Abstract
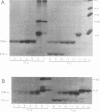
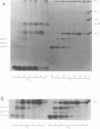
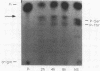
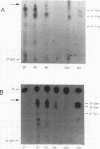
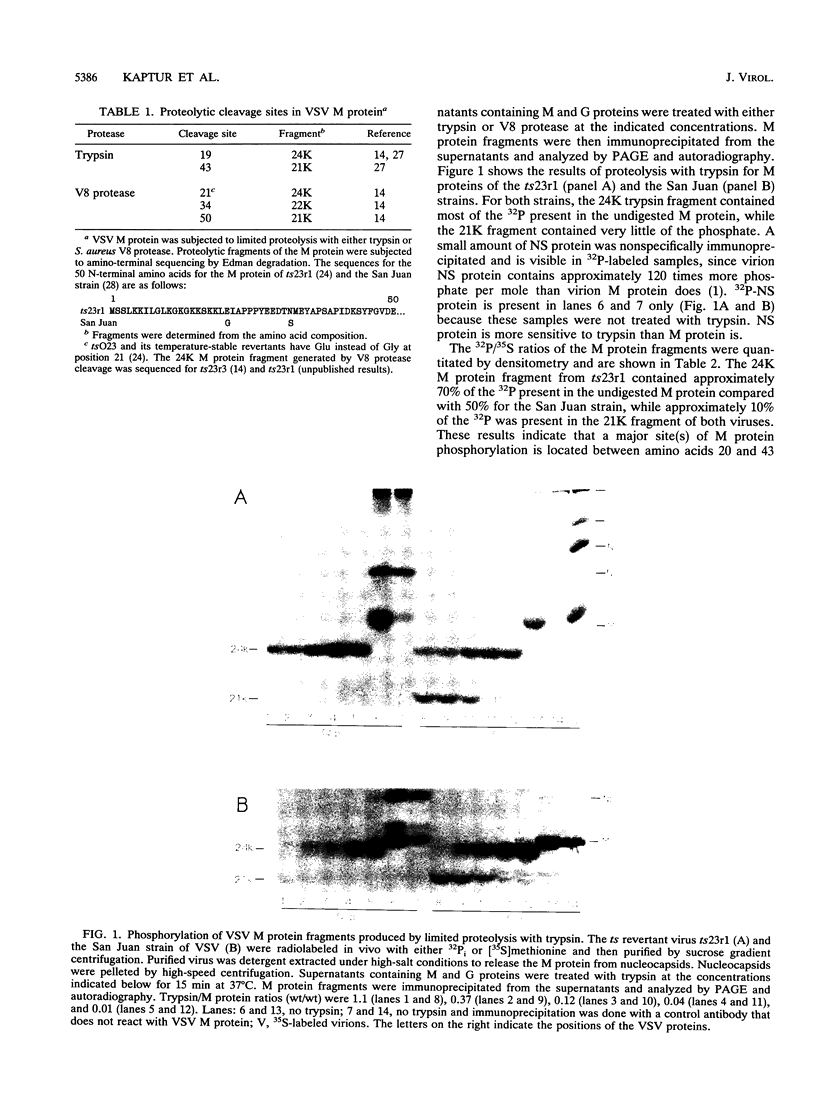
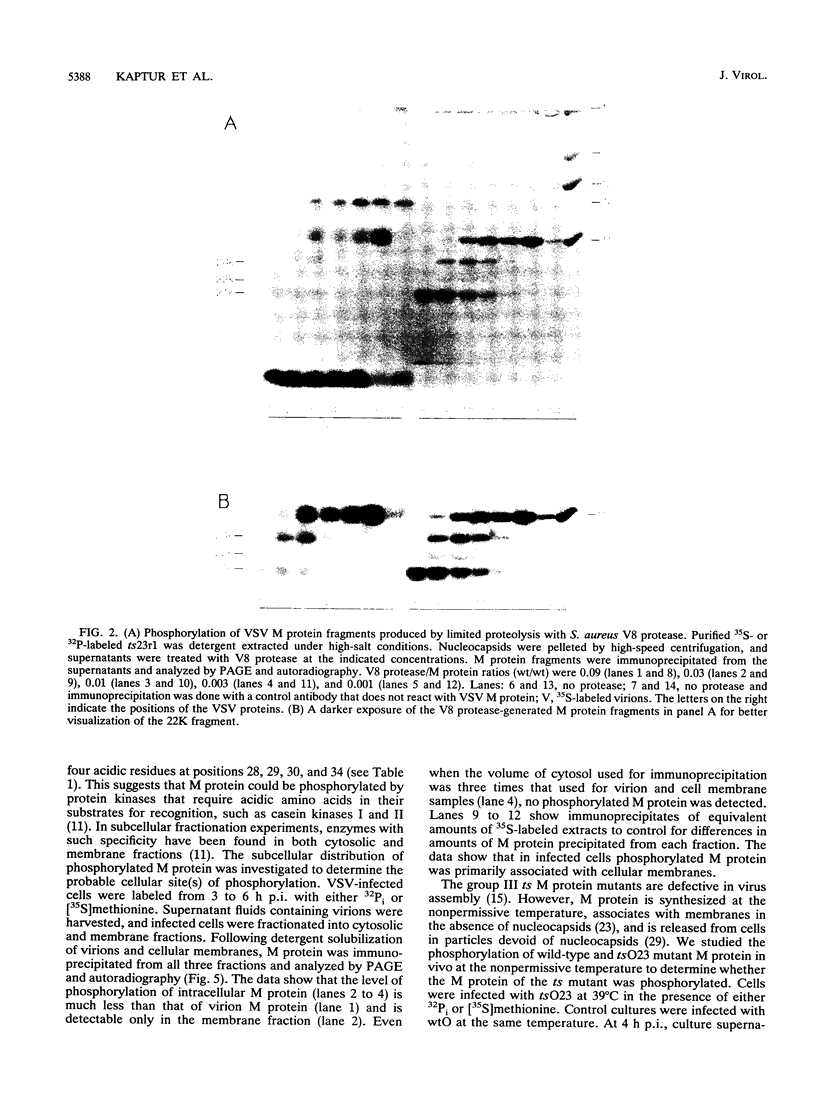
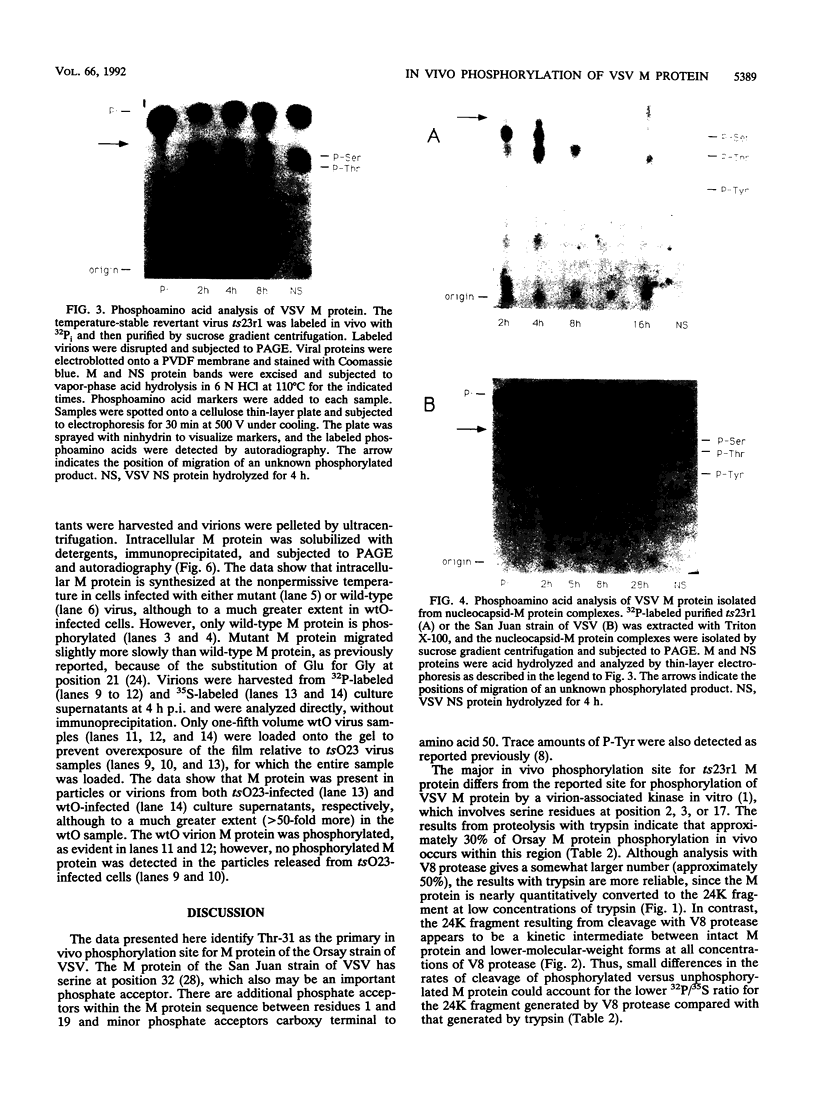
We mapped the in vivo phosphorylation sites for the matrix (M) protein of the Orsay and San Juan strains of vesicular stomatitis virus, Indiana serotype, using limited proteolysis and phosphoamino acid analysis. M protein was solubilized from 32P-labeled virions by using detergent and high-salt conditions, then treated with either trypsin or Staphylococcus aureus V8 protease, and analyzed by polyacrylamide gel electrophoresis and autoradiography to determine which fragments contained phosphate residues. The M protein fragment extending from amino acid 20 to the carboxy terminus contained approximately 70% of the control 32P label, while the fragment extending from amino acid 35 to the carboxy terminus had only trace amounts of label. These data indicate that the major phosphorylation site was between amino acids 20 and 34 in the Orsay strain M protein. Phosphoamino acid analysis of M protein by thin-layer electrophoresis showed the presence of phosphothreonine and phosphoserine and that phosphothreonine continued to be released after prolonged vapor-phase acid hydrolysis. These data identify Thr-31 as the primary in vivo phosphate acceptor for M protein of the Orsay strain of vesicular stomatitis virus. The San Juan strain M protein has serine at position 32, which may also be an important phosphate acceptor. In addition, phosphorylation at Ser-2, -3, or -17 occurs to a greater extent in the San Juan strain M protein than in the Orsay strain M protein. The subcellular distribution of phosphorylated M protein was investigated to determine a probable intracellular site(s) of phosphorylation. Phosphorylated M protein was associated primarily with cellular membranes, suggesting phosphorylation by a membrane-associated kinase. Virion M protein was phosphorylated to a greater extent than membrane-bound M protein, indicating that M protein phosphorylation occurs at a late stage in virus assembly. Phosphorylation of wild-type and temperature-sensitive mutant M protein was studied in vivo at the nonpermissive temperature. The data show that phosphorylated M protein was detected only in wild-type virus-infected cells and virions, suggesting that association with nucleocapsids may be required for M protein phosphorylation or that misfolding of mutant M protein at the nonpermissive temperature prevents phosphorylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckes J. D., Childers L. C., Perrault J. Phosphorylation of vesicular stomatitis virus M protein: evidence for a second virion-associated protein serine kinase activity. Virology. 1989 Mar;169(1):161–171. doi: 10.1016/0042-6822(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Beckes J. D., Perrault J. Two distinct protein kinase activities in vesicular stomatitis virions phosphorylate the NS transcription factor. Virology. 1991 Sep;184(1):383–386. doi: 10.1016/0042-6822(91)90854-5. [DOI] [PubMed] [Google Scholar]
- Blondel D., Dezelee S., Wyers F. Vesicular stomatitis virus growth in Drosophila melanogaster cells. II. Modifications of viral protein phosphorylation. J Gen Virol. 1983 Aug;64(Pt 8):1793–1799. doi: 10.1099/0022-1317-64-8-1793. [DOI] [PubMed] [Google Scholar]
- Bylund D. B., Huang T. S. Decomposition of phosphoserine and phosphothreonine during acid hydrolysis. Anal Biochem. 1976 Jun;73(2):477–485. doi: 10.1016/0003-2697(76)90197-4. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Phosphoproteins of vesicular stomatitis virus: identity and interconversion of phosphorylated forms. Virology. 1979 Nov;99(1):84–94. doi: 10.1016/0042-6822(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Duclos B., Marcandier S., Cozzone A. J. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and/or electrophoresis. Methods Enzymol. 1991;201:10–21. doi: 10.1016/0076-6879(91)01004-l. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P. Determination of phosphoamino acid composition by acid hydrolysis of protein blotted to Immobilon. Methods Enzymol. 1991;201:21–27. doi: 10.1016/0076-6879(91)01005-m. [DOI] [PubMed] [Google Scholar]
- Kaptur P. E., Rhodes R. B., Lyles D. S. Sequences of the vesicular stomatitis virus matrix protein involved in binding to nucleocapsids. J Virol. 1991 Mar;65(3):1057–1065. doi: 10.1128/jvi.65.3.1057-1065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella D. A., Lenard J. Interactions of wild-type and mutant M protein of vesicular stomatitis virus with viral nucleocapsid and envelope in intact virions. Evidence from [125I]iodonaphthyl azide labeling and specific cross-linking. Biochemistry. 1981 Nov 24;20(24):6872–6877. doi: 10.1021/bi00527a020. [DOI] [PubMed] [Google Scholar]
- Matsumura S., Murakami N., Yasuda S., Kumon A. Site-specific phosphorylation of brain myosin light chains by calcium-dependent and calcium-independent myosin kinases. Biochem Biophys Res Commun. 1982 Dec 15;109(3):683–688. doi: 10.1016/0006-291x(82)91994-5. [DOI] [PubMed] [Google Scholar]
- McCreedy B. J., Jr, Lyles D. S. Distribution of M protein and nucleocapsid protein of vesicular stomatitis virus in infected cell plasma membranes. Virus Res. 1989 Nov;14(3):189–205. doi: 10.1016/0168-1702(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Morita K., Vanderoef R., Lenard J. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J Virol. 1987 Feb;61(2):256–263. doi: 10.1128/jvi.61.2.256-263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Dubois-Dalcq M. E., Schubert M., Lazzarini R. A. A mutated membrane protein of vesicular stomatitis virus has an abnormal distribution within the infected cell and causes defective budding. J Virol. 1987 May;61(5):1332–1341. doi: 10.1128/jvi.61.5.1332-1341.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Lodish H. F. Noninfectious vesicular stomatitis virus particles deficient in the viral nucleocapsid. J Virol. 1979 Feb;29(2):443–447. doi: 10.1128/jvi.29.2.443-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmerman H. K., Collins J. H., Theibert J. L., Wegener A. D., Jones L. R. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986 Oct 5;261(28):13333–13341. [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]