Abstract
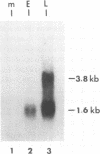
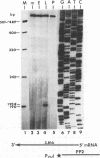
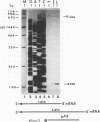
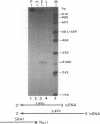
The DNA sequence of 3,240 nucleotides of the XbaI G fragment located in the unique long (UL) region of the equine herpesvirus 1 genome revealed two major open reading frames (ORFs) designated UL3 and UL4. The UL3 ORF of 470 amino acids (aa) maps at nucleotides (nt) 4450 to 3038 from the long terminus, and its predicted 51.4-kDa protein product exhibits significant homology to the ICP27 alpha regulatory protein of herpes simplex virus type 1 (HSV-1; 32% identity) and to the ORF4 protein of varicella-zoster virus (13% identity). Interestingly, a zinc finger motif is conserved in the C-terminal domains of both ICP27 of HSV-1 (aa 483 to 508) and UL3 of equine herpesvirus 1 (aa 441 to 466). The UL4 ORF of 343 aa maps at nt 5618 to 4587 and could encode a protein of 38.1 kDa which exhibits significant homology to the UL53 protein (cell fusion protein or glycoprotein K) of HSV-1 (26% identity) and to the ORF5 protein of varicella-zoster virus (33% identity). Analyses of the UL4 amino acid sequence revealed domains characteristic of a membrane-bound glycoprotein and included potential signature sequences for (i) a signal sequence, (ii) two N-linked glycosylation sites, and (iii) four transmembrane domains. Nucleotide sequence analyses also revealed potential TATA boxes located upstream of the UL3 and UL4 ORFs. However, only a single polyadenylation signal (nt 2988 to 2983) was detected downstream of the UL3 ORF. Northern (RNA) blot hybridization and S1 nuclease analyses were used to map and characterize the UL3 and UL4 mRNAs. Metabolic inhibitors were used to identify the kinetic class of these two genes. The data revealed that UL3 is an early gene that encodes a 1.6-kb mRNA, while UL4 is a late gene encoding a 3.8-kb mRNA that overlaps the UL3 transcript. Both transcripts were shown by S1 nuclease analyses to initiate 24 to 26 nt downstream of their respective TATA boxes and to have a common transcription termination signal as a pair of 3'-coterminal mRNAs.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Braun D. K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984 Oct;52(1):108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., Coogle L. D. Characterization of an equine herpesvirus type 1 gene encoding a glycoprotein (gp13) with homology to herpes simplex virus glycoprotein C. J Virol. 1988 Aug;62(8):2850–2858. doi: 10.1128/jvi.62.8.2850-2858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., Yeargan M. R. Use of lambda gt11 and monoclonal antibodies to map the genes for the six major glycoproteins of equine herpesvirus 1. J Virol. 1987 Aug;61(8):2454–2461. doi: 10.1128/jvi.61.8.2454-2461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audonnet J. C., Winslow J., Allen G., Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990 Dec;71(Pt 12):2969–2978. doi: 10.1099/0022-1317-71-12-2969. [DOI] [PubMed] [Google Scholar]
- Baumann R. P., Staczek J., O'Callaghan D. J. Cloning and fine mapping the DNA of equine herpesvirus type one defective interfering particles. Virology. 1986 Sep;153(2):188–200. doi: 10.1016/0042-6822(86)90022-x. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Colle C. F., 3rd, Flowers C. C., O'Callaghan D. J. Open reading frames encoding a protein kinase, homolog of glycoprotein gX of pseudorabies virus, and a novel glycoprotein map within the unique short segment of equine herpesvirus type 1. Virology. 1992 Jun;188(2):545–557. doi: 10.1016/0042-6822(92)90509-n. [DOI] [PubMed] [Google Scholar]
- Dauenhauer S. A., Robinson R. A., O'Callaghan D. J. Chronic production of defective-interfering particles by hamster embryo cultures of herpesvirus persistently infected and oncogenically transformed cells. J Gen Virol. 1982 May;60(Pt 1):1–14. doi: 10.1099/0022-1317-60-1-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Debroy C., Pederson N., Person S. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology. 1985 Aug;145(1):36–48. doi: 10.1016/0042-6822(85)90199-0. [DOI] [PubMed] [Google Scholar]
- Elton D. M., Halliburton I. W., Killington R. A., Meredith D. M., Bonass W. A. Sequence analysis of the 4.7-kb BamHI-EcoRI fragment of the equine herpesvirus type-1 short unique region. Gene. 1991 May 30;101(2):203–208. doi: 10.1016/0378-1119(91)90412-5. [DOI] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Flowers C. C., Eastman E. M., O'Callaghan D. J. Sequence analysis of a glycoprotein D gene homolog within the unique short segment of the EHV-1 genome. Virology. 1991 Jan;180(1):175–184. doi: 10.1016/0042-6822(91)90021-3. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., Caughman G. B., O'Callaghan D. J., Staczek J. Regulation of equine herpesvirus type 1 gene expression: characterization of immediate early, early, and late transcription. Virology. 1987 May;158(1):79–87. doi: 10.1016/0042-6822(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., O'Callaghan D. J., Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987 Sep;8(3):233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Yalamanchili R., Raengsakulrach B., Baumann R. P., Staczek J., O'Callaghan D. J. Viral transcripts in cells infected with defective interfering particles of equine herpesvirus type 1. Virology. 1989 Sep;172(1):1–10. doi: 10.1016/0042-6822(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Green L. M., Berg J. M. A retroviral Cys-Xaa2-Cys-Xaa4-His-Xaa4-Cys peptide binds metal ions: spectroscopic studies and a proposed three-dimensional structure. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4047–4051. doi: 10.1073/pnas.86.11.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F. J., Baumann R. P., O'Callaghan D. J. DNA sequence and comparative analyses of the equine herpesvirus type 1 immediate early gene. Virology. 1989 Sep;172(1):223–236. doi: 10.1016/0042-6822(89)90124-4. [DOI] [PubMed] [Google Scholar]
- Hardwicke M. A., Vaughan P. J., Sekulovich R. E., O'Conner R., Sandri-Goldin R. M. The regions important for the activator and repressor functions of herpes simplex virus type 1 alpha protein ICP27 map to the C-terminal half of the molecule. J Virol. 1989 Nov;63(11):4590–4602. doi: 10.1128/jvi.63.11.4590-4602.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty R. N., Colle C. F., Grundy F. J., O'Callaghan D. J. Mapping the termini and intron of the spliced immediate-early transcript of equine herpesvirus 1. J Virol. 1989 Dec;63(12):5101–5110. doi: 10.1128/jvi.63.12.5101-5110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. E., Robinson R. A., Dauenhauer S. A., Atherton S. S., Hayward G. S., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 1. Virology. 1981 Nov;115(1):97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. ICP22 homolog of equine herpesvirus 1: expression from early and late promoters. J Virol. 1992 Feb;66(2):664–673. doi: 10.1128/jvi.66.2.664-673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L., Goldsmith K., Snoddy D., Ghosh H., Graham F. L., Johnson D. C. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J Virol. 1992 Sep;66(9):5603–5609. doi: 10.1128/jvi.66.9.5603-5609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McMahan L., Schaffer P. A. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990 Jul;64(7):3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Hyde J. M., Gentry G. A., Randall C. C. Kinetics of viral deoxyribonucleic acid, protein, and infectious particle production and alterations in host macromolecular syntheses in equine abortion (herpes) virus-infected cells. J Virol. 1968 Aug;2(8):793–804. doi: 10.1128/jvi.2.8.793-804.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Randall C. C. Molecular anatomy of herpesviruses: recent studies. Prog Med Virol. 1976;22:152–210. [PubMed] [Google Scholar]
- Pogue-Geile K. L., Spear P. G. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology. 1987 Mar;157(1):67–74. doi: 10.1016/0042-6822(87)90314-x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy R., Holland T. C. In vitro characterization of the HSV-1 UL53 gene product. Virology. 1992 Feb;186(2):579–587. doi: 10.1016/0042-6822(92)90024-j. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988 Oct;62(10):3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Su L. S., Knipe D. M. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J Virol. 1989 Aug;63(8):3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson G. R., Scott N. A., Miller J. M., Sabine M., Zheng M., Bell C. W., Whalley J. M. Sequence characteristics of a gene in equine herpesvirus 1 homologous to glycoprotein H of herpes simplex virus. DNA Seq. 1991;1(4):241–249. doi: 10.3109/10425179109020779. [DOI] [PubMed] [Google Scholar]
- Robinson R. A., Tucker P. W., Dauenhauer S. A., O'Callaghan D. J. Molecular cloning of equine herpesvirus type 1 DNA: analysis of standard and defective viral genomes and viral sequences in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6684–6688. doi: 10.1073/pnas.78.11.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. L., Hardwicke M. A., Sandri-Goldin R. M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992 Jan;186(1):74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Sekulovich R. E., Hardwicke M. A., Sandri-Goldin R. M. Mutations in the activation region of herpes simplex virus regulatory protein ICP27 can be trans dominant. J Virol. 1991 Jul;65(7):3656–3666. doi: 10.1128/jvi.65.7.3656-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. H., Caughman G. B., O'Callaghan D. J. Characterization of the regulatory functions of the equine herpesvirus 1 immediate-early gene product. J Virol. 1992 Feb;66(2):936–945. doi: 10.1128/jvi.66.2.936-945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Sullivan D. C., Allen G. P., O'Callaghan D. J. Synthesis and processing of equine herpesvirus type 1 glycoprotein 14. Virology. 1989 Dec;173(2):638–646. doi: 10.1016/0042-6822(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Scott N. A., Hudson G. C., Bell C. W., Woodworth L. M. Identification and nucleotide sequence of a gene in equine herpesvirus 1 analogous to the herpes simplex virus gene encoding the major envelope glycoprotein gB. J Gen Virol. 1989 Feb;70(Pt 2):383–394. doi: 10.1099/0022-1317-70-2-383. [DOI] [PubMed] [Google Scholar]
- Whittaker G. R., Riggio M. P., Halliburton I. W., Killington R. A., Allen G. P., Meredith D. M. Antigenic and protein sequence homology between VP13/14, a herpes simplex virus type 1 tegument protein, and gp10, a glycoprotein of equine herpesvirus 1 and 4. J Virol. 1991 May;65(5):2320–2326. doi: 10.1128/jvi.65.5.2320-2326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker G. R., Wheldon L. A., Giles L. E., Stocks J. M., Halliburton I. W., Killington R. A., Meredith D. M. Characterization of the high Mr glycoprotein (gP300) of equine herpesvirus type 1 as a novel glycoprotein with extensive O-linked carbohydrate. J Gen Virol. 1990 Oct;71(Pt 10):2407–2416. doi: 10.1099/0022-1317-71-10-2407. [DOI] [PubMed] [Google Scholar]
- Yalamanchili R. R., Raengsakulrach B., Baumann R. P., O'Callaghan D. J. Identification of the site of recombination in the generation of the genome of DI particles of equine herpesvirus type 1. Virology. 1990 Apr;175(2):448–455. doi: 10.1016/0042-6822(90)90429-u. [DOI] [PubMed] [Google Scholar]
- Yalamanchili R. R., Raengsakulrach B., O'Callaghan D. J. Equine herpesvirus 1 sequence near the left terminus codes for two open reading frames. Virus Res. 1991 Mar;18(2-3):109–116. doi: 10.1016/0168-1702(91)90012-k. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]