Abstract
Acinetobacter baumannii has been increasingly reported as a significant causative organism of various nosocomial infections. Here we describe an outbreak of carbapenem-resistant A. baumannii (CRAB) in the ICUs of a Korean university hospital, along with a successful outbreak control program. From October 2007 through July 2008, CRAB was isolated from 57 ICU patients. Nineteen patients were diagnosed as being truly infected with CRAB, four of whom were presumed to have died due to CRAB infection, producing a case-fatality rate of 21.1%. In surveillance of the environment and the healthcare workers (HCWs), CRAB was isolated from 24 (17.9%) of 135 environmental samples and seven (10.9%) of 65 HCWs. The pulsed field gel electrophoresis patterns showed that the isolates from patients, HCWs, and the environment were genetically related. Control of the outbreak was achieved by enforcing contact precautions, reducing environmental contamination through massive cleaning, and use of a closed-suctioning system. By August 2008 there were no new cases of CRAB in the ICUs. This study shows that the extensive spread of CRAB can happen through HCWs and the environmental contamination, and that proper strategies including strict contact precautions, massive environmental decontamination, and a closed-suctioning system can be effective for controlling CRAB outbreaks.
Keywords: Acinetobacter baumannii, Disease Outbreaks, Infection Control
INTRODUCTION
Nosocomial infections are mostcommonly encountered in intensive care units (ICUs). Acinetobacter baumannii has been increasingly reported as a significant causative organism of various nosocomial infections, including pneumonia, sepsis, wound infection, urinary tract infection, and post-neurosurgical meningitis, especially among critically ill patients in ICUs (1, 2). A. baumannii is able to survive for long periods on dry surfaces, and this ability to tolerate desiccation (3), as well as development of multi-drug resistance within the strain, may contribute to its persistence in hospitals (4, 5). Carbapenems are usually the antimicrobial agents of choice for treating serious A. baumannii infections, but resistance to these compounds is increasing worldwide and, in some instances, is associated with high morbidity and mortality rates (6, 7). Outbreaks of nosocomial infections due to infection with carbapenem-resistant A. baumannii (CRAB) have been described previously (8, 9). Risk factors associated with CRAB acquisitions include antibiotic exposure, length of stay in an ICU, mechanical ventilation, and being housed in a trauma ICU (10-12). Investigations of several outbreaks of CRAB infection have implicated contamination of the inanimate hospital environment and person-to-person contact in the spread of these carbapenem-resistant strains during hospital outbreaks (8, 13). The application of meticulous environmental decontamination and strict adherence to infection control practices are vital to interrupting transmission, but in some outbreaks the complete closure of units has been necessary (8, 14).
From October 2007 through July 2008, in the two ICUs of Korea University Ansan Hospital, Ansan-si, Korea, an increased number of infections or colonizations due to CRAB strains was noted. In this study, we describe the outbreak of CRAB in the two ICUs and the infection control program that proved successful in reducing the spread of CRAB.
MATERIALS AND METHODS
Hospital setting
Korea University Ansan Hospital is a 609-bed teaching hospital that houses two ICUs: an 18-bed medical intensive care unit (MICU) that has two single isolation rooms and an 18-bed surgical intensive care unit (SICU) that has one single isolation room. The two ICUs are on the same floor, but are separated from one another.
Case definition
Patients with at least one clinical sample positive for CRAB were defined as cases of CRAB colonization or infection. Clinical episodes of colonization or infection were considered only if the specimen for culture was acquired 48 hr after ICU admission. Nosocomial infections were identified on the basis of the standard Centers for Disease Control and Prevention (CDC) definitions (15). Isolates resistant to imipenem and meropenem (minimum inhibitory concentration >8 mg/L) were considered carbapenem-resistant regardless of their susceptibility to other antibiotics. All patients infected or colonized with CRAB over the outbreak period were included in the study.
Culture surveillance and microbiological methods
Samples were obtained from the surroundings of patients infected or colonized with CRAB, including bed rails, bedside tables, infusion pumps, blood pressure cuffs, external surfaces of the endotracheal tubes of all patients receiving mechanical ventilation, ventilator control panels, and condensate formed in the inspiratory phase tubing of the ventilator circuits. A total of 135 swab samples were taken from the environment of patients infected or colonized with CRAB. The hands of health care workers (HCWs), including doctors, nurses, and nursing aides, were also sampled to assess the potential of hand carriage, given the multiple contacts with ventilators and other patient-care items. A total of 65 staff members were screened, including two doctors, 47 nurses, and 16 nursing aides.
Samples were taken using sterile cotton-tipped swabs moistened with sterile distilled water. They were immediately inoculated onto 5% sheep blood agar plates and incubated overnight in air at 37℃.
During the study period, various clinical samples (e.g., blood, tracheal secretions, wounds, drainage fluid, and urine) were also regularly obtained for culture from all patients. All isolates underwent species identification and antibiotic susceptibility testing using a MicroScan automated instrument (Dade Behring, West Sacramento, CA, USA).
Molecular typing
The genetic relationships of the CRAB isolates were analyzed using pulse-field gel electrophoresis (PFGE) (16). Chromosomal DNA from A. baumannii was prepared in agarose blocks and was cleaved with ApaI. Electrophoresis was performed with the CHEF DR II system (Bio-Rad, Hercules, CA, USA) in 0.5× TBE buffer. The electrophoretic conditions were 6 V/cm at 12-14℃, with altering pulses at 120° in a 5-15 sec gradient for 10 hr and then a 15-45 sec gradient for 15 hr. The Lambda Ladder Standard (New England Biolabs, Ipswich, MA, USA) was used as a size marker, and band images were captured using the Molecular Imager Gel Doc XR system (Bio-Rad). Electrokaryotypes were compared and classified using the following criteria: identical bands, isolate is part of outbreak; differences in 2-3 bands, isolate is probably part of the outbreak; differences in 4-6 bands, the isolate is possibly part of the outbreak; and differences in more than 7 bands, the isolate is unrelated to the outbreak (17).
Review of the research plan
This study was performed with approval of the Institutional Review Board (IRB) of Korea University Ansan Hospital (IRB No.: AS 09089), and consent was waived.
RESULTS
Outbreak description
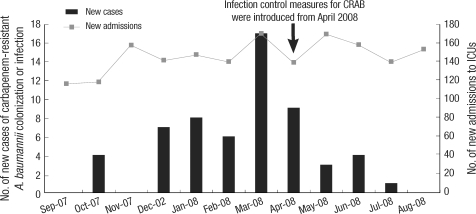
In October 2007, the number of new cases of CRAB colonization/infection in the ICUs increased to four cases per month (3.39 cases per 100 new ICU admissions per month) (Fig. 1). Because the number of new cases of CRAB colonization/infection in the ICUs was zero to two per month (0-1.54 cases per 100 new admissions to the ICUs per month) during the nine-month period from January to September 2007, the increase in CRAB colonizations/infections was defined as an outbreak. The index case was a 57-yr-old female patient who had been hospitalized in the general ward for ten days before being admitted to the MICU with septic shock and pneumonia following an operation for a burst fracture of the C6 vertebra and spondylodiscitis of the C5-6 intervertebral disc. The patient shared a nursing staff with the second case.
Fig. 1.
Time course of the outbreak of colonization/infection with carbapenem-resistant A. baumannii (CRAB) in two intensive care units (ICUs). The graph displays the number of new cases of CRAB infection and colonization and the number of new patients admitted to ICUs. Infection control measures were introduced from April 2008.
During the outbreak period (from October 2007 through July 2008), a total of 204 specimens from 57 patients were positive for CRAB: 42 MICU patients and 15 SICU patients. The incidence of CRAB colonization/infection was 38.6 cases per 1,000 ICU patients. The mean age of the affected patients was 59.09±20.65 yr (range 1-83) and 41 (71.9%) of the patients were male. The average length of stay in the ICUs before isolation of CRAB was 7.98±6.08 days (range 2-26). Among the 204 CRAB isolates, 155 (76.0%) were recovered from the respiratory tract, 21 (10.3%) from blood, seven (3.4%) from wounds, four (2.0%) from hemovac, three (1.5%) from urine, and 14 (6.9%) from other specimens.
Clinical impact of the outbreak
Among the affected patients (n=57), 19 (33.3%) patients were diagnosed as being truly infected with CRAB: 17 (89.4%) had pneumonia, one (5.3%) had peritonitis, and one (5.3%) had a pancreatic pseudocyst infection. Three patients also had bacteremia with CRAB. Among the patients who were colonized or infected with CRAB, 20 (35.1%) died during hospitalization, and four patients were presumed to have died due to CRAB infection, producing a case-fatality rate of CRAB infection of 21.1%. In 2007, before the outbreak occurred, the overall mortality rate in both ICUs was 8.2%. During the CRAB outbreak, the mortality rate increased to 9.9%.
Microbiological investigation of the environment and HCWs
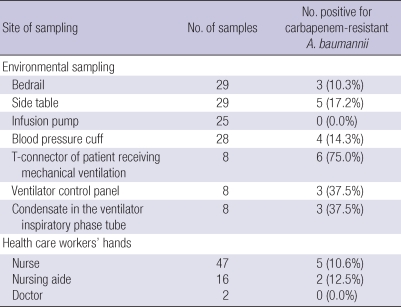
Twenty-four (17.9%) of the 135 environmental screening samples were positive for CRAB (Table 1), which was isolated from all of the types of environmental reservoirs tested, except infusion pumps. The hands of seven of the 65 HCWs (10.9%; five nurses and two nursing aides) were positive for CRAB; the two doctors were negative for CRAB.
Table 1.
Distribution of the cultures from environmental sampling and health care workers' hands
Susceptibility patterns and molecular typing of the isolates
All of the isolates were resistant to imipenem and meropenem. The isolates from 15 patients were resistant to all kinds of antibiotics except colistin, and the isolates from 42 patients were resistant or intermediately susceptible to ampicillin/sulbactam, amikacin, tobramycin, ceftazidime, cefepime, ciprofloxacin, or trimethoprim/sulfamethoxazol.
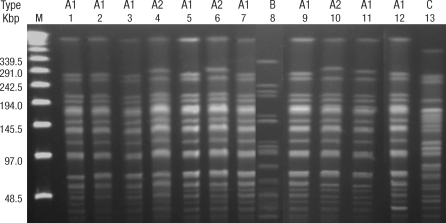
The PFGE patterns for the isolates are presented in Fig. 2. Pulsed field gel electrophoresis analysis grouped the isolates into three distinct clonal types, with one of them containing two subtypes. This PFGE patterns showed that the isolates taken from patients, from the hands of health care workers, and from the ward environment were genetically related and so were considered to be involved in the outbreak. The data suggest that type A1 CRAB was probably the major causative organism of the outbreak.
Fig. 2.
Banding patterns determined by pulsed field gel electrophoresis. The patterns show the genetic relatedness of the isolates of carbapanem-resistant Acinetobacter baumannii recovered from patients, health care workers, and the environment. Lanes 1-5 are from patients, 6-11 are from environmental samples, and 12 and 13 are from the hands of HCWs. Electrokaryotypes were compared and classified using the band difference criteria described in the Methods section.
Outbreak control measures
During the outbreak, an outbreak control team was organized and included infectious disease physicians, an infection control nurse, and senior members of the nursing staff of both ICUs. The team established the outbreak control strategies and implemented them beginning in April 2008.
The first outbreak control strategy was to prevent the handborne transmission of CRAB via HCWs through strict contact precautions. The second strategy was to reduce environmental contamination by cleaning all areas of the ward environment including the equipment. The third strategy was to prevent transmission and environmental contamination of CRAB during endotracheal suctioning by use of a closed-suctioning system. Education was provided for all staff in both ICUs, emphasizing the importance of hand hygiene and of controlling environmental contamination.
According to the strategies, all affected patients were isolated in single rooms or cohort areas with strict contact precautions. Disposable gowns and gloves were introduced for all HCWs caring for the affected patients, and only the minimum number of staff members required for patient care was allowed to enter the affected rooms. Any member of the nursing staff that was caring for affected patients was not allowed to care for any unaffected patients. Contact precautions were continued until two negative cultures were reported with a one-week interval. When a patient left an ICU, areas where the affected patient had been were thoroughly cleaned and disinfected, and new patients were admitted to the areas only if the environmental samples were confirmed to be negative for CRAB.
The environment of the ICU and the surrounding areas were cleaned thoroughly with 100 ppm sodium dichloroisocyanurate. A higher concentration (200 ppm) was used to clean the environment in which the CRAB patients were hospitalized.
Because CRAB was isolated from the respiratory tract in most cases, the improper technique of open-suctioning was presumed to be the main cause of environmental contamination. A closed-suctioning system was introduced for all patients receiving mechanical ventilation, and for those who did not receive mechanical ventilation, new guidelines for aseptic techniques of open suctioning were introduced.
The hospital has a computerized antibiotic prescription system with a restriction policy for the use of specific agents including imipenem and meropenem so that prescription of either drug requires approval from infectious disease specialists. During the period of the outbreak, prudent use of antibiotics in the ICU was emphasized while maintaining the antibiotic policy.
Results of outbreak control measures
The number of newly diagnosed cases per month increased to a maximum of 17 in March 2008 and began to decrease after the introduction of outbreak control measures. By August 2008, there were no new cases of CRAB colonization or infection in either ICU.
DISCUSSION
An outbreak is defined as an increase in the number of infections caused by a particular pathogen above baseline levels (18). The rise in rates of a particular pathogen may be due to lapses in infection control measures, resulting in an increase in cross-transmission between patients (19). Outbreaks of multidrug-resistant A. baumannii strains in ICUs have been described previously (8, 10, 14), and several of these studies reported that environmental contamination and hand carriage by HCWs were the main sources of transmission of this bacteria during the outbreaks. Acinetobacter spp. can stay alive on dry surfaces or in environmental samples for months and, thus, have a high potential to spread (20, 21). Furthermore, dissemination of this pathogen is facilitated by high colonization rates among hospitalized patients and frequent contamination of the hands of HCWs (10, 14).
In the ICU outbreak reported here, microbiological investigations of the patients' immediate environment and hands of HCWs showed extensive CRAB contamination. Moreover, molecular testing showed that CRAB isolates of the same genotype were recovered from the clinical and environmental samples as well as from HCWs' hands. These data indicate that the patient-to-patient cross transmission of CRAB was related to contact with contaminated items in the environment and to transient colonization of HCWs' hands. Moreover, these data support the view that infection control measures for CRAB require multifactorial implementation of specific control measures, strict adherence to infection control precautions for patients and HCWs, application of meticulous environmental decontamination, and use of a closed-suctioning for intubated patients.
In the current outbreak, the spread of CRAB was presumed to be mainly through HCWs' hands and the environment. Although CRAB was isolated only from nurses and nursing aides, doctors could also have been the source of transmission. The two ICUs are located on separate areas of the same floor, with nurses and nursing aides being limited to only one or the other ICU. Therefore, the spread of CRAB between the two ICUs could not be explained by only hand carriage by nurses or nursing aides. Unlike the nurses and nursing aides, doctors care for patients in both ICUs and so could have been a conduit of transmission of CRAB. Similarly, other HCWs such as medical technologists, radiological technologists, or physical therapists who care for patients in both ICUs could have been a source of transmission. These findings indicate that the most important strategy in the control of an outbreak is to follow strict contact precautions.
The main clinical diagnosis of CRAB infection in this outbreak was pneumonia. This observation was the reason why we introduced the closed-suctioning system for control of the outbreak. The effect of the closed-suctioning system for prevention of ventilator-associated pneumonia still remains controversial, though a few studies have reported that this type of system reduces the risk of respiratory infection (22). However, a closed-suctioning system can decrease environmental contamination (23, 24) and has been recommended as a measure for the control of multidrug-resistant A. baumannii (25). Although we could not assess the true effect of the closed-suctioning system for prevention of CRAB pneumonia, it is possible that it was helpful in decreasing environmental contamination and patient-to-patient transmission of CRAB.
In some previous studies, complete closure of units was necessary to control outbreaks (8, 14). However, closing the ICUs during the outbreak in this hospital was not possible because the hospital is the only referral facility in the region. With this outbreak, we demonstrated that the closure of the contaminated units is not essential, and that it is sufficient to practice strict contact precautions and to reduce environmental contamination to control an outbreak of CRAB.
The PFGE results demonstrated that the patient isolates belonged to a single genotype. This finding agrees with previous observations that the majority of A. baumannii isolates in specific institutional outbreaks belonged to a single clone (26), while polyclonal outbreaks and sporadic strains coexisting with epidemic strains have also been reported (27, 28). As compared to isolates from patients, isolates from the environmental samples had diverse genotypes. This result indicates that a specific genotype might be more virulent than the others. To date, only a few virulence factors have been reported for this particular bacterial strain (29, 30). More studies are needed to identify other associated factors.
The present study was limited by the lack of routine surveillance culture for all patients newly admitted to the ICUs. As a result, we could not discriminate new cases who acquired CRAB in the ICUs from imported cases who acquired CRAB prior to admittance to the ICUs. Another limitation was that the control measures were introduced at short intervals, which did not allow for an assessment of which strategy was the most effective.
This study shows that the extensive spread of CRAB can occur through contact with HCWs and the environment, and that proper strategies including strict contact precautions, massive environmental decontamination, and use of a closed-suctioning system can be effective for the control of a CRAB outbreak.
References
- 1.Cefai C, Richards J, Gould FK, McPeake P. An outbreak of Acinetobacter respiratory tract infection resulting from incomplete disinfection of ventilatory equipment. J Hosp Infect. 1990;15:177–182. doi: 10.1016/0195-6701(90)90128-b. [DOI] [PubMed] [Google Scholar]
- 2.Siegman-Igra Y, Bar-Yosef S, Gorea A, Avram J. Nosocomial acinetobacter meningitis secondary to invasive procedures: report of 25 cases and review. Clin Infect Dis. 1993;17:843–849. doi: 10.1093/clinids/17.5.843. [DOI] [PubMed] [Google Scholar]
- 3.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aygun G, Demirkiran O, Utku T, Mete B, Urkmez S, Yilmaz M, Yasar H, Dikmen Y, Ozturk R. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect. 2002;52:259–262. doi: 10.1053/jhin.2002.1300. [DOI] [PubMed] [Google Scholar]
- 5.Levin AS, Gobara S, Mendes CM, Cursino MR, Sinto S. Environmental contamination by multidrug-resistant Acinetobacter baumannii in an intensive care unit. Infect Control Hosp Epidemiol. 2001;22:717–720. doi: 10.1086/501852. [DOI] [PubMed] [Google Scholar]
- 6.Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2007;28:713–719. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 7.Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, Ryu SY, Heo ST, Jung DS, Rhee JY, Shin SY, Ko KS, Peck KR, Lee NY. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007;59:525–530. doi: 10.1093/jac/dkl499. [DOI] [PubMed] [Google Scholar]
- 8.Markogiannakis A, Fildisis G, Tsiplakou S, Ikonomidis A, Koutsoukou A, Pournaras S, Manolis EN, Baltopoulos G, Tsakris A. Cross-transmission of multidrug-resistant Acinetobacter baumannii clonal strains causing episodes of sepsis in a trauma intensive care unit. Infect Control Hosp Epidemiol. 2008;29:410–417. doi: 10.1086/533545. [DOI] [PubMed] [Google Scholar]
- 9.Wybo I, Blommaert L, De Beer T, Soetens O, De Regt J, Lacor P, Pierard D, Lauwers S. Outbreak of multidrug-resistant Acinetobacter baumannii in a Belgian university hospital after transfer of patients from Greece. J Hosp Infect. 2007;67:374–380. doi: 10.1016/j.jhin.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 10.El Shafie SS, Alishaq M, Leni Garcia M. Investigation of an outbreak of multidrug-resistant Acinetobacter baumannii in trauma intensive care unit. J Hosp Infect. 2004;56:101–105. doi: 10.1016/j.jhin.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65:204–211. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005;11:22–29. doi: 10.3201/eid1101.040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanetti G, Blanc DS, Federli I, Raffoul W, Petignat C, Maravic P, Francioli P, Berger MM. Importation of Acinetobacter baumannii into a burn unit: a recurrent outbreak of infection associated with widespread environmental contamination. Infect Control Hosp Epidemiol. 2007;28:723–725. doi: 10.1086/517956. [DOI] [PubMed] [Google Scholar]
- 14.Simor AE, Lee M, Vearncombe M, Jones-Paul L, Barry C, Gomez M, Fish JS, Cartotto RC, Palmer R, Louie M. An outbreak due to multiresistant Acinetobacter baumannii in a burn unit: risk factors for acquisition and management. Infect Control Hosp Epidemiol. 2002;23:261–267. doi: 10.1086/502046. [DOI] [PubMed] [Google Scholar]
- 15.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections,1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang JA, Park DW, Sohn JW, Yang IS, Kim KH, Kim MJ. Molecular analysis of isoleucyl-tRNA synthetase mutations in clinical isolates of methicillin-resistant Staphylococcus aureus with low-level mupirocin resistance. J Korean Med Sci. 2006;21:827–832. doi: 10.3346/jkms.2006.21.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zana S, Jarvis WR. Investigations of outbreaks. In: Mayhall CG, editor. Hospital epidemiology and infection control. Baltimore, MD: Williams & Wilkins; 1996. pp. 106–113. [Google Scholar]
- 19.D'Agata EM, Thayer V, Schaffner W. An outbreak of Acinetobacter baumannii: the importance of cross-transmission. Infect Control Hosp Epidemiol. 2000;21:588–591. doi: 10.1086/501808. [DOI] [PubMed] [Google Scholar]
- 20.Catalano M, Quelle LS, Jeric PE, Di Martino A, Maimone SM. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J Hosp Infect. 1999;42:27–35. doi: 10.1053/jhin.1998.0535. [DOI] [PubMed] [Google Scholar]
- 21.Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siempos II, Vardakas KZ, Falagas ME. Closed tracheal suction systems for prevention of ventilator-associated pneumonia. Br J Anaesth. 2008;100:299–306. doi: 10.1093/bja/aem403. [DOI] [PubMed] [Google Scholar]
- 23.Cobley M. Environmental contamination during tracheal suction - a comparison of disposable conventional catheters with a multiple-use closed system device anaesthesia. Anaesthesia. 1991;46:957–961. doi: 10.1111/j.1365-2044.1991.tb09858.x. [DOI] [PubMed] [Google Scholar]
- 24.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006;130:251–260. doi: 10.1378/chest.130.1.251. [DOI] [PubMed] [Google Scholar]
- 25.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, Martinez-Martinez L, Bou G, Pachon J. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25:819–824. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 27.Marchaim D, Navon-Venezia S, Leavitt A, Chmelnitsky I, Schwaber MJ, Carmeli Y. Molecular and epidemiologic study of polyclonal outbreaks of multidrug-resistant Acinetobacter baumannii infection in an Israeli hospital. Infect Control Hosp Epidemiol. 2007;28:945–950. doi: 10.1086/518970. [DOI] [PubMed] [Google Scholar]
- 28.Villers D, Espaze E, Coste-Burel M, Giauffret F, Ninin E, Nicolas F, Richet H. Nosocomial Acinetobacter baumannii infections: microbiological and clinical epidemiology. Ann Intern Med. 1998;129:182–189. doi: 10.7326/0003-4819-129-3-199808010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Braun G, Vidotto MC. Evaluation of adherence, hemagglutination, and presence of genes codifying for virulence factors of Acinetobacter baumannii causing urinary tract infection. Mem Inst Oswaldo Cruz. 2004;99:839–844. doi: 10.1590/s0074-02762004000800010. [DOI] [PubMed] [Google Scholar]
- 30.Boujaafar N, Freney J, Bouvet PJ, Jeddi M. Cell surface hydrophobicity of 88 clinical strains of Acinetobacter baumannii. Res Microbiol. 1990;141:477–482. doi: 10.1016/0923-2508(90)90073-y. [DOI] [PubMed] [Google Scholar]