Summary
Background
Whether surgery is beneficial for patients with asymptomatic carotid stenosis is controversial. Better methods of identifying patients who are likely to develop stroke would improve the risk–benefit ratio for carotid endarterectomy. We aimed to investigate whether detection of asymptomatic embolic signals by use of transcranial doppler (TCD) could predict stroke risk in patients with asymptomatic carotid stenosis.
Methods
The Asymptomatic Carotid Emboli Study (ACES) was a prospective observational study in patients with asymptomatic carotid stenosis of at least 70% from 26 centres worldwide. To detect the presence of embolic signals, patients had two 1 h TCD recordings from the ipsilateral middle cerebral artery at baseline and one 1 h recording at 6, 12, and 18 months. Patients were followed up for 2 years. The primary endpoint was ipsilateral stroke and transient ischaemic attack. All recordings were analysed centrally by investigators masked to patient identity.
Findings
482 patients were recruited, of whom 467 had evaluable recordings. Embolic signals were present in 77 of 467 patients at baseline. The hazard ratio for the risk of ipsilateral stroke and transient ischaemic attack from baseline to 2 years in patients with embolic signals compared with those without was 2·54 (95% CI 1·20–5·36; p=0·015). For ipsilateral stroke alone, the hazard ratio was 5·57 (1·61–19·32; p=0·007). The absolute annual risk of ipsilateral stroke or transient ischaemic attack between baseline and 2 years was 7·13% in patients with embolic signals and 3·04% in those without, and for ipsilateral stroke was 3·62% in patients with embolic signals and 0·70% in those without. The hazard ratio for the risk of ipsilateral stroke and transient ischaemic attack for patients who had embolic signals on the recording preceding the next 6-month follow-up compared with those who did not was 2·63 (95% CI 1·01–6·88; p=0·049), and for ipsilateral stroke alone the hazard ratio was 6·37 (1·59–25·57; p=0·009). Controlling for antiplatelet therapy, degree of stenosis, and other risk factors did not alter the results.
Interpretation
Detection of asymptomatic embolisation on TCD can be used to identify patients with asymptomatic carotid stenosis who are at a higher risk of stroke and transient ischaemic attack, and also those with a low absolute stroke risk. Assessment of the presence of embolic signals on TCD might be useful in the selection of patients with asymptomatic carotid stenosis who are likely to benefit from endarterectomy.
Funding
British Heart Foundation.
Background
About 15% of strokes are caused by carotid artery stenosis. In patients with symptomatic carotid stenosis greater than 50–70%, carotid endarterectomy reduces ipsilateral stroke risk by about 75%1 and is generally accepted as being cost effective. However, the situation in patients with asymptomatic carotid stenosis is less clear. Asymptomatic carotid stenosis is more benign than symptomatic carotid stenosis and has an ipsilateral stroke risk of 2% or less per year.2 Two large randomised trials, the Asymptomatic Carotid Atherosclerosis Study (ACAS)3 and the Asymptomatic Carotid Surgery Trial (ACST),4 reported that about 32 patients needed to have carotid endarterectomy to prevent disabling stroke or death in one patient over a 5-year period. The cost-effectiveness of surgery for asymptomatic carotid stenosis has been questioned,5 and recently the benefit of surgery has been suggested to be even less because of the availability of more effective medical therapies.6, 7 Nevertheless, asymptomatic carotid stenosis accounts for a large burden of stroke. Only 15% of strokes are preceded by transient ischaemic attack (TIA) and therefore waiting for stenoses to become symptomatic fails to prevent most strokes caused by carotid stenosis.
Risk–benefit and cost–benefit ratios of carotid endarterectomy in asymptomatic carotid stenosis would be improved if surgery was only done in patients with asymptomatic carotid stenosis who are at particularly high risk of stroke.8 In patients with symptomatic carotid stenosis, the stroke risk increases markedly over the few months after symptom onset, and the mechanism of stroke is believed to be primarily embolic.9 If clinical embolism is a good predictor of the subsequent stroke risk, asymptomatic cerebral emboli might also predict clinical stroke risk. Transcranial doppler ultrasound (TCD) is a non-invasive technique that can be used to detect circulating emboli. These emboli appear as short-direction, high-intensity embolic signals and are accompanied by a characteristic chirping sound. This technique of detecting circulating emboli has high sensitivity and specificity in vivo10 and in vitro.11
In patients with symptomatic carotid stenosis, the presence of embolic signals is an independent predictor of future stroke risk.12, 13, 14 Detection of embolic signals on TCD might be similarly predictive in asymptomatic carotid stenosis, but previous studies have been inconclusive and have reported different results.13, 15, 16 We established the Asymptomatic Carotid Emboli Study (ACES) to assess whether detection of asymptomatic embolic signals by use of TCD could predict stroke risk in patients with asymptomatic carotid stenosis.
Methods
Patients
ACES was a multicentre, international, prospective observational study. The full protocol has been published previously.17 Patients were enrolled between July, 1999, and August, 2007. Patients from 26 centres worldwide were included in the study. Inclusion criteria were at least 70% carotid stenosis, assessed by ultrasound, with no symptoms in the carotid artery territory for at least 2 years. If patients had previously had symptoms in the contralateral carotid artery territory or in the vertebrobasilar territory, they were eligible if those symptoms occurred more than 2 years ago. Patients with contralateral carotid endarterectomy were eligible 1 year after endarterectomy, assuming that they had been asymptomatic in the territory of the operated artery since the operation.
Exclusion criteria were other disease likely to limit life expectancy to less than 3 years; patient, physician, or surgeon unwilling to manage asymptomatic carotid stenosis medically; absence of an acoustic window necessary for TCD; and presence of non-biological prosthetic heart valves (because these can be associated with large numbers of presumed gaseous embolic signals18).
Procedures
At baseline, two 1 h TCD recordings, separated by 1 week, were taken from the ipsilateral middle cerebral artery. In symptomatic carotid stenosis, the risk of recurrent stroke declines rapidly to a low level by 6 months;1 therefore, wherever possible, we did 1 h repeat TCD recordings at the 6, 12, and 18 month follow-up visits to find out whether the presence of embolic signals at the start of each 6-month period predicted risk over the subsequent 6 months.
A standard TCD recording protocol was followed by all centres, which was based on the recommendations of the International Consensus Group on Microembolus Detection.19 A 2 MHz transducer was used to insonate the middle cerebral artery ipsilateral to the asymptomatic carotid stenosis at a depth of between 45 mm and 55 mm. A standard axial sample volume of 5 mm was used. All embolic signal data were recorded onto digital audiotape and analysed centrally by investigators who were masked to clinical information. For central data analysis, the audio signal was played back into the same doppler machine (Pioneer, EME/Nicolet, Madison, USA) with standard settings, and fast Fourier transform spectral analysis was done with an overlap of at least 60%. Standard consensus criteria on embolic signal identification were used in addition to an intensity threshold of 7 dB.20 Embolic signals were identified visually and audibly. All potential embolic signals were then reviewed by a second observer (HSM) to ensure consistency in reporting of whether or not signals were present.
Cardiovascular risk factors were recorded at study entry and brain imaging (CT or MRI) was done. In cases of stroke or TIA during follow-up, repeat brain CT or MRI was done. In addition, all patients had carotid duplex ultrasound at entry and at 12 months, which was done according to a standard protocol17 and recorded onto videotape for central image analysis.
All patients were assessed for hypertension, which was defined as taking antihypertensive drugs, systolic blood pressure over 140 mm Hg, or diastolic blood pressure over 90 mm Hg. We also recorded whether patients had diabetes mellitus, classed as a clinical diagnosis of type 1 or type 2 diabetes. Patients were defined as having ischaemic heart disease if they had a history of angina or myocardial infarction and as having peripheral vascular disease if they had a history of symptomatic disease. Smoking history (present, previous, or never) was also recorded. Atrial fibrillation was recorded as being present if there was a past history of it, or if it was present on electrocardiogram at entry. We noted all drugs each patient was receiving at each follow-up visit.
The primary endpoint was ipsilateral TIA and stroke. Secondary endpoints were ipsilateral stroke, any stroke, and any stroke or cardiovascular death. All endpoints were centrally assessed by review of clinical details and brain imaging by investigators who were masked to the results of the embolic signals recordings.
The primary study hypothesis was that the presence of embolic signals (defined as at least one embolic signal on TCD) on recordings at study entry would predict ipsilateral TIA and stroke risk over the following 2 years. The secondary hypothesis was that the presence of embolic signals on a 1 h recording at baseline or at 6, 12, or 18 months predicted ipsilateral TIA and stroke risk over the subsequent 6-month period.
All patients gave written informed consent and the study was approved by the local ethics committees.
Statistical analysis
Because of the paucity of data from large patient groups on the prevalence of embolic signals in asymptomatic carotid stenosis at the time of study design, sample size calculations were revised in year 3 of the study (March, 2003) by use of interim analysis of baseline recordings from the first 132 patients in whom both first and second initial recordings had been analysed.17 The investigators remained masked to patient identity during this analysis. At this timepoint, 28 of 132 patients had embolic signals during either of two 1 h baseline recordings. We therefore used a prevalence of 20% in calculations for the primary hypothesis. At this interim timepoint, the 2-year combined ipsilateral stroke and TIA rate was 6·6%. The following assumption was made: the risk of stroke or TIA in patients with embolic signals is three-times that seen in unselected asymptomatic carotid stenosis. A relative risk of 3·0 was used because this would be a clinically useful level of relative risk for selection of patients for endarterectomy. For sample size calculations, a power of 0·9 and p value of 0·05 was used. Using these values, a sample size of 440 would be needed to answer our primary hypothesis. Assuming a dropout of 8%, the required sample size was increased to 478. We therefore chose a total sample size of 480.
For the secondary hypothesis, we used the first of the entry recordings and the 1 h recordings made at the 6, 12, and 18 month follow-up visits from 132 patients. Based on prospective data after clinical (ie, symptomatic) emboli in patients with carotid stenosis,9 we assumed that 40% of clinical events would occur in the first 6 months in patients with embolic signals. If each patient's exposure at 6, 12, and 18 months was reclassified according to the presence or absence of embolic signals at these timepoints, then the risk ratio that we would need to detect is RR1=1·6×RR, where RR is the risk ratio over the whole 2-year period. The previous risk ratio of 3·0 corresponds to a new RR1 of 4·8. From our interim analysis, 16 of 132 patients had embolic signals on the first of the two baseline recordings and we therefore assumed a prevalence of 10% for our power calculations. Using these values, a sample size of 270 would be needed to answer our secondary hypothesis. Assuming that 80% of patients would have repeat recordings, the required sample size increased from 270 to 338. In addition, assuming a dropout of 8%, the required sample size was increased to 367. Further details on power calculations are presented in the methodology paper.17
We investigated whether the presence of embolic signals predicted ipsilateral stroke or TIA by use of Kaplan-Meier survival analysis to enable data from patients who did not complete the full 2-year study protocol to be included. For each outcome, event rates per 100 person-years were calculated and used to estimate the absolute annual risk using the formula: annual risk=(1−(exp(−event rate×time)). Hazard ratios and their 95% CIs for the presence of embolic signals were estimated using Cox proportional hazard regression models. Age and sex were controlled for in all models and variables that were associated with embolic signals at baseline were also controlled for. Hazard ratios for patients with embolic signals compared with those without at baseline and 6, 12, and 18 months for the risk of having an endpoint over the subsequent 6-month period were estimated using time-dependent proportional hazards regression models with the same adjustments as described earlier.
The relation between embolic signals at baseline and clinical parameters has been previously published.17 In brief, the use of antiplatelet drugs was the only independent predictor of embolic signals at baseline (odds ratio 0·37, 95% CI 0·19–0·72, p=0·003) after controlling for age, sex, hypertension, diabetes, current smoking, lipid-lowering agents, and antihypertensive drugs. Therefore, antiplatelet use was additionally controlled for in the analyses.
We did a meta-analysis combining the ACES results with those from other prospective studies that reported the relation between the presence of embolic signals and ipsilateral stroke risk and also the relation with ipsilateral stroke and TIA, using previously described methods.13 In brief, Medline, Embase, and PubMed were searched between Jan 1, 1990, and Feb 10, 2010. Only articles in English that reported results in human beings were included. Search terms were (transcranial doppler OR TCD OR ultrasound OR ultrasonography) AND (embolic signals OR HITS OR emboli OR cerebral embolism OR embolic particles OR MES OR microembolic signals) AND (stroke OR transient ischemic attack OR transient ischaemic attack OR amaurosis fugax OR death). Reference lists of articles fulfilling the inclusion criteria and reviews were also searched for relevant references. Data were only included from prospective studies. Case series including fewer than five patients were excluded. Data from each study were extracted independently by two researchers, and meta-analyses were done using RevMan5 software by use of a random effects model.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication. HSM had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
We recruited 482 patients with asymptomatic carotid stenosis. Five patients were excluded for the following reasons: two had mechanical prosthetic heart valves, one had stenosis less than 70%, one dropped out before any TCD recording, and one had unpredictable TCD recordings because of a poor acoustic window. Baseline demographics and cardiovascular risk factors of all recruited patients are shown in table 1.
Table 1.
Demographics and baseline characteristics
| Patients with ACS (n=482) | ||
|---|---|---|
| Age (years) | 71·5 (8·1) | |
| Women | 125 (26%) | |
| Smoking | ||
| Current | 70 (15%) | |
| Previous | 224 (46%) | |
| Never | 188 (39%) | |
| Hypertension | 433 (90%) | |
| Diabetes mellitus | 99 (21%) | |
| Ischaemic heart disease | 178 (40%) | |
| Atrial fibrillation | 35 (7%) | |
| Carotid artery stenosis | ||
| <70% | 1 (0%) | |
| 70–79% | 244 (51%) | |
| 80–89% | 138 (29%) | |
| 90–99% | 99 (21%) | |
| Contralateral carotid stenosis ≥70%* | 94 (20%) | |
| History of ipsilateral ischaemia in study artery territory | 37 (8%) | |
Data are mean (SD) or number (%).
472 patients with data available. ACS=asymptomatic carotid stenosis.
The first baseline TCD recording was done in all 477 patients, and 459 of the recordings could be analysed. The second baseline TCD recording was done in 425 of 477 patients, and 407 of the recordings could be analysed. 467 patients had at least one baseline recording of sufficient quality for embolic signal analysis. 48 of 459 (10%) patients had embolic signals on recording 1 and 44 of 407 (11%) had embolic signals on recording 2. 399 patients had both baseline recordings: of these, 43 had embolic signals on the first recording and 43 on the second recording. 15 of 43 patients had embolic signals on both recordings and 328 of 356 patients did not have embolic signals on either recording. There was a significant association between patients having similar embolic signals status on the two recordings (p<0·0001). At baseline, 77 of 467 patients had embolic signals on either of the two baseline recordings. The mean number of embolic signals in patients with embolic signals detected was 2·63 (median 1, range 1–20) on recording 1 and 2·23 (2, 1–11) on recording 2.
There were 32 primary endpoints during follow-up (26 ipsilateral TIAs and six ipsilateral strokes; table 2). Four of the 26 patients who had ipsilateral TIA later had ipsilateral stroke; thus, there was a total of ten ipsilateral strokes during follow-up. The secondary endpoint of any stroke occurred in 18 patients, and 37 had any stroke or cardiovascular death. During follow-up, 34 patients had carotid endarterectomy: 16 after ipsilateral TIA, one after ipsilateral stroke, and 17 for asymptomatic stenosis.
Table 2.
Primary and secondary endpoints
|
Primary endpoint |
Secondary endpoint |
|||
|---|---|---|---|---|
| Ipsilateral stroke or TIA | Ipsilateral stroke | Any stroke | Any stroke or cardiovascular death | |
| Primary analysis | ||||
| No embolic signals (n=390) | 22 | 5 | 13 | 31 |
| Embolic signals present (n=77) | 10 | 5 | 5 | 6 |
| Total | 32 | 10 | 18 | 37 |
| Secondary analysis | ||||
| No embolic signals (n=1333) | 25 | 6 | 14 | 20 |
| Embolic signals present (n=111) | 5 | 3 | 3 | 5 |
| Total | 30 | 9 | 17 | 25 |
TIA=transient ischaemic attack.
The hazard ratio for the risk of ipsilateral stroke and TIA from baseline to 2 years for patients with embolic signals at baseline compared with those without was 2·54 (95% CI 1·20–5·36; p=0·015; table 3); after controlling for presence versus absence of antiplatelet therapy at baseline, the hazard ratio was 2·39 (1·12–5·11; p=0·025). The absolute annual risk of ipsilateral stroke or TIA was 7·13% in patients with embolic signals and 3·04% in patients without embolic signals. The hazard ratio for the risk of ipsilateral stroke alone at 2 years for patients with embolic signals at baseline compared with those without was 5·57 (1·61–19·32; p=0·007); after controlling for baseline antiplatelet therapy, the hazard ratio was 5·90 (1·68–20·72; p=0·006). The absolute annual risk of ipsilateral stroke was 3·62% in patients with embolic signals and 0·70% for those without. The negative predictive value was 94·4% and the positive predictive value was 13·0%. Controlling for degree of stenosis and other risk factors (as specified in the protocol) did not significantly alter the results (table 4). In addition, there was no effect of the use of statins at baseline (test for interaction p=0·53). There was no association between the presence of embolic signals and risk of any stroke (p=0·14), or any stroke and cardiovascular death (p=0·87; table 3). Kaplan-Meier survival plots for the different outcomes are shown in figure 1.
Table 3.
Primary analysis
|
Number of events |
Person-years |
Event rate (per 100 person-years) |
Adjusted for age and sex |
Adjusted for age, sex, and antiplatelet therapy |
|||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | ||||
| Ipsilateral stroke and TIA | |||||||
| Embolic signals absent | 22 | 713·1 | 3·09 | 1·0 | 1·0 | ||
| Embolic signals present | 10 | 135·0 | 7·40 | 2·54 (1·20–5·36) | 0·015 | 2·39 (1·12–5·11) | 0·025 |
| Ipsilateral stroke | |||||||
| Embolic signals absent | 5 | 716·3 | 0·70 | 1·0 | 1·0 | ||
| Embolic signals present | 5 | 135·6 | 3·69 | 5·57 (1·61–19·32) | 0·007 | 5·90 (1·68–20·72) | 0·006 |
| Any stroke | |||||||
| Embolic signals absent | 13 | 714·5 | 1·82 | 1·0 | 1·0 | ||
| Embolic signals present | 5 | 135·6 | 3·69 | 2·19 (0·78–6·15) | 0·14 | 2·36 (0·83–6·67) | 0·11 |
| Any stroke or cardiovascular death | |||||||
| Embolic signals absent | 31 | 714·8 | 4·34 | 1·0 | 1·0 | ||
| Embolic signals present | 6 | 135·6 | 4·43 | 1·08 (0·45–2·59) | 0·87 | 1·12 (0·46–2·71) | 0·80 |
TIA=transient ischaemic attack.
Table 4.
Association between embolic signals at baseline and risk of stroke or TIA over subsequent 2 years with adjustment for a-priori risk factors
|
Ipsilateral stroke and TIA |
Ipsilateral stroke |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age and sex | 2·54 (1·20–5·36) | 0·015 | 5·57 (1·61–19·32) | 0·007 |
| Age, sex, and antiplatelet therapy | 2·39 (1·12–5·11) | 0·025 | 5·90 (1·68–20·72) | 0·006 |
| Age, sex, and hypertension | 2·73 (1·29–5·79) | 0·009 | 5·47 (1·57–19·04) | 0·008 |
| Age, sex, and diabetes | 2·48 (1·17–5·25) | 0·017 | 5·59 (1·61–19·43) | 0·007 |
| Age, sex, and smoking | 2·54 (1·20–5·40) | 0·015 | 5·24 (1·49–18·35) | 0·010 |
| Age, sex, and degree of stenosis | 2·53 (1·20–5·37) | 0·015 | 5·08 (1·45–17·76) | 0·011 |
TIA=transient ischaemic attack.
Figure 1.
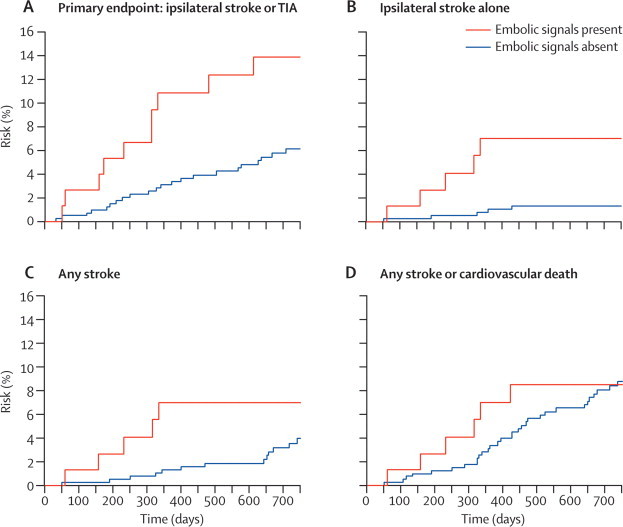
Survival plots for the association between the presence of embolic signals and cumulative event rates for the analysis of whether embolic signals at baseline predict risk
77 patients had embolic signals and 390 did not. TIA=transient ischaemic attack.
For the analysis of 6-monthly time periods, recordings of sufficient quality for analysis were available for 1444 6-monthly periods from 470 patients (233 had four recordings, 102 had three recordings, 71 had two recordings, and 64 had one recording) and embolic signals were present in 111 of these. The hazard ratio for the risk of ipsilateral stroke and TIA for patients with embolic signals at the recording preceding the next 6-month period compared with those without embolic signals at that timepoint was 2·63 (95% CI 1·01–6·88; p=0·049; table 5); after controlling for presence versus absence of antiplatelet therapy at time of the TCD recording, the hazard ratio was 2·65 (1·01–7·00; p=0·049). The absolute annual risk of ipsilateral stroke or TIA was 9·04% in patients with embolic signals and 3·66% in those without. The hazard ratio for the risk of ipsilateral stroke alone for patients with embolic signals at the recording preceding the next 6-month period compared with those without embolic signals at that timepoint was 6·37 (1·59–25·57; p=0·009); after controlling for antiplatelet therapy at time of the TCD recording, the hazard ratio was 6·56 (1·60–26·86; p=0·009). Controlling for degree of stenosis did not alter the results (table 6). The absolute annual risk of ipsilateral stroke was 5·50% in patients with embolic signals and 0·89% in those without embolic signals. The positive predictive value was 94·2% and the negative predictive value was 14·6%. The hazard ratio for the risk of any stroke for patients with embolic signals at the recording preceding the next 6-month period compared with those without embolic signals at that timepoint was 2·88 (0·83–10·04; p=0·10) and for the risk of any stroke or cardiovascular death the hazard ratio was 3·37 (1·26–8·98; p=0·015; table 5). Kaplan-Meier survival plots for the different outcomes are shown in figure 2.
Table 5.
Secondary analysis
| Number of events | Person-years | Event rate (per 100 person-years) |
Adjusted for age and sex |
Adjusted for age, sex, and antiplatelet therapy |
|||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | ||||
| Ipsilateral stroke and TIA | |||||||
| Embolic signals absent | 25 | 670·9 | 3·73 | 1·0 | 1·0 | ||
| Embolic signals present | 5 | 52·8 | 9·47 | 2·63 (1·01–6·88) | 0·049 | 2·65 (1·01–7·00) | 0·049 |
| Ipsilateral stroke | |||||||
| Embolic signals absent | 6 | 673·8 | 0·89 | 1·0 | 1·0 | ||
| Embolic signals present | 3 | 53·0 | 5·66 | 6·37 (1·59–25·57) | 0·009 | 6·56 (1·60–26·86) | 0·009 |
| Any stroke | |||||||
| Embolic signals absent | 14 | 672·1 | 2·08 | 1·0 | 1·0 | ||
| Embolic signals present | 3 | 53·0 | 5·66 | 2·88 (0·83–10·04) | 0·10 | 3·10 (0·88–10·89) | 0·078 |
| Any stroke or cardiovascular death | |||||||
| Embolic signals absent | 20 | 672·7 | 2·97 | 1·0 | 1·0 | ||
| Embolic signals present | 5 | 53·0 | 9·43 | 3·37 (1·26–8·98) | 0·015 | 3·52 (1·31–9·47) | 0·013 |
TIA=transient ischaemic attack.
Table 6.
Embolic signals at the start of each 6-month period and risk over the subsequent 6 months
|
Ipsilateral stroke and TIA |
Ipsilateral stroke |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age and sex | 2·63 (1·01–6·88) | 0·049 | 6·37 (1·59–25·57) | 0·009 |
| Age, sex, and antiplatelet therapy | 2·65 (1·01–7·00) | 0·049 | 6·56 (1·60–26·86) | 0·009 |
| Age, sex, and hypertension | 2·62 (1·00–6·85) | 0·050 | 6·48 (1·62–25·99) | 0·008 |
| Age, sex, and diabetes | 2·64 (1·01–6·90) | 0·048 | 6·37 (1·59–25·55) | 0·009 |
| Age, sex, and smoking | 2·63 (1·01–6·86) | 0·049 | 6·36 (1·58–25·52) | 0·009 |
| Age, sex, and degree of stenosis | 2·60 (1·00–6·82) | 0·051 | 6·03 (1·49–24·40) | 0·012 |
TIA=transient ischaemic attack.
Figure 2.
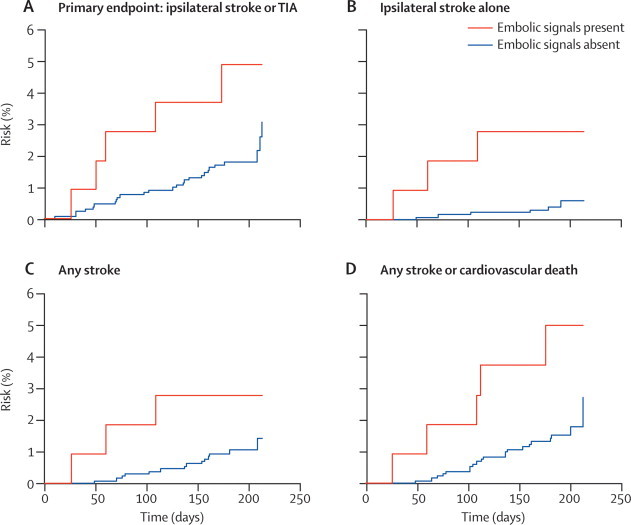
Survival plots for the association between the presence of embolic signals and cumulative event rates for the analysis of whether embolic signals at the start of each 6-month interval predict risk over the subsequent 6-month period
111 recordings had embolic signals and 1333 did not. TIA=transient ischaemic attack.
We did a meta-analysis of the results of the previous published studies15, 16, 21, 22, 23 as well as the primary endpoint data (2-year follow-up analysis) from ACES (figure 3). Data were available for a total of 1144 patients. The cut-off for defining a patient as having embolic signals varied: one or more embolic signals for ACES and three other studies16, 21, 22 and at least two embolic signals for two studies.15, 23 The hazard ratio for the risk of ipsilateral stroke for those with embolic signals compared with those without was 6·63 (95% CI 2·85–15·44; p<0·0001) and there was no heterogeneity between studies (p=0·33). The hazard ratio for the risk of ipsilateral stroke and TIA for those with embolic signals compared with those without was 7·57 (2·32–24·69; p=0·0008), but there was heterogeneity in this analysis (p=0·002).
Figure 3.
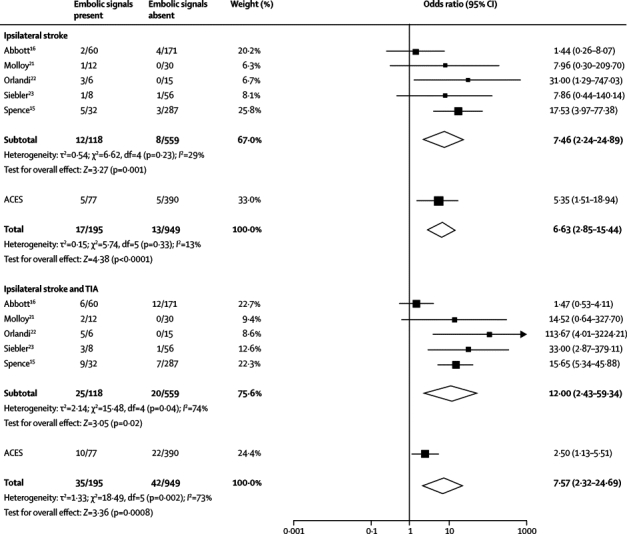
Meta-analyses of ACES with previous studies of the association of embolic signals with future risk of ipsilateral stroke or ipsilateral stroke and TIA
TIA=transient ischaemic attack.
Discussion
In this international multicentre study, the presence of asymptomatic embolisation, detected by TCD, predicted subsequent ipsilateral stroke and TIA and also ipsilateral stroke alone. This suggests that TCD might be useful to identify patients with asymptomatic carotid stenosis who are at increased risk of stroke or TIA, and also to identify patients at low risk in whom surgical intervention will not be beneficial. The technique enabled identification of a group of patients who did not have embolic signals in whom the annual risk of stroke was less than 1%; at this level of risk carotid endarterectomy is not associated with benefit and might actually incur risk.6
Previous studies in symptomatic carotid stenosis have shown that embolic signals predict future stroke risk.12, 13 However, data from asymptomatic carotid stenosis, in which there is a greater potential clinical application, have been less conclusive.13, 15, 16 One study in 319 patients, of whom 210 were available for analysis at 2 years, reported a significant association between embolic signals at baseline and future risk of stroke and TIA.15 In this single-centre study, analysis of embolic signals was done online, rather than by subsequent masked analysis as in ACES; however, the proportion of individuals with embolic signals was similar to that in ACES (10% on a single baseline recording). A second study in 202 individuals with subsequent offline analysis of embolic signals found no association between embolic signals at baseline and subsequent stroke risk.16 Repeat recordings were done in some individuals at 6-monthly intervals and there seemed to be fewer events in patients who consistently did not have embolic signals. The hazard ratio for stroke associated with the presence of embolic signals in ACES was midway between these two studies. Meta-analysis of the results of the primary analysis from ACES with all previous studies showed a significant association between embolic signals and subsequent stroke risk. Meta-analysis also showed a significant association between embolic signals and the combined endpoint of stroke and TIA, although there was heterogeneity between studies for this analysis.
Optimal management of asymptomatic carotid stenosis remains controversial, and practices vary between different clinicians and in different countries. Data from large randomised trials have shown a significant effect of endarterectomy in preventing future stroke, but with a small absolute benefit.3, 4 Recent analyses6, 7 have reported that, with improved medical treatment, the annual risk of stroke in patients with asymptomatic carotid stenosis is lower than that reported in the carotid endarterectomy trials (nearer to 1% in these trials compared with 2·3% in ACAS),3 which further reduces the benefit of surgical intervention. This is consistent with the annual risk of ipsilateral stroke of 1·2% (ten ipsilateral strokes over 2 years) reported in ACES. This improvement in natural history with better medical therapy might make surgical intervention hazardous.6 Carotid stenting has been suggested as an alternative to endarterectomy, but as yet there are no data showing it is safer than endarterectomy for asymptomatic carotid stenosis.
Despite the small absolute benefit from intervention, asymptomatic carotid stenosis accounts for a substantial stroke burden. Most patients with carotid stenosis will not have TIA or minor stroke before disabling stroke. Therefore, an appealing approach is to identify the small group of patients with asymptomatic carotid stenosis and high risk of stroke who would benefit most from surgical intervention. Several markers of high risk have been suggested, including clinical risk factors, degree of carotid stenosis, and plaque characteristics on imaging. However, none have been consistently supported by data from prospective studies.2 The ACES results show that TCD embolic signal detection can be used to identify a high-risk group.
We chose our primary endpoint to allow sufficient numbers of endpoints to detect an association within our sample size. However, we were able to also detect an association with the more robust endpoint of stroke alone. This association was stronger, perhaps because diagnosis of stroke is more reliable than that of TIA. The risk of further stroke in patients with symptomatic carotid stenosis is greatest within the first few weeks and rapidly reduces over the first 6 months.9 We hypothesised that a similar relation might exist with embolic signals; that is, their presence would be associated with an early high risk which would rapidly reduce over a period of weeks to months. For this reason, we assessed whether the presence of embolic signals at the start of each 6-month period predicted risk over the subsequent 6 months. This analysis confirmed associations between the presence of embolic signals and subsequent TIA and stroke risk, and there was also a significant association with the risk of any stroke and cardiovascular death. However, the difference in hazard ratios between prediction over 6 months and our primary analysis of prediction over 2 years was small and our results suggest that the presence of embolic signals is associated with risk over a longer follow-up period.
An important consideration with any risk stratification technique is whether it provides additional information over conventional risk factors. Controlling for whether the patient was treated with antiplatelet therapy, which was associated with the presence of embolic signals at baseline, did not markedly alter the association between embolic signals and future stroke risk. In addition, controlling for other risk factors that were specified in the study protocol17 but that were not associated with the presence of embolic signals at baseline had little effect on the associations with stroke and TIA or stroke alone. This supports the use of embolic signals as an independent predictor of stroke risk.
ACES is the first prospective, multicentre, international study of the predictive value of the detection of embolic signals. ACES included over 20 centres from different health-care systems, both academic and non-academic, and therefore its results are widely applicable. Analysis of embolic signals was done centrally by investigators who were masked to clinical information, and endpoint assessment was done centrally by investigators masked to the results of embolic signals analysis. There was only a small amount of missing data and no patients were lost to follow-up. There is a paucity of large multicentre studies evaluating the clinical impact of new neurovascular ultrasound techniques, which has resulted in uncertainty over their clinical application. However ACES, and other multicentre studies on embolic signal detection,14, 24 therapeutic ultrasound,25 and diagnostic ultrasound,26 show that such multicentre studies are feasible.
A potential limitation of ACES is that bias could have occurred in those cases of asymptomatic carotid stenosis where the surgeon was unwilling to enrol the patient, which could have led to exclusion of a higher risk group of patients. However, embolic signal results were not available at the time this decision was made and therefore would not have influenced the decision as to whether to enrol a patient in the study. A second limitation is that there were only ten strokes during follow-up, although there were 32 ipsilateral strokes or TIA.
If TCD is to be used as a clinical tool for risk stratification, improved methods of automated detection of embolic signals are needed. TCD recording itself is simple, non-invasive, and widely used in clinical practice worldwide. However, review of data for the presence of embolic signals is time consuming and relies on trained observers. Inter-observer reproducibility studies have reported that there is a high reproducibility among trained observers in detection of embolic signals, but that without adequate training some centres interpret the criteria incorrectly.27 The rate of embolisation we detected was low, with a median count of one per hour in those patients with embolic signals. Therefore, such automated systems need to be both sensitive and specific. Automated systems have been developed that have high sensitivity and specificity for detecting the higher intensity embolic signals seen in patients with symptomatic stenosis and in the immediate period after carotid endarterectomy.28 However, these systems were less sensitive to the lower intensity embolic signals found in asymptomatic carotid stenosis.29 Further work is needed to develop more sensitive systems, although there are several promising image analysis techniques that could be used in such systems. In our study we did all TCD recordings for 1 h. Further analysis of ACES data, and in particular an individual patient meta-analysis of studies to date, might allow us to identify the optimal duration of recording.
In summary, ACES shows that detection of embolic signals by TCD can identify groups of patients with asymptomatic carotid stenosis who are at low or high risk of future stroke. This technique might be a useful risk predictor for identifying those patients who might benefit from intervention with carotid endarterectomy.
Acknowledgments
Acknowledgments
The study was funded by a British Heart Foundation Programme Grant (RG99/073 and renewal RG04/002). We thank Peter Rothwell for assistance with Figure 1, Figure 2.
Contributors
HSM had the idea for the study, designed the study, obtained funding, was the principal investigator for the study, and wrote the first draft of the paper. AK was a trial coordinator and did the data management and analysis of results. MS was the study statistician and was involved in study design, analysis of results, and writing of the paper. RT did the data management and analysis of results. MC and SR were trial coordinators. NMB and AS recruited patients and were involved in the writing of the paper.
Study personnel
St George's University of London, London Hugh Markus (principal investigator), Alice King (study coordinator), Jennifer Siegel (study coordinator), Sheila Reihill (study coordinator), Marisa Cullinane (study coordinator), Helen McCorie, Emma Morgan, Sun Kwon, Raffi Topakian, Kelly Jones, Ruth Keating. University College, London, London Martin Shipley (statistician).
Study centres
Austria Wagner-Jauregg Hospital, Linz (Franz Aichner, Stefan Guggenberger; number of patients: 1). China Harbin Medical University, Harbin (Song-Bin Qu; 20); Prince of Wales Hospital, Hong Kong Special Administrative Region (Lawrence Wong, Sunny Qing Hao, Roxanna Liu; 3). Croatia University Hospital Zagreb, Zagreb (Vida Demarin, Vlasta Vukovic; 12). France Bretonneau Hospital, Paris (Francois Tranquart, Aurore Bleuzen; 2). Georgia State Medical Academy, Tbilisi (Marina Alpaidze, Nana Metreveli; 12). Germany J W Goethe University, Frankfurt (Matthias Sitzer, Oliver Singer; 14); University of Dusseldorf, Dusseldorf (Mario Siebler, Holger Schade, Torge Brosig, Christina Boettcher, Verica Jovanovic; 8); University of Münster, Münster (E Bernd Ringelstein, Martin Ritter, Ralf Dittrich; 19). Ireland James Connolly Memorial Hospital, Dublin (Dermot Fitzgerald, Nuala McMahon; 8). Israel Rabin Medical Centre, Petah Tikva, Israel (Jonathan Streifler, Tilda Sabah; 7); Tel Aviv Sourasky Medical Centre, Israel (Natan Bornstein, Alex Gur, Sigal Lorenz; 46). Italy San Martino Hospital Genova, Genove (Giulia Brusa, Vittorio Montano, Gian Andrea Ottonello; 21). Lithuania Vilnius University Hospital, Vilnius (Dalius Jatuzis; 19). Netherlands Martini Ziekenhuis Groningen, Groningen (Arjen Schaafsma, An Fokkens; 84). Poland Institute of Psychiatry and Neurology, Warsaw (Anna Czlonkowskia, Anna Rozenfeld, Anna Piorkowska, Marta Skowronska; 5). Singapore Singapore General Hospital Campus, National Neuroscience Institute (Hui-Meng Chang, Moi Pin Lee, Meng Cheong Wong, Christopher P L H Chen; 15). Slovenia University Medical Centre Ljubljana, Ljubljana (Bojana Zvan, Janja Pretnar; 4). Spain University Hospital Josep Trueta, Girona (Joaquin Serena, Xavier Ustrell; 19). UK Charing Cross Hospital, London (Alun Davies; 6); Kings College Hospital, London (Paul Baskerville, Colin Deane, David Goss; 31); Leicester Royal Infirmary, Leicester (Ross Naylor, Jo Walker; 23); St George's University of London, London (coordinating centre) (Hugh Markus, Jennifer Siegel; 71); UCL Institute of Neurology, London (Martin M Brown; 1); University Hospital of South Manchester, Manchester (Charles McCollum, Sarah Welsh, Zoe Bonner; 26). USA University of California Los Angeles School of Medicine, CA (Jeffrey Saver, Gina Paek; 5)
Conflicts of interest
We have no conflicts of interest.
References
- 1.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ, for the Carotid Endarterectomy Trialists Collaboration Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 2.Abbott AL, Bladin C, Levi C, Chambers B. What should we do about asymptomatic carotid stenosis? Int J Stroke. 2007;2:27–39. doi: 10.1111/j.1747-4949.2007.00096.x. [DOI] [PubMed] [Google Scholar]
- 3.Asymptomatic Carotid Atherosclerosis Study Group Carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.Halliday A, Mansfield A, Marro J, MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 5.Naylor AR, Gaines PA, Rothwell PM. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg. 2009;37:625–632. doi: 10.1016/j.ejvs.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573–e578. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 7.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke. 2010;41:e11–e17. doi: 10.1161/STROKEAHA.109.561837. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides AN. Asymptomatic carotid stenosis and risk of stroke. Identification of a high-risk group. Int Angiol. 1995;14:21–23. [PubMed] [Google Scholar]
- 9.European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results in the MRC European carotid surgery trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 10.Russell D, Madden KP, Clark WM, Sandset PM, Zivin JA. Detection of arterial emboli using doppler ultrasound in rabbits. Stroke. 1991;22:253–258. doi: 10.1161/01.str.22.2.253. [DOI] [PubMed] [Google Scholar]
- 11.Markus HS, Brown MM. Differentiation between different pathological cerebral embolic materials using transcranial doppler in an in vitro model. Stroke. 1993;24:1–5. doi: 10.1161/01.str.24.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Markus HS, MacKinnon A. Asymptomatic embolisation, detected by doppler ultrasound, predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36:971–975. doi: 10.1161/01.STR.0000162717.62684.40. [DOI] [PubMed] [Google Scholar]
- 13.King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and meta-analysis. Stroke. 2009;40:3711–3717. doi: 10.1161/STROKEAHA.109.563056. [DOI] [PubMed] [Google Scholar]
- 14.Markus HS, Droste DW, Kaps M. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 15.Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG. Absence of microemboli on transcranial doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke. 2005;36:2373–2378. doi: 10.1161/01.STR.0000185922.49809.46. [DOI] [PubMed] [Google Scholar]
- 16.Abbott AL, Chambers BR, Stork JL, Levi CR, Bladin CF, Donnan GA. Embolic signals and prediction of ipsilateral stroke or transient ischemic attack in asymptomatic carotid stenosis: a multicenter prospective cohort study. Stroke. 2005;36:1128–1133. doi: 10.1161/01.STR.0000166059.30464.0a. [DOI] [PubMed] [Google Scholar]
- 17.ACES Investigators The asymptomatic carotid emboli study: study design and baseline results. Int J Stroke. 2009;4:398–405. doi: 10.1111/j.1747-4949.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 18.Georgiadis D, Mackay TG, Kelman AW, Grosset DG, Wheatley DJ, Lees KR. Differentiation between gaseous and formed embolic materials in vivo: application in prosthetic heart valve patients. Stroke. 1994;25:1559–1563. doi: 10.1161/01.str.25.8.1559. [DOI] [PubMed] [Google Scholar]
- 19.Ringelstein EB, Droste DW, Babikian VL. International Consensus Group on Microembolus Detection. Consensus on microembolus detection by TCD. Stroke. 1998;29:725–729. doi: 10.1161/01.str.29.3.725. [DOI] [PubMed] [Google Scholar]
- 20.Markus HS, Molloy J. The use of a decibel threshold in the detection of embolic signals. Stroke. 1997;28:692–695. doi: 10.1161/01.str.28.4.692. [DOI] [PubMed] [Google Scholar]
- 21.Molloy J, Markus HS. Asymptomatic embolisation predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke. 1999;30:1440–1443. doi: 10.1161/01.str.30.7.1440. [DOI] [PubMed] [Google Scholar]
- 22.Orlandi G, Fanucchi S, Sartucci F, Murri L. Can microembolic signals identify unstable plaques affecting symptomatology in carotid stenosis? Stroke. 2002;33:1744–1746. doi: 10.1161/01.str.0000020967.64837.f3. [DOI] [PubMed] [Google Scholar]
- 23.Siebler M, Nachtmann A, Sitzer M. Cerebral microembolism and the risk of ischaemia in asymptomatic high-grade internal carotid artery ischaemia. Stroke. 1995;26:2184–2186. doi: 10.1161/01.str.26.11.2184. [DOI] [PubMed] [Google Scholar]
- 24.Wong LKS, Chen C, Fu J, for the CLAIR study investigators Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–497. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov AV, Molina CA, Grotta JC, CLOTBUST Investigators Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 26.Allendoerfer J, Goertler M, von Reutern GM, Neurosonology in Acute Ischemic Stroke Study Group Prognostic relevance of ultra-early doppler sonography in acute ischaemic stroke: a prospective multicentre study. Lancet Neurol. 2006;5:835–840. doi: 10.1016/S1474-4422(06)70551-8. [DOI] [PubMed] [Google Scholar]
- 27.Markus HS, Ackerstaff R, Babikian V. Inter-centre agreement in reading doppler embolic signals: a multicentre international study. Stroke. 1997;28:1307–1310. doi: 10.1161/01.str.28.7.1307. [DOI] [PubMed] [Google Scholar]
- 28.Cullinane M, Reid G, Dittrich R. Evaluation of a new on-line automated embolic signal detection algorithm, including comparison with a panel of international experts. Stroke. 2000;31:1335–1341. doi: 10.1161/01.str.31.6.1335. [DOI] [PubMed] [Google Scholar]
- 29.Cullinane M, Kaposzta Z, Reihill R, Markus HS. On-line automated detection of cerebral embolic signals from a variety of embolic sources. Ultrasound Med Biol. 2002;28:1271–1277. doi: 10.1016/s0301-5629(02)00615-4. [DOI] [PubMed] [Google Scholar]


