Abstract
Somatostatin analogs were initially developed for the control of hormonal syndromes associated with neuroendocrine tumors (NETs). In recent years, accumulating data has supported their role as antiproliferative agents, capable of stabilizing tumor growth in patients with metastatic neuroendocrine malignancies, including carcinoid and pancreatic endocrine tumors. A phase III, randomized, placebo-controlled trial has now demonstrated that octreotide long-acting repeatable (LAR) 30 mg can significantly prolong time to tumor progression among patients with metastatic midgut NETs regardless of functional status, chromogranin A level or age. In addition to significantly lengthening time to tumor progression in the overall study population, subset analysis suggests that patients with low tumor burden are most likely to experience disease stabilization with octreotide LAR 30 mg, supporting the early use of octreotide LAR in patients with metastatic disease. Further research efforts are underway to evaluate the use of somatostatin analogs as antiproliferative agents in other types of gastroenteropancreatic-NETs. Ongoing studies are also evaluating novel somatostatin analogs and somatostatin analogs in combination with other anti-tumor therapies.
Keywords: Somatostatin analogues, Neuroendocrine tumors, Antiproliferative
INTRODUCTION
The human hormone somatostatin was first isolated in 1973 and identified as a hypothalamic inhibitor of growth hormone[1-4]. It was subsequently discovered in multiple tissues, including the central nervous system, endocrine system and gastrointestinal tract[3]. Somatostatin has been characterized as a universal endocrine “off-switch” due to its exocrine, endocrine, paracrine and autocrine inhibitory effects[5-7]. In the digestive tract, it reduces secretion and motility, decreases portal blood flow, inhibits gallbladder contraction and reduces the secretion of other gastrointestinal hormones[8]. The effects of somatostatin are mediated through interaction with five somatostatin receptors (sst1-5)[9], belonging to a family of G-protein coupled receptors with seven transmembrane domains.
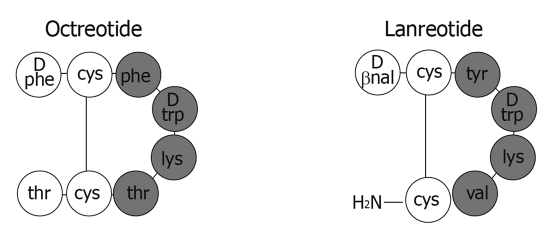
The clinical utility of native human somatostatin is limited by its short half life of approximately two minutes. Both bioactive forms of the hormone, the fourteen-peptide somatostatin-14 and a C-terminally extended form, somatostatin-28, contain multiple enzymatic cleavage sites resulting in rapid circulatory degradation[6]. In order to improve the pharmacokinetic profile, synthetic somatostatin analogs (SSAs) have been developed by shortening the polypeptide chain while retaining binding affinity to somatostatin receptors (Figure 1)[10]. The two commercially available analogs, octreotide and lanreotide, are octapeptides that bind with high affinity to somatostatin receptor subtype 2 (sst2) and with moderate affinity to sst5 (Table 1).
Figure 1.
Chemical structure of the synthetic somatostatin analogs octreotide and lanreotide (adapted from[11]).
Table 1.
Receptor binding affinities of somatostatin, octreotide and lanreotide
Octreotide has been used in clinical practice since data emerged in the 1980s confirming its ability to palliate carcinoid syndrome[14], as well as other hormonal syndromes caused by metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Octreotide was initially available in an immediate-release formulation suitable for deep subcutaneous or intravenous administration[15]. Octreotide subcutaneous (sc) has been tested primarily at doses ranging from 100 to 500 μg two to three times daily. During the past decade, a long-acting repeatable (LAR) depot formulation of octreotide (Sandostatin LAR®) has been available, which allows monthly intramuscular dosing. Octreotide LAR has demonstrated similar efficacy to octreotide sc in the control of flushing and diarrhea associated with carcinoid syndrome[16]. The dose of octreotide sc and octreotide LAR should be titrated per symptom control for optimal patient care[17]. A second somatostatin analog, lanreotide, was licensed in Europe in 1998 for the treatment of symptoms associated with neuroendocrine (particularly carcinoid) tumors. A long-acting formulation of lanreotide (Somatuline Autogel®)[18] has also been developed as a deep subcutaneous injection.
Early on, clinical trials of SSAs tested their ability to inhibit the release of neuroendocrine hormones such as serotonin, glucagon, insulin, gastrin and vasoactive intestinal peptide (VIP)[14,19-22]. These trials formed the basis for the approval of octreotide and lanreotide as antisecretory agents indicated for treatment of hormonally active GEP-NETs. It was not until several years after the approval of octreotide that evidence of antineoplastic activity emerged. Although objective radiographic responses associated with SSAs were rare, many cases of prolonged disease stability were documented in the literature, leading to the hypothesis that SSAs exert an inhibitory effect on tumor growth. Recently, this hypothesis was tested in a Phase III, randomized, placebo-controlled clinical trial evaluating octreotide LAR 30 mg. This review summarizes the preclinical and clinical evidence supporting the role of SSAs as antiproliferative agents in the treatment of patients with GEP-NETs. To date, most data (including the results from the only phase III randomized, placebo-controlled trial) have been generated in studies evaluating octreotide sc and LAR.
BIOLOGICAL BASIS FOR THE ANTIPROLIFERATIVE EFFECTS OF SSAS
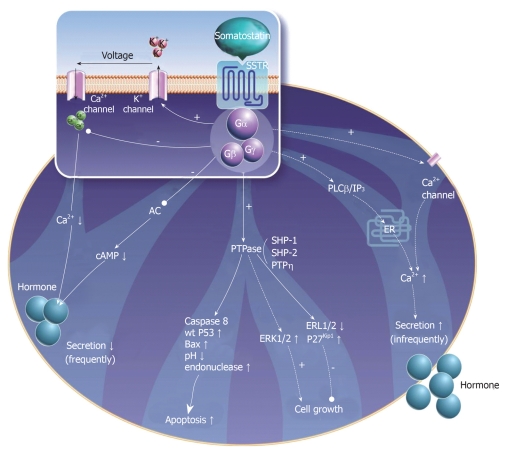
Over the past two decades there has been significant progress in our understanding of the molecular basis for the antiproliferative effects of somatostatin and its analogs. Antitumoral activity appears to be mediated via direct and indirect mechanisms. Direct mechanisms involve the activation of somatostatin receptors on tumor cells leading to modulation of intracellular signaling transduction pathways. Multiple in vitro studies using cell lines transfected with somatostatin receptors indicate that all receptor subtypes (sst1-5) may mediate inhibition of cell proliferation[23], whereas specific receptor subtypes (sst2,3) may mediate apoptosis (Table 2)[24-26]. These actions appear to be regulated primarily via the MAP-kinase signaling pathway and through activation of phosphotyrosine phosphatases (Figure 2)[27-29]. Indirect antiproliferative mechanisms include inhibition of mitogenic growth factors such as insulin-like growth factor (IGF), as well as inhibition of tumor angiogenesis through interaction with somatostatin receptors on endothelial cells and monocytes[30].
Table 2.
Receptor mediation of cell proliferation
| sst1 | sst2 | sst3 | sst4 | sst5 | |
| Induction of G1 cell cycle arrest | + | + | + | + | |
| Induction of apoptosis | + | + |
Figure 2.
Somatostatin receptor-mediated effects on neuroendocrine cells (adapted from[23]).
Activation of phosphotyrosine phosphatases
Several phosphotyrosine phosphatases (PTPs), including SHP-1 and SHP-2, have emerged as important regulators of intracellular signaling pathways[27]. Somatostatin receptor-mediated activation of SHP-1 results in arrest of cell proliferation in various cell lines, including cells derived from pancreatic, breast and prostate carcinomas[31,32]. In pituitary adenoma cells, activation of sst2 inhibits PI3 kinase activity and causes cell growth arrest via stimulation of SHP-1[33]. The enzymatic activity of SHP-1 has also been implicated in sst3-dependent apoptosis in transfected Chinese Hamster Ovary (CHO) cells[34]. Stimulation of SHP-1 in sst2-expressing CHO cells has led to G1 cell cycle arrest via induction of the cyclin-dependent kinase inhibitor p27[35]. SHP-2 has also been identified as a mediator of the antiproliferative effects of somatostatin receptors, primarily through inactivation of tyrosine kinase receptors for insulin and epidermal growth factors[36]. Moreover, activation of PTPs has been shown to down-regulate Raf-1[37] and block the MAP-kinase pathway[38].
Modulation of the mitogen activated protein-kinase pathway
Both inhibition and stimulation of the mitogen activated protein (MAP)-kinase pathway have been linked to the antiproliferative effects of somatostatin and its analogs. In a glioma cell line, the receptor-like PTP, PTPeta, mediated the antiproliferative effects of somatostatin through inhibition of ERK1/2[39]. Conversely, another study of sst1-expressing CHO cells demonstrated that somatostatin robustly activated MAP-kinase, which in turn enhanced the expression of the cyclin-dependent kinase inhibitor p21, thereby inhibiting cell proliferation[40]. Another study in CHO cells demonstrated that activation of p38 MAP-kinase via sst2 and sst4 mediated the inhibitory effects of somatostatin on fibroblast growth factor induced proliferation[41].
Indirect antiproliferative mechanisms
Suppression of tumor growth may occur via inhibition of various circulating growth factors, including insulin-like growth factor (IGF), epidermal growth factor (EGF) and growth hormone (GH). Inhibition of GH is thought to be mediated primarily via sst2 and sst5, which are strongly expressed in the anterior pituitary[42-44]. Octreotide has been shown to suppress circulating levels of IGF-1, both via suppression of pituitary secretion of GH as well as through direct inhibition of IGF-1 production in the liver[45,46].
The antiangiogenic effects of octreotide have been demonstrated in multiple in vitro tumor models[47,48]. Octreotide has been shown to inhibit proliferating endothelial cells that over-express sst2 and sst5[49]. The primary mechanism of angiogenesis inhibition may be suppression of endothelial nitric oxide release[50]. Inhibition of circulating vascular-endothelial growth factor (VEGF) appears to also play a role in suppression of peritumoral vessel growth[51,52].
EARLY CLINICAL EVIDENCE FOR THE ANTIPROLIFERATIVE EFFECTS OF SSAS
Since the introduction of SSAs, multiple phase II trials and retrospective series have demonstrated that SSA treatment is associated with prolonged survival and disease stabilization in a large proportion of patients. For example, a single-institution retrospective study of 146 patients with metastatic mid-gut NETs, 91% of whom received long term octreotide treatment, demonstrated a 5-year survival rate of 75% (compared to 19% historically)[53]. Additionally, an analysis of the US-based Surveillance, Epidemiology and End Results (SEER) database found a significant increase in survival from 1988 to 2004 compared with 1973 to 1987, coinciding with the introduction of octreotide[54].
In general, early clinical studies evaluating the disease-stabilizing effect of SSAs in patients with GEP-NETs are characterized by their lack of randomized design and enrollment of heterogeneous populations of patients with GEP-NETs. Although objective radiographic response rates have been rare (generally < 5%), the rate of tumor stabilization observed in most studies has ranged from 40%-60%, with higher rates observed in patients without documented disease progression at onset of treatment[55].
Among the first prospective studies documenting the antiproliferative effects of SSAs in GEP-NETs was one conducted by the German Sandostatin Study Group[56]. In this study, 103 patients with metastatic carcinoid and pancreatic endocrine tumors were treated with octreotide 200 μg thrice daily until evidence of radiographic progression. Among patients who had disease progression documented at treatment outset, the rate of disease stability lasting at least 3 mo was 37%, whereas among patients with documented stable disease at treatment outset, disease stability lasting at least 12 mo was documented in 54% of patients[57]. No objective tumor responses were observed. Another phase II clinical trial testing octreotide as an antiproliferative agent in 34 patients with progressive metastatic NETs demonstrated a disease stabilization rate of 50% lasting a median of 5 mo[58].
The antiproliferative effect of intramuscular lanreotide SR 30 mg every 10 or 14 d was evaluated in a phase II trial of 46 patients with carcinoid and pancreatic endocrine tumors. Two patients (4%) achieved an objective radiographic response while 19 patients (41%) experienced stable disease for a mean duration of 9.5 mo[59]. In another phase II study of lanreotide SR 30 mg in 55 patients with GEP-NETs (48 with carcinoid tumors, six with gastrinomas and one with a VIPoma), 7% of 31 assessable patients achieved a partial response and 81% experienced disease stability[60]. In one study of patients with progressive tumors, participants received either octreotide LAR 30 mg or lanreotide SR 60 mg (this study considered all patients as a single cohort). Among 31 assessable patients, 14 (45%) achieved disease stability vs 55% who continued to progress radiographically[61]. Overall survival was considerably prolonged among patients with stable vs progressive disease. In multivariate analysis, pancreatic endocrine tumors appeared significantly less likely to achieve disease stabilization compared to intestinal carcinoid tumors. Extra-hepatic metastases were also associated with a poor prognosis. Table 3 summarizes the results of multiple non-randomized clinical trials evaluating the antineoplastic effects of octreotide and lanreotide in GEP-NETs.
Table 3.
Summary of non-randomized clinical trials evaluating the antiproliferative effect of somatostatin analogs n (%)
| Analog | Author | n | CR/PR | SD | PD |
| Patients with documented tumor progression | |||||
| Lanreotide | Faiss et al[62], 2003 | 22 | 1 (4) | 7 (32) | 14 (64) |
| Lanreotide | Aparicio et al[63], 2001 | 35 | 1 (3) | 20 (57) | 14 (40) |
| Octreotide | Arnold et al[64], 1993 | 52 | 0 (0) | 19 (36) | 33 (63) |
| Octreotide | Saltz et al[58], 1993 | 34 | 0 (0) | 17 (50) | 17 (50) |
| Octreotide | di Bartolomeo et al[19], 1996 | 58 | 2 (3) | 27 (46) | 29 (50) |
| 201 | 4 (1) | 90 (45) | 107 (53) | ||
| Patients without documented tumor progression | |||||
| Lanreotide | Wymenga et al[60], 1999 | 31 | 2 (6) | 25 (80) | 4 (13) |
| Lanreotide | Ducreux et al[59], 2000 | 39 | 2 (5) | 21 (54) | 16 (41) |
| Lanreotide | Eriksson et al[65], 1997 | 19 | 1 (5) | 12 (63) | 6 (32) |
| Lanreotide | Tomasetti et al[66], 1998 | 18 | 0 (0) | 14 (77) | 4 (22) |
| Octreotide | Tomasetti et al[67], 2000 | 16 | 0 (0) | 14 (87) | 2 (12) |
| Octreotide | Ricci et al[68], 2000 | 15 | 1 (6) | 6 (40) | 8 (53) |
| 138 | 6 (4) | 92 (67) | 40 (29) | ||
CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
THE PROMID TRIAL
Although providing initial evidence for the antitumor effects of SSAs, studies described in the previous section have a number of features that prevent them from providing conclusive evidence. Examples of these features include relatively small patient cohorts, lack of a randomized placebo control group, and analysis of heterogeneous populations. As such, to prove or to disprove an antiproliferative effect of octreotide LAR 30 mg, the PROMID (Placebo-controlled, Prospective, Randomized study in patients with metastatic neuroendocrine midgut tumors) study was initiated. This randomized, double-blind, placebo-controlled, phase III trial, was among the very few randomized trials performed in patients with this rare tumor type. To avoid confounding variables, only patients with well-differentiated inoperable or metastatic midgut tumors were included. Additionally, octreotide LAR 30 mg was the only dose of octreotide LAR evaluated.
High-level evidence of the antiproliferative effects of octreotide emerged after publication of the PROMID trial[69]. Eighty-five participants with well-differentiated carcinoid tumors originating in the distal intestine and proximal colon were randomized to receive either octreotide LAR 30 mg or placebo until radiographic evidence of progression or death. The primary endpoint was time to tumor progression. Most patients (75%) had evidence of somatostatin receptor expression as evidenced by radiotracer uptake on Octreoscan. Nearly half of patients (38%) manifested the carcinoid syndrome (flushing and/or diarrhea associated with elevation in urine 5-HIAA). Only patients with mild carcinoid syndrome who tolerated flushing without intervention or responded to treatment with loperamide and/or cholestyramine in cases of diarrhea were included.
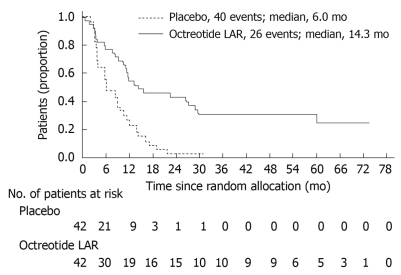
Median time to tumor progression was 14.3 mo in the octreotide LAR 30 mg group vs 6.0 mo in the placebo group (P = 0.000072, Figure 3). This significantly lengthened time-to-tumor progression was seen in the overall study population, regardless of tumor functionality, chromogranin A level or age. At 6 mo, tumor progression was observed in 24% of patients on the octreotide LAR 30 mg arm vs 66% of patients receiving placebo (P = 0.0079). Serious adverse events were nearly evenly balanced (11 patients in the octreotide LAR 30 mg arm and 10 patients in the placebo arm). On multivariate analysis, the highest rates of disease stabilization were observed in patients with low hepatic tumor load (< 10%) and resected primary tumor, however both of these subgroups contained the majority of study patients. Even patients with higher hepatic tumor burden (> 10%) experienced a near doubling in time to progression on the octreotide LAR arm of the study. The small number of deaths in both treatment arms (seven in the octreotide LAR 30 mg arm; nine in the placebo arm) precluded any analysis of differences in survival.
Figure 3.
Kaplan Meier curve demonstrating time to tumor progression in patients treated with octreotide long-acting repeatable (LAR) vs placebo[69]. Log-rank test stratified by functional activity: P = 0.000072, HR = 0.34 (95% CI: 0.20-0.59).
FUTURE DIRECTIONS
Novel somatostatin analogs
NETs generally express multiple somatostatin receptors[13,70], all of which may mediate the antiproliferative effects of SSAs. These receptor subtypes can undergo heterodimerization with each other and with other receptor families (such as the dopamine receptor family), enhancing their binding affinities and internalization[71,72]. Thus, novel SSAs that bind to multiple receptor subtypes as well as analogs capable of binding to different families of receptors may prove to be effective antisecretory and antiproliferative agents in patients refractory to octreotide or lanreotide.
Pasireotide is one such novel somatostatin analog; it binds avidly to four of the five somatostatin receptors (sst1,2,3 and sst5). Compared with octreotide, pasireotide has a 40-, 30- and 5-fold higher binding affinity for sst5, sst1 and sst3, and a slightly lower affinity for sst2[73]. Pasireotide also has a 2-times higher binding affinity for sst5 than endogenous somatostatin[73]. In an in vitro study evaluating the use of octreotide and pasireotide on HEK293 cells expressing somatostatin receptor subtype sst2 on the cell membrane, treatment with octreotide resulted in an internalization of sst2 receptors at 30 min whereas treatment with pasireotide did not lead to sst2 internalization. Such findings may suggest that a persistent and more durable efficacy could be obtained with pasireotide[74].
An open-label trial evaluated the activity of pasireotide sc in patients with carcinoid syndrome whose symptoms (flushing and diarrhea) were inadequately controlled with octreotide LAR[75]. Preliminary data indicated activity in this refractory population. Future clinical trials are being designed to test the antiproliferative effects of pasireotide in neuroendocrine carcinomas. Other compounds capable of interacting with sst2 as well as with the dopamine D2 receptor (DAD2) are in clinical development[76].
Radioactive labeling of SSAs is another promising approach to treatment of neuroendocrine malignancies which express high levels of somatostatin receptors. Early clinical trials employing 111In-pentetreotide produced limited objective responses, probably due to the small particle range and short tissue penetration of Auger electrons emitted by the 111In isotope[77]. The next generation of radiolabled SSAs used 90Y-DOTATOC, a β-particle emitter with a tissue range of approximately 12 mm[78-80]. Objective response rates of 10%-30% were reported in phase I and II clinical trials. Dose-limiting side effects included bone marrow and renal toxicity.
The latest research efforts in radiolabeled SSAs have focused on 177Lu octreotate, a β-and γ-emitting radionuclide with a shorter range of tissue penetration (2 mm) than 90Y. A recent phase II clinical trial reported an objective radiographic response rate of 30% among 310 patients with GEP-NETs, and a median progression-free survival duration of 40 mo[81].
CONCLUSION
SSAs were initially developed as antisecretory agents used primarily for the control of hormonal syndromes associated with NETs. In recent years, accumulating laboratory and clinical data has supported their role as antiproliferative agents, capable of stabilizing tumor growth in a large proportion of patients with metastatic carcinoid and pancreatic endocrine tumors. The recently-published PROMID study provides high-level evidence validating the role of octreotide LAR 30 mg as an antiproliferative agent in patients with metastatic carcinoid tumors of the midgut. Subset analysis suggests that patients with low tumor burden are most likely to experience disease stabilization, supporting the early use of octreotide LAR 30 mg in patients with metastatic disease. Further research efforts are underway to evaluate the use of novel SSAs, SSAs as antiproliferative agents in other types of GEP-NETs, and SSAs in combination with other anti-tumor agents.
Footnotes
Peer reviewer: Loes van Keimpema, MSc, Department of Gastroenterology and Hepatology, Radboud University Nijmegen Medical Center, PO Box 9101, 6500 HB, Nijmegen, The Netherlands
S-Editor Wang YR L-Editor O'Neill M E-Editor Wu PZ
References
- 1.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 2.Brazeau P, Gullemin R. Editorial: Somatostatin: newcomer from the hypothalamus. N Engl J Med. 1974;290:963–964. doi: 10.1056/NEJM197404252901711. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 4.Reichlin S. Somatostatin (second of two parts) N Engl J Med. 1983;309:1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- 5.Evers BM, Parekh D, Townsend CM Jr, Thompson JC. Somatostatin and analogues in the treatment of cancer. A review. Ann Surg. 1991;213:190–198. doi: 10.1097/00000658-199103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47 Suppl 2:30–39. doi: 10.1159/000049159. [DOI] [PubMed] [Google Scholar]
- 7.Weckbecker G, Raulf F, Stolz B, Bruns C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol Ther. 1993;60:245–264. doi: 10.1016/0163-7258(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 9.Maurer R, Reubi JC. Somatostatin receptors. JAMA. 1985;253:2741. [PubMed] [Google Scholar]
- 10.Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31:1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 11.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 12.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 13.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 14.Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 15.Sandostatin® Injection [prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2005. [Google Scholar]
- 16.Rubin J, Ajani J, Schirmer W, Venook AP, Bukowski R, Pommier R, Saltz L, Dandona P, Anthony L. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 17.Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 18.Ruszniewski P, Ish-Shalom S, Wymenga M, O'Toole D, Arnold R, Tomassetti P, Bax N, Caplin M, Eriksson B, Glaser B, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–251. doi: 10.1159/000082875. [DOI] [PubMed] [Google Scholar]
- 19.di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L, Zilembo N, di Leo A. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77:402–408. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Vezzosi D, Bennet A, Rochaix P, Courbon F, Selves J, Pradere B, Buscail L, Susini C, Caron P. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152:757–767. doi: 10.1530/eje.1.01901. [DOI] [PubMed] [Google Scholar]
- 21.O'Dorisio TM, Gaginella TS, Mekhjian HS, Rao B, O'Dorisio MS. Somatostatin and analogues in the treatment of VIPoma. Ann N Y Acad Sci. 1988;527:528–535. doi: 10.1111/j.1749-6632.1988.tb27006.x. [DOI] [PubMed] [Google Scholar]
- 22.Boden G, Ryan IG, Eisenschmid BL, Shelmet JJ, Owen OE. Treatment of inoperable glucagonoma with the long-acting somatostatin analogue SMS 201-995. N Engl J Med. 1986;314:1686–1689. doi: 10.1056/NEJM198606263142606. [DOI] [PubMed] [Google Scholar]
- 23.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 24.Sharma K, Srikant CB. G protein coupled receptor signaled apoptosis is associated with activation of a cation insensitive acidic endonuclease and intracellular acidification. Biochem Biophys Res Commun. 1998;242:134–140. doi: 10.1006/bbrc.1997.7933. [DOI] [PubMed] [Google Scholar]
- 25.Sharma K, Srikant CB. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int J Cancer. 1998;76:259–266. doi: 10.1002/(sici)1097-0215(19980413)76:2<259::aid-ijc14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Lattuada D, Casnici C, Venuto A, Marelli O. The apoptotic effect of somatostatin analogue SMS 201-995 on human lymphocytes. J Neuroimmunol. 2002;133:211–216. doi: 10.1016/s0165-5728(02)00364-8. [DOI] [PubMed] [Google Scholar]
- 27.Florio T. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol. 2008;286:40–48. doi: 10.1016/j.mce.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Florio T, Thellung S, Arena S, Corsaro A, Bajetto A, Schettini G, Stork PJ. Somatostatin receptor 1 (SSTR1)-mediated inhibition of cell proliferation correlates with the activation of the MAP kinase cascade: role of the phosphotyrosine phosphatase SHP-2. J Physiol Paris. 2000;94:239–250. doi: 10.1016/s0928-4257(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 29.Barbieri F, Pattarozzi A, Gatti M, Aiello C, Quintero A, Lunardi G, Bajetto A, Ferrari A, Culler MD, Florio T. Differential efficacy of SSTR1, 2 and 5 agonists in the inhibition of C6 glioma growth in nude mice. Am J Physiol Endocrinol Metab. 2009:Epub ahead of print. doi: 10.1152/ajpendo.00292.2009. [DOI] [PubMed] [Google Scholar]
- 30.Florio T, Morini M, Villa V, Arena S, Corsaro A, Thellung S, Culler MD, Pfeffer U, Noonan DM, Schettini G, et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology. 2003;144:1574–1584. doi: 10.1210/en.2002-220949. [DOI] [PubMed] [Google Scholar]
- 31.Zapata PD, Ropero RM, Valencia AM, Buscail L, López JI, Martín-Orozco RM, Prieto JC, Angulo J, Susini C, López-Ruiz P, et al. Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab. 2002;87:915–926. doi: 10.1210/jcem.87.2.8194. [DOI] [PubMed] [Google Scholar]
- 32.Thangaraju M, Sharma K, Liu D, Shen SH, Srikant CB. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999;59:1649–1654. [PubMed] [Google Scholar]
- 33.Theodoropoulou M, Zhang J, Laupheimer S, Paez-Pereda M, Erneux C, Florio T, Pagotto U, Stalla GK. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006;66:1576–1582. doi: 10.1158/0008-5472.CAN-05-1189. [DOI] [PubMed] [Google Scholar]
- 34.Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688–1696. doi: 10.1210/mend.10.12.8961277. [DOI] [PubMed] [Google Scholar]
- 35.Pagès P, Benali N, Saint-Laurent N, Estève JP, Schally AV, Tkaczuk J, Vaysse N, Susini C, Buscail L. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186–15193. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 36.Held-Feindt J, Forstreuter F, Pufe T, Mentlein R. Influence of the somatostatin receptor sst2 on growth factor signal cascades in human glioma cells. Brain Res Mol Brain Res. 2001;87:12–21. doi: 10.1016/s0169-328x(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 37.Dent P, Wang Y, Gu YZ, Wood SL, Reardon DB, Mangues R, Pellicer A, Schonbrunn A, Sturgill TW. S49 cells endogenously express subtype 2 somatostatin receptors which couple to increase protein tyrosine phosphatase activity in membranes and down-regulate Raf-1 activity in situ. Cell Signal. 1997;9:539–549. doi: 10.1016/s0898-6568(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 38.Reardon DB, Dent P, Wood SL, Kong T, Sturgill TW. Activation in vitro of somatostatin receptor subtypes 2, 3, or 4 stimulates protein tyrosine phosphatase activity in membranes from transfected Ras-transformed NIH 3T3 cells: coexpression with catalytically inactive SHP-2 blocks responsiveness. Mol Endocrinol. 1997;11:1062–1069. doi: 10.1210/mend.11.8.9960. [DOI] [PubMed] [Google Scholar]
- 39.Massa A, Barbieri F, Aiello C, Iuliano R, Arena S, Pattarozzi A, Corsaro A, Villa V, Fusco A, Zona G, et al. The phosphotyrosine phosphatase eta mediates somatostatin inhibition of glioma proliferation via the dephosphorylation of ERK1/2. Ann N Y Acad Sci. 2004;1030:264–274. doi: 10.1196/annals.1329.033. [DOI] [PubMed] [Google Scholar]
- 40.Florio T, Yao H, Carey KD, Dillon TJ, Stork PJ. Somatostatin activation of mitogen-activated protein kinase via somatostatin receptor 1 (SSTR1) Mol Endocrinol. 1999;13:24–37. doi: 10.1210/mend.13.1.0224. [DOI] [PubMed] [Google Scholar]
- 41.Alderton F, Humphrey PP, Sellers LA. High-intensity p38 kinase activity is critical for p21(cip1) induction and the antiproliferative function of G(i) protein-coupled receptors. Mol Pharmacol. 2001;59:1119–1128. doi: 10.1124/mol.59.5.1119. [DOI] [PubMed] [Google Scholar]
- 42.Koch BD, Schonbrunn A. The somatostatin receptor is directly coupled to adenylate cyclase in GH4C1 pituitary cell membranes. Endocrinology. 1984;114:1784–1790. doi: 10.1210/endo-114-5-1784. [DOI] [PubMed] [Google Scholar]
- 43.Schonbrunn A, Dorflinger LJ, Koch BD. Mechanisms of somatostatin action in pituitary cells. Adv Exp Med Biol. 1985;188:305–324. doi: 10.1007/978-1-4615-7886-4_17. [DOI] [PubMed] [Google Scholar]
- 44.Schettini G, Florio T, Meucci O, Landolfi E, Lombardi G, Marino A. Somatostatin inhibition of anterior pituitary adenylate cyclase activity: different sensitivity between male and female rats. Brain Res. 1988;439:322–329. doi: 10.1016/0006-8993(88)91489-8. [DOI] [PubMed] [Google Scholar]
- 45.Ambler GR, Butler AA, Padmanabhan J, Breier BH, Gluckman PD. The effects of octreotide on GH receptor and IGF-I expression in the GH-deficient rat. J Endocrinol. 1996;149:223–231. doi: 10.1677/joe.0.1490223. [DOI] [PubMed] [Google Scholar]
- 46.Serri O, Brazeau P, Kachra Z, Posner B. Octreotide inhibits insulin-like growth factor-I hepatic gene expression in the hypophysectomized rat: evidence for a direct and indirect mechanism of action. Endocrinology. 1992;130:1816–1821. doi: 10.1210/endo.130.4.1547711. [DOI] [PubMed] [Google Scholar]
- 47.Woltering EA, Watson JC, Alperin-Lea RC, Sharma C, Keenan E, Kurozawa D, Barrie R. Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs. 1997;15:77–86. doi: 10.1023/a:1005774713202. [DOI] [PubMed] [Google Scholar]
- 48.Danesi R, Agen C, Benelli U, Paolo AD, Nardini D, Bocci G, Basolo F, Campagni A, Tacca MD. Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201-995) Clin Cancer Res. 1997;3:265–272. [PubMed] [Google Scholar]
- 49.Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer. 2005;92:1493–1498. doi: 10.1038/sj.bjc.6602503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arena S, Pattarozzi A, Corsaro A, Schettini G, Florio T. Somatostatin receptor subtype-dependent regulation of nitric oxide release: involvement of different intracellular pathways. Mol Endocrinol. 2005;19:255–267. doi: 10.1210/me.2004-0280. [DOI] [PubMed] [Google Scholar]
- 51.Kumar M, Liu ZR, Thapa L, Qin RY. Anti-angiogenic effects of somatostatin receptor subtype 2 on human pancreatic cancer xenografts. Carcinogenesis. 2004;25:2075–2081. doi: 10.1093/carcin/bgh216. [DOI] [PubMed] [Google Scholar]
- 52.Kumar M, Liu ZR, Thapa L, Chang Q, Wang DY, Qin RY. Antiangiogenic effect of somatostatin receptor subtype 2 on pancreatic cancer cell line: Inhibition of vascular endothelial growth factor and matrix metalloproteinase-2 expression in vitro. World J Gastroenterol. 2004;10:393–399. doi: 10.3748/wjg.v10.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89:471–476. doi: 10.1159/000197899. [DOI] [PubMed] [Google Scholar]
- 54.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 55.Plöckinger U, Wiedenmann B. Treatment of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451 Suppl 1:S71–S80. doi: 10.1007/s00428-007-0446-z. [DOI] [PubMed] [Google Scholar]
- 56.Arnold R, Benning R, Neuhaus C, Rolwage M, Trautmann ME. Gastroenteropancreatic endocrine tumors: effect of Sandostatin on tumor growth. The German Sandostatin Study Group. Metabolism. 1992;41:116–118. doi: 10.1016/0026-0495(92)90044-b. [DOI] [PubMed] [Google Scholar]
- 57.Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C, Jürgensen R, Stein K, Schäfer H, Bruns C, et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430–438. doi: 10.1136/gut.38.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y, Kelsen D. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 59.Ducreux M, Ruszniewski P, Chayvialle JA, Blumberg J, Cloarec D, Michel H, Raymond JM, Dupas JL, Gouerou H, Jian R, et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol. 2000;95:3276–3281. doi: 10.1111/j.1572-0241.2000.03210.x. [DOI] [PubMed] [Google Scholar]
- 60.Wymenga AN, Eriksson B, Salmela PI, Jacobsen MB, Van Cutsem EJ, Fiasse RH, Välimäki MJ, Renstrup J, de Vries EG, Oberg KE. Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J Clin Oncol. 1999;17:1111. doi: 10.1200/JCO.1999.17.4.1111. [DOI] [PubMed] [Google Scholar]
- 61.Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 62.Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 63.Aparicio T, Ducreux M, Baudin E, Sabourin JC, De Baere T, Mitry E, Schlumberger M, Rougier P. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37:1014–1019. doi: 10.1016/s0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 64.Arnold R, Neuhaus C, Benning R, Schwerk WB, Trautmann ME, Joseph K, Bruns C. Somatostatin analog sandostatin and inhibition of tumor growth in patients with metastatic endocrine gastroenteropancreatic tumors. World J Surg. 1993;17:511–519. doi: 10.1007/BF01655111. [DOI] [PubMed] [Google Scholar]
- 65.Eriksson B, Renstrup J, Imam H, Oberg K. High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumors: clinical and biological effects. Ann Oncol. 1997;8:1041–1044. doi: 10.1023/a:1008205415035. [DOI] [PubMed] [Google Scholar]
- 66.Tomassetti P, Migliori M, Gullo L. Slow-release lanreotide treatment in endocrine gastrointestinal tumors. Am J Gastroenterol. 1998;93:1468–1471. doi: 10.1111/j.1572-0241.1998.465_q.x. [DOI] [PubMed] [Google Scholar]
- 67.Tomassetti P, Migliori M, Corinaldesi R, Gullo L. Treatment of gastroenteropancreatic neuroendocrine tumours with octreotide LAR. Aliment Pharmacol Ther. 2000;14:557–560. doi: 10.1046/j.1365-2036.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 68.Ricci S, Antonuzzo A, Galli L, Ferdeghini M, Bodei L, Orlandini C, Conte PF. Octreotide acetate long-acting release in patients with metastatic neuroendocrine tumors pretreated with lanreotide. Ann Oncol. 2000;11:1127–1130. doi: 10.1023/a:1008383132024. [DOI] [PubMed] [Google Scholar]
- 69.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 70.Nasir A, Stridsberg M, Strosberg J, Su PH, Livingston S, Malik HA, Kelley ST, Centeno BA, Coppola D, Malafa ME, et al. Somatostatin receptor profiling in hepatic metastases from small intestinal and pancreatic neuroendocrine neoplasms: immunohistochemical approach with potential clinical utility. Cancer Control. 2006;13:52–60. doi: 10.1177/107327480601300108. [DOI] [PubMed] [Google Scholar]
- 71.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 72.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 73.Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. doi: 10.1016/j.mce.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Van Vugt HH, Schmid HA, Sailer AW. Ligand-dependent internalization of somatostatin receptors. Endocrine Abstracts. 2008;16:P659. [Google Scholar]
- 75.Kvols L, Wiedenmann B, Oberg K, Glusman J, O'Dorisio T, de Herder WW, Gao B, Arnold R, Anthony L The SOM230 Carcinoid Study Group. Safety and efficacy of pasireotide (SOM230) in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR: Results of a phase II study. ASCO GI Cancers Symposium 2006; abst 171. [Google Scholar]
- 76.Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J, Kim S, Moreau JP, Enjalbert A, Culler MD. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol. 2005;153:135–141. doi: 10.1530/eje.1.01950. [DOI] [PubMed] [Google Scholar]
- 77.Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 78.Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, Kwekkeboom DJ, Bouterfa H, Krenning EP. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147–156. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, Haldemann A, Mueller-Brand J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 80.Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, Bartolomei M, Orsi F, De Cicco C, Mäcke HR, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–434. doi: 10.1007/s002590100490. [DOI] [PubMed] [Google Scholar]
- 81.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]