxide synthase is downregulated.20 Nitric oxide synthase downregulation is a common situation within ischaemic tissues and also one in which allopurinol is liable to be particularly advantageous because it can favourably alter both enzymes.
By contrast, we previously reported that allopurinol had no effect on exercise capacity in chronic heart failure.31 There are several good explanations for this difference. First, the cause of exercise incapacity is completely different in chronic heart failure and in coronary artery disease. In chronic heart failure, it is due mainly to dyspnoea resulting from skeletal muscle underperfusion and ergoreflex-induced hyperventilation, whereas in coronary artery disease it is due to myocardial ischaemia. This difference between the two types of cardiopathies can also be shown by the fact that treatments that improve exercise capacity in heart failure (angiotensin-converting-enzyme inhibitors, spironolactone) are very different from the treatments that have antianginal effects in coronary artery disease. Second, we used a lower dose of allopurinol (300 mg) in the previous study, and the dose response is steep between this dose and the 600 mg used in patients in the current study.6 Third, the primary endpoint was a mild 6 min walking test in our previous study,31 whereas it was a demanding Bruce protocol in the present study. The choice of exercise tests was appropriate for each disease but it is another reason not to draw too many comparisons between studies of patients with chronic heart failure and coronary artery disease.
At what stage might allopurinol fit in the treatment of angina pectoris? Chronic stable angina contributes to a substantial reduction in the quality of life, and nearly one in three patients has angina at least once per week.32 Thus, many patients still do not meet the guideline goal according to the American College of Cardiology and American Heart Association of complete absence of exertional angina episodes.33 Therefore, new treatments are needed. Allopurinol might now be regarded as a potential drug for angina. It has many advantages compared with several other available antianginal drugs. Allopurinol is inexpensive compared with some other antianginal drugs such as ranolazine and ivabradine, and has a favourable long-term (>40 years) safety record for the treatment of gout. Compared with older antianginal drugs (nitrates, β blockers), allopurinol is better tolerated because it does not reduce the blood pressure or the heart rate, and does not cause many side-effects, such as headaches and tiredness, that occur frequently with nitrates and β blockers. More data are needed to compare and contrast the use of allopurinol with other treatment options in patients with angina.
The treatments (nitrates, calcium antagonists, and angioplasty) that relieve chest pain on exertion in angina pectoris are usually different from those (statins, aspirin, and angiotensin-converting-enzyme inhibitors) that reduce future cardiovascular events and mortality. Only β blockers seem to improve symptoms and survival. Our results show that high-dose allopurinol improved symptom relief during exercise, which alone is a treatment goal in angina since it is highly relevant to everyday life in patients with this symptom. We assessed this goal in the best way possible by using an objective standardised degree of exercise to assess whether allopurinol prolonged the time to key endpoints during exercise. This method is a more objective way of assessment of exercise capacity than is the use of diary cards since patients with angina often reduce activity in their normal daily life to reduce their chest pain. Furthermore, in general, our data are in accord with those from a study in which allopurinol reduced troponin release during ST-elevation myocardial infarction.34 The limitations of our study are the small sample size (although the size of the effect and the p values are impressive). In the OPT-CHF trial,35 oxypurinol was ineffective in patients with heart failure, probably because the dose (81 mg allopurinol equivalent) used was very low compared with the dose of allopurinol used in our study, and because drugs that have anti-ischaemic effects in angina seldom alter exercise capacity or number of deaths in patients with heart failure.
In conclusion, on the basis of our results, allopurinol is a useful anti-ischaemic treatment option in patients with angina that has the advantage of being inexpensive, well tolerated and safe in the long term. The precise place of allopurinol in the management of angina pectoris now needs to be explored further, but this drug might be especially appealing for use in developing countries where coronary artery disease is rapidly increasing in frequency and where access to expensive drugs or invasive treatments (angioplasty and bypass surgery) is often restricted.
Acknowledgments
Acknowledgments
This work was supported by the British Heart Foundation (FS/06/029). We thank June Anderson for assistance during the exercise tests (data collection).
Contributors
ADS was responsible for the concept and design of the study, and obtained funding. AN was responsible for acquisition of data. SO and AN did the statistical analysis. ADS, AN, and CCL supervised the study. DSCA, SO, and AN provided administrative, technical, and material support. All authors participated in the analysis and interpretation of data, and critical revision of the report for intellectual content; and provided final approval of the submitted version.
Conflicts of interest
The University of Dundee and ADS have applied for a patent for the use of xanthine oxidase inhibitors to treat anginal chest pain. The other authors declare that they have no conflicts of interest.
References
- 1.Ekelund UE, Harrison RW, Shokek O. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 2.Ukai T, Cheng CP, Tachibana H. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;103:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- 3.Cappola TP, Kass DA, Nelson GS. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 4.Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;83:423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- 5.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;95:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 6.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 7.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. 2009;10:27. doi: 10.1186/1745-6215-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grizzle JE. The two-period change-over design and its use in clinical trials. Biometrics. 1965;21:467–480. [PubMed] [Google Scholar]
- 9.Hollander M, Wolfe D. Nonparametric statistical inference. John Wiley and Sons; New York: 1973. pp. 27–33. [Google Scholar]
- 10.Knight CJ, Fox KM. Amlodipine versus diltiazem as additional antianginal treatment to atenolol. Centralised European Studies in Angina Research (CESAR) Investigators. Am J Cardiol. 1998;81:133–136. doi: 10.1016/s0002-9149(97)00893-x. [DOI] [PubMed] [Google Scholar]
- 11.Dunselman PH, van Kempen LH, Bouwens LH, Holwerda KJ, Herweijer AH, Bernink PJ. Value of the addition of amlodipine to atenolol in patients with angina pectoris despite adequate beta blockade. Am J Cardiol. 1998;81:128–132. doi: 10.1016/s0002-9149(97)00877-1. [DOI] [PubMed] [Google Scholar]
- 12.Halcox JP, Nour KR, Zalos G. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- 13.Fox KM, Thadani U, Ma PT. Sildenafil citrate does not reduce exercise tolerance in men with erectile dysfunction and chronic stable angina. Eur Heart J. 2003;24:2206–2212. doi: 10.1016/j.ehj.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Thadani U, Smith W, Nash S. The effect of vardenafil, a potent and highly selective phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction, on the cardiovascular response to exercise in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:2006–2012. doi: 10.1016/s0735-1097(02)02563-9. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95:311–316. doi: 10.1016/j.amjcard.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Campeau L. Grading of angina pectoris. Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- 17.Tardif JC, Ponikowski P, Kahan T. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–548. doi: 10.1093/eurheartj/ehn571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellin V, Isabelle M, Oudot A. Transient reduction in myocardial free oxygen radical levels is involved in the improved cardiac function and structure after long-term allopurinol treatment initiated in established chronic heart failure. Eur Heart J. 2005;26:1544–1550. doi: 10.1093/eurheartj/ehi305. [DOI] [PubMed] [Google Scholar]
- 19.Khatib SY, Farah H, El-Migdadi F. Allopurinol enhances adenine nucleotide levels and improves myocardial function in isolated hypoxic rat heart. Biochemistry (Mosc) 2001;66:328–333. doi: 10.1023/a:1010264216357. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra WF, Paolocci N, St John ME. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 21.Saugstad OD. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98:103–107. [PubMed] [Google Scholar]
- 22.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 23.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30(1 part 1):57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 24.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 25.Doehner W, Schoene N, Rauchhaus M. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 26.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 27.O'Driscoll JG, Green DJ, Rankin JM, Taylor RR. Nitric oxide-dependent endothelial function is unaffected by allopurinol in hypercholesterolaemic subjects. Clin Exp Pharmacol Physiol. 1999;26:779–783. doi: 10.1046/j.1440-1681.1999.03125.x. [DOI] [PubMed] [Google Scholar]
- 28.Mercuro G, Vitale C, Cerquetani E. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Baldus S, Koster R, Chumley P. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan F, George J, Wong K, McSwiggan S, Struthers AD, Belch JJ. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc Ther. 2008;26:247–252. doi: 10.1111/j.1755-5922.2008.00057.x. [DOI] [PubMed] [Google Scholar]
- 31.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–1499. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- 33.Fraker TD, Jr, Fihn SD, Gibbons RJ. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50:2264–2274. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Rentoukas E, Tsarouhas K, Tsitsimpikou C, Lazaros G, Deftereos S, Vavetsi S. The prognostic impact of allopurinol in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.08.037. published online Sept 21. [DOI] [PubMed] [Google Scholar]
- 35.George J, Struthers A. The OPT-CHF (Oxypurinol Therapy for Congestive Heart Failure) trial: a question of dose. J Am Coll Cardiol. 2009;53:2405. doi: 10.1016/j.jacc.2008.07.076. [DOI] [PubMed] [Google Scholar]


