Abstract
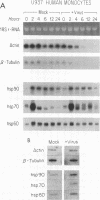
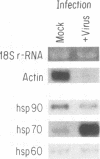
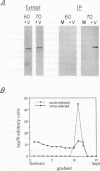
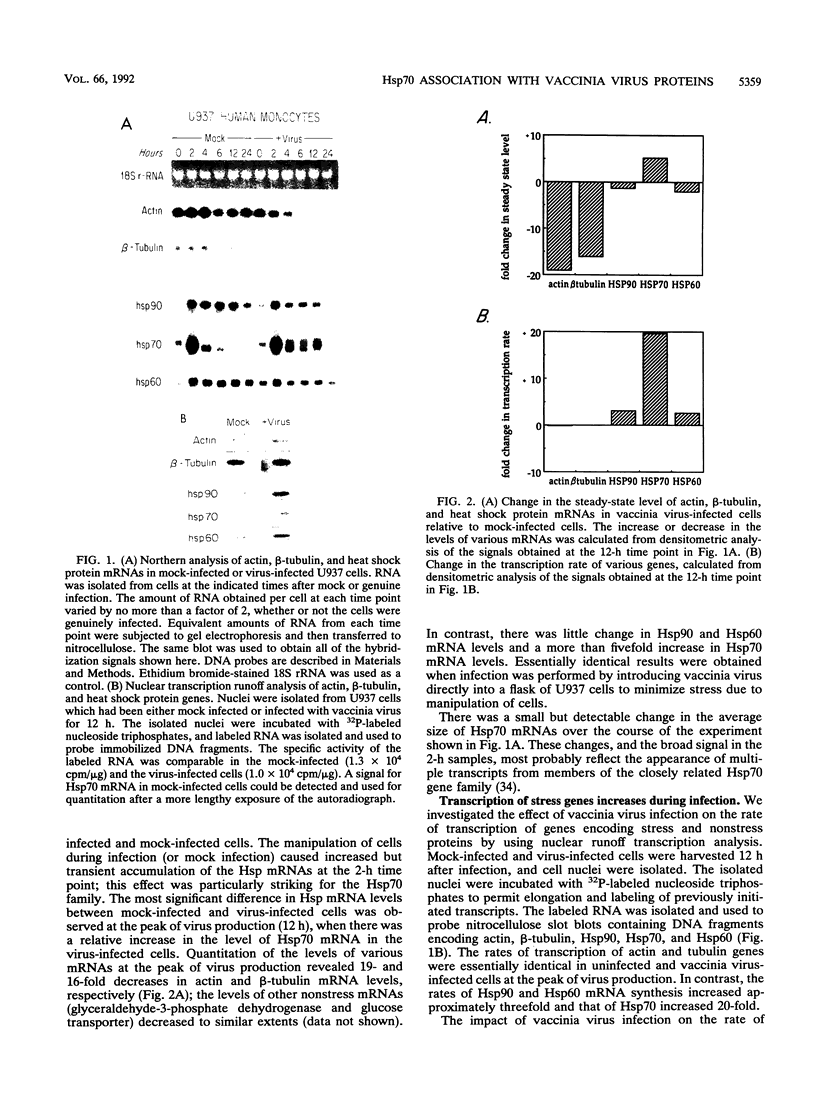
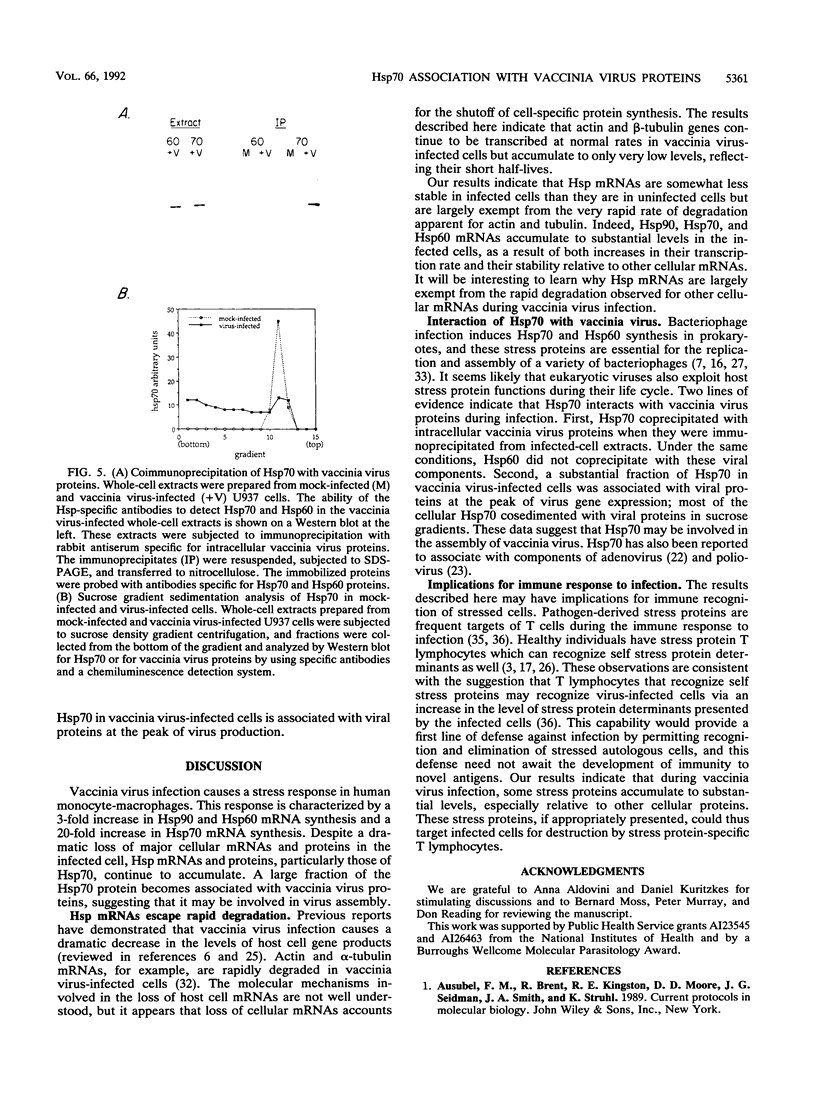
We studied the impact of vaccinia virus infection on stress protein gene expression in human cells and investigated the possibility that eukaryotic heat shock proteins interact with viral components during assembly. Infection of human monocyte-macrophages by vaccinia virus caused a dramatic decrease in levels of cellular mRNAs such as those encoding actin and tubulin. In contrast, infection did not cause a significant reduction in the levels of Hsp90 and Hsp60 mRNAs and led to substantially increased levels of Hsp70 mRNAs. The accumulation of these stress protein mRNAs was due both to increases in their transcription rate and to their stability relative to other cellular mRNAs. The relative levels of the heat shock proteins and the other cellular proteins reflected the relative levels of their mRNAs. These results indicate that stress protein gene expression is relatively refractory to the generally deleterious effects of vaccinia virus infection on host cell gene expression. The continued expression of some of these stress proteins may be beneficial to the virus; the observations that the levels of Hsp70 are greatest at the peak of viral gene expression and that a large fraction of cellular Hsp70 is associated with vaccinia virus proteins suggest that Hsp70 is involved in vaccinia virus assembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Reciprocity in the interactions between the poxviruses and their host cells. Annu Rev Microbiol. 1990;44:173–192. doi: 10.1146/annurev.mi.44.100190.001133. [DOI] [PubMed] [Google Scholar]
- Drahos D. J., Hendrix R. W. Effect of bacteriophage lambda infection on synthesis of groE protein and other Escherichia coli proteins. J Bacteriol. 1982 Mar;149(3):1050–1063. doi: 10.1128/jb.149.3.1050-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Norton P., Ivanyi J. Distribution in tissue sections of the human groEL stress-protein homologue. APMIS. 1990 May;98(5):437–441. doi: 10.1111/j.1699-0463.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Ulug E. T., Bose H. R., Jr Induction of stress proteins in Sindbis virus- and vesicular stomatitis virus-infected cells. Virology. 1983 Sep;129(2):319–332. doi: 10.1016/0042-6822(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Sadis S., Smale G., Weber L. A. Molecular cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986;43(1-2):147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan J., Murialdo H. Stimulation of groE synthesis in Escherichia coli by bacteriophage lambda infection. J Bacteriol. 1982 Mar;149(3):1166–1170. doi: 10.1128/jb.149.3.1166-1170.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Thangue N. B., Latchman D. S. A cellular protein related to heat-shock protein 90 accumulates during herpes simplex virus infection and is overexpressed in transformed cells. Exp Cell Res. 1988 Sep;178(1):169–179. doi: 10.1016/0014-4827(88)90388-6. [DOI] [PubMed] [Google Scholar]
- LaThangue N. B., Shriver K., Dawson C., Chan W. L. Herpes simplex virus infection causes the accumulation of a heat-shock protein. EMBO J. 1984 Feb;3(2):267–277. doi: 10.1002/j.1460-2075.1984.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Macejak D. G., Luftig R. B. Association of HSP70 with the adenovirus type 5 fiber protein in infected HEp-2 cells. Virology. 1991 Jan;180(1):120–125. doi: 10.1016/0042-6822(91)90015-4. [DOI] [PubMed] [Google Scholar]
- Macejak D. G., Sarnow P. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J Virol. 1992 Mar;66(3):1520–1527. doi: 10.1128/jvi.66.3.1520-1527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab J. C., Orr A., La Thangue N. B. Cellular proteins expressed in herpes simplex virus transformed cells also accumulate on herpes simplex virus infection. EMBO J. 1985 Dec 1;4(12):3223–3228. doi: 10.1002/j.1460-2075.1985.tb04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Notarianni E. L., Preston C. M. Activation of cellular stress protein genes by herpes simplex virus temperature-sensitive mutants which overproduce immediate early polypeptides. Virology. 1982 Nov;123(1):113–122. doi: 10.1016/0042-6822(82)90299-9. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Breitwieser G. E., Szabo G. Activation of muscarinic potassium currents by ATP gamma S in atrial cells. Science. 1988 Oct 21;242(4877):443–445. doi: 10.1126/science.3051383. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Roberts B. E. Vaccinia virus induces cellular mRNA degradation. J Virol. 1983 Sep;47(3):529–539. doi: 10.1128/jvi.47.3.529-539.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]