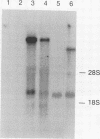
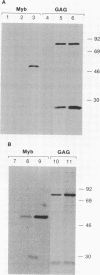
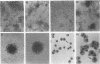
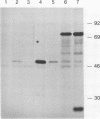
Abstract
To test the effect of long terminal repeat (LTR) regulatory sequences on the transforming capability of the v-myb oncogene from avian myeloblastosis virus (AMV), we have constructed replication-competent avian retroviral vectors with nearly identical structural genes that express v-myb from either AMV or Rous sarcoma virus (RSV) LTRs. After transfection into chicken embryo fibroblasts, virus-containing cell supernatants were used to infect chicken myelomonocytic target cells from preparations of 16-day-old embryonic spleen cells. Both wild-type AMV and the virus expressing v-myb from AMV LTRs (RCAMV-v-myb) were able to transform the splenocyte cultures into a population of immature myelomonocytic cells. The transformed cells expressed the p48v-Myb oncoprotein and formed compact foci when grown in soft agar. In contrast, the virus expressing v-myb from RSV LTRs (RCAS-v-myb) was repeatedly unable to transform the same splenocyte cells, despite being able to infect fibroblasts with high efficiency. This difference in the transforming activities of v-myb-expressing viruses with different LTRs most likely results from the presence of a factor (or factors) within the appropriate myelomonocytic target cell that promotes specific expression from the AMV but not from the RSV LTR.
Full text
PDF


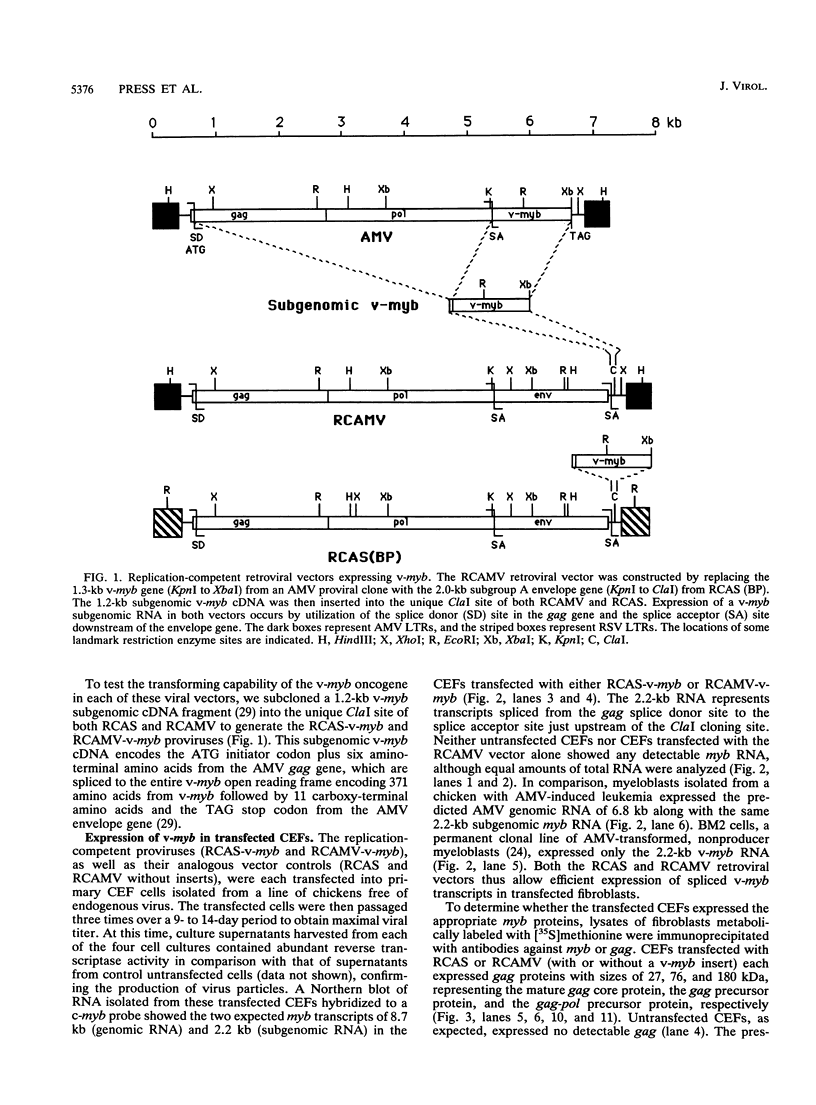
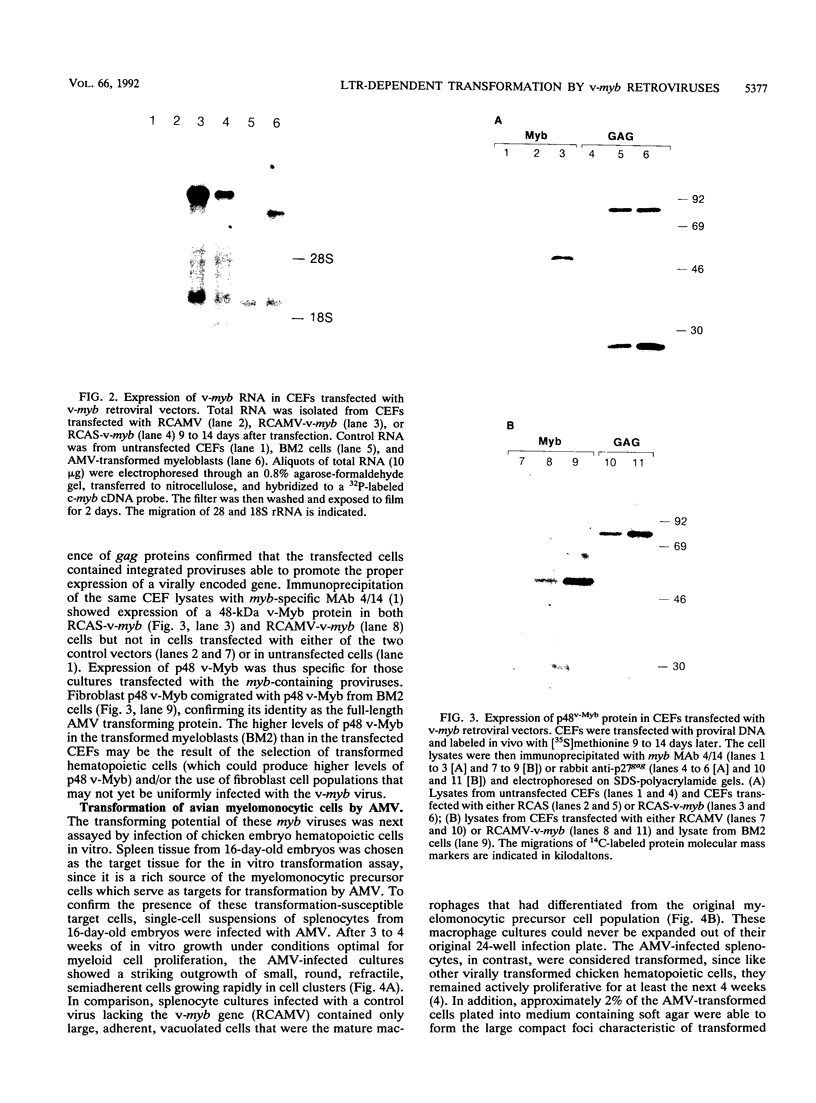
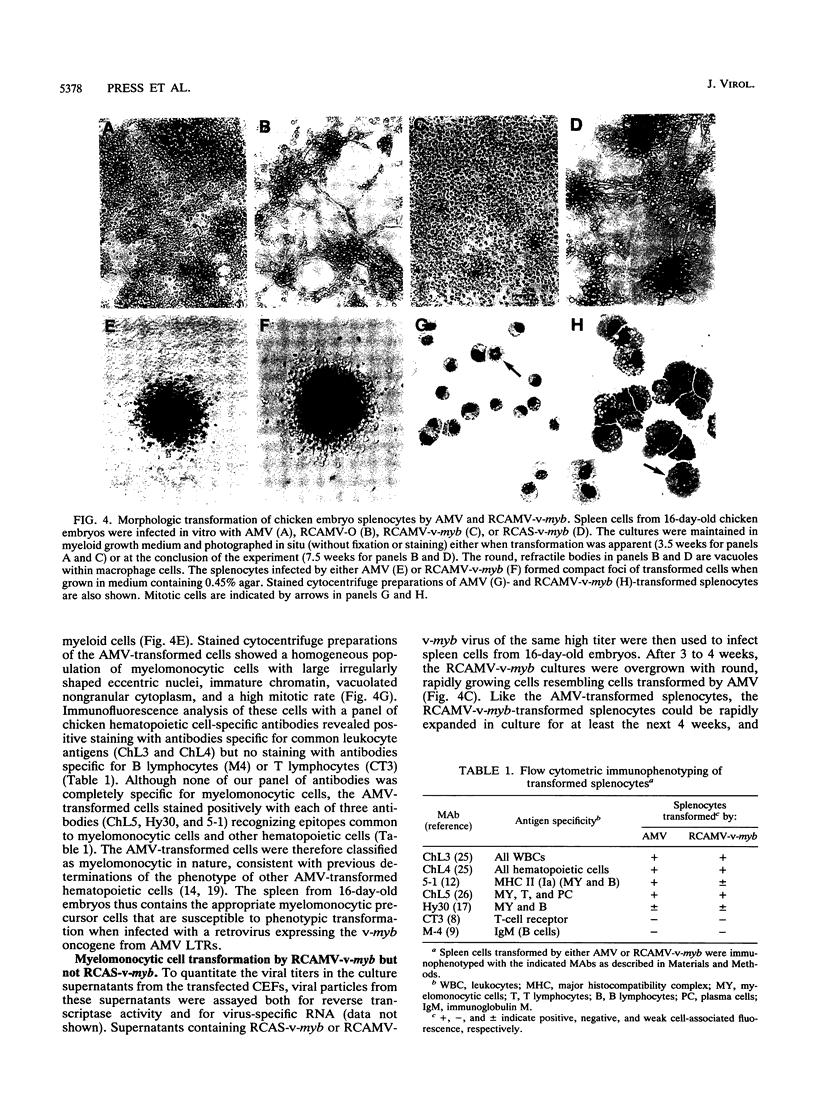




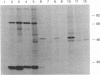
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bading H., Hansen J., Moelling K. Selective DNA binding of the human cellular myb protein isolated by immunoaffinity chromatography using a monoclonal antibody. Oncogene. 1987;1(4):395–401. [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Brown D. W., Blais B. P., Robinson H. L. Long terminal repeat (LTR) sequences, env, and a region near the 5' LTR influence the pathogenic potential of recombinants between Rous-associated virus types 0 and 1. J Virol. 1988 Sep;62(9):3431–3437. doi: 10.1128/jvi.62.9.3431-3437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Robinson H. L. Influence of env and long terminal repeat sequences on the tissue tropism of avian leukosis viruses. J Virol. 1988 Dec;62(12):4828–4831. doi: 10.1128/jvi.62.12.4828-4831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Lehmeyer J. E., Cooper M. D. Evidence for an IgD homologue on chicken lymphocytes. J Immunol. 1982 Dec;129(6):2580–2585. [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Transcriptional activity of avian retroviral long terminal repeats directly correlates with enhancer activity. J Virol. 1985 Feb;53(2):515–521. doi: 10.1128/jvi.53.2.515-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert D. L., Munchus M. S., Chen C. L., Cooper M. D. Analysis of structural properties and cellular distribution of avian Ia antigen by using monoclonal antibody to monomorphic determinants. J Immunol. 1984 May;132(5):2524–2530. [PubMed] [Google Scholar]
- Goodwin G. H. Identification of three sequence-specific DNA-binding proteins which interact with the Rous sarcoma virus enhancer and upstream promoter elements. J Virol. 1988 Jun;62(6):2186–2190. doi: 10.1128/jvi.62.6.2186-2190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse J. J., Petropoulos C. J., Crittenden L. B., Hughes S. H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988 Dec;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer F. A., Graf T., Lipsick J. S. Protein truncation is required for the activation of the c-myb proto-oncogene. Mol Cell Biol. 1991 Aug;11(8):3987–3996. doi: 10.1128/mcb.11.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. L., Ramsay R. G., Kanei-Ishii C., Ishii S., Gonda T. J. Transformation by carboxyl-deleted Myb reflects increased transactivating capacity and disruption of a negative regulatory domain. Oncogene. 1991 Sep;6(9):1549–1553. [PubMed] [Google Scholar]
- Huffnagle G. B., Ratcliffe M. J., Humphries E. H. Bu-2, a novel avian cell surface antigen on B cells and a population of non-lymphoid cells, is expressed homogeneously in germinal centers. Hybridoma. 1989 Dec;8(6):589–604. doi: 10.1089/hyb.1989.8.589. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Kanter M. R., Smith R. E., Hayward W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol. 1988 Apr;62(4):1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl W. M., Bender T. P., Stafford J., McClinton D., Segal S., Dmitrovsky E. Expression and function of the c-myb oncogene during hematopoietic differentiation. Curr Top Microbiol Immunol. 1988;141:318–323. doi: 10.1007/978-3-642-74006-0_42. [DOI] [PubMed] [Google Scholar]
- Lavu S., Reddy E. P. Structural organization and nucleotide sequence of mouse c-myb oncogene: activation in ABPL tumors is due to viral integration in an intron which results in the deletion of the 5' coding sequences. Nucleic Acids Res. 1986 Jul 11;14(13):5309–5320. doi: 10.1093/nar/14.13.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Olson W. C., Ewert D. L. Markers of B lymphocyte differentiation in the chicken. Hybridoma. 1990 Aug;9(4):331–350. doi: 10.1089/hyb.1990.9.331. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Hughes S. H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991 Jul;65(7):3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson D. Effects of 5' and 3' truncations of the myb gene on the transforming ability of avian myeloblastosis virus (AMV). Virology. 1990 Apr;175(2):562–567. doi: 10.1016/0042-6822(90)90441-s. [DOI] [PubMed] [Google Scholar]
- Ruddell A., Linial M. L., Groudine M. Tissue-specific lability and expression of avian leukosis virus long terminal repeat enhancer-binding proteins. Mol Cell Biol. 1989 Dec;9(12):5660–5668. doi: 10.1128/mcb.9.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Reddy E. P., Souza L. M., Baluda M. A., Chirikjian J. G., Papas T. S. Nucleotide sequence analysis of the long terminal repeat of avian myeloblastosis virus and adjacent host sequences. J Virol. 1982 Jun;42(3):840–846. doi: 10.1128/jvi.42.3.840-846.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Potter M., Mushinski J. F., Lavu S., Reddy E. P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984 Nov 30;226(4678):1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Baluda M. A. Identification of the avian myeloblastosis virus genome. I. Identification of restriction endonuclease fragments associated with acute myeloblastic leukemia. J Virol. 1980 Nov;36(2):317–324. doi: 10.1128/jvi.36.2.317-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Lerner T. L., Hanafusa H. Polymerase-defective mutant of the Bryan high-titer strain of Rous sarcoma virus. Nucleic Acids Res. 1986 Mar 11;14(5):2391–2405. doi: 10.1093/nar/14.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Enhancer activity correlates with the oncogenic potential of avian retroviruses. EMBO J. 1985 Apr;4(4):949–956. doi: 10.1002/j.1460-2075.1985.tb03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]