Abstract
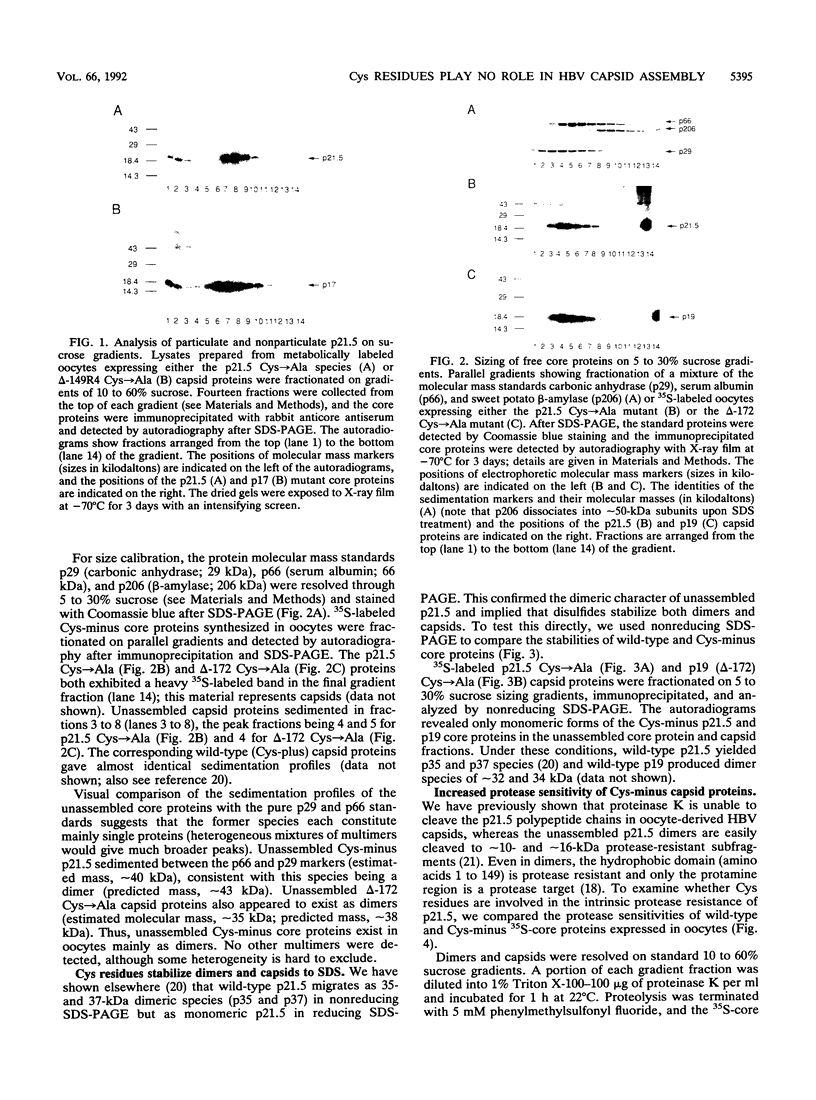
In the spherical capsid of hepatitis B virus (HBV), intermolecular disulfide bonds cross-link the approximately 180 p21.5 capsid protein subunits into a stable lattice. In this study, we used mutant capsid proteins to investigate the role that disulfide bonds and the four p21.5 Cys residues (positions 48, 61, 107, and 185) play in capsid assembly and/or stabilization. p21.5 Cys residues were either replaced by Ala or removed (Cys-185) by carboxyl-terminal truncation, creating Cys-minus mutants which were expressed in Xenopus oocytes via microinjected synthetic mRNAs. Fractionation of radiolabeled oocyte extracts on 10 to 60% sucrose gradients revealed that Cys-minus core proteins resolved into the nonparticulate and capsid forms seen for wild-type p21.5. On 5 to 30% sucrose gradients, nonparticulate Cys-minus core proteins sedimented as dimers of approximately 40 kDa. We conclude that Cys residues and disulfides are not required for the assembly of either HBV capsids or the dimers that provide the precursors for capsid assembly. Since assembly presumably demands an appropriate p21.5 tertiary structure, it is unlikely that Cys residues are required for proper p21.5 folding. However, Cys residues stabilize isolated p21.5 structures, as evidenced by the marked reduction in stability of Cys-minus dimers and capsids (i) in nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and (ii) upon protease digestion. We discuss these results in the context of the HBV life cycle and the role of Cys residues in other proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum F., Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990 Jul;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Disulphide bonds and protein stability. Bioessays. 1988 Feb-Mar;8(2):57–63. doi: 10.1002/bies.950080204. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., Milanesi G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J Virol. 1989 Nov;63(11):4645–4652. doi: 10.1128/jvi.63.11.4645-4652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton T., Zhou S., Standring D. N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in viral replication. J Virol. 1992 Sep;66(9):5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. H., Matsumura M., Faber H. R., Matthews B. W. Structure of a stabilizing disulfide bridge mutant that closes the active-site cleft of T4 lysozyme. Protein Sci. 1992 Jan;1(1):46–57. doi: 10.1002/pro.5560010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng K. S., Hu C. P., Chang C. M. Differential formation of disulfide linkages in the core antigen of extracellular and intracellular hepatitis B virus core particles. J Virol. 1991 Jul;65(7):3924–3927. doi: 10.1128/jvi.65.7.3924-3927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Onodera S., Ohori H., Yamaki M., Ishida N. Electron microscopy of human hepatitis B virus cores by negative staining-carbon film technique. J Med Virol. 1982;10(2):147–155. doi: 10.1002/jmv.1890100209. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Q., Walter M., Standring D. N. Hepatitis B virus p25 precore protein accumulates in Xenopus oocytes as an untranslocated phosphoprotein with an uncleaved signal peptide. J Virol. 1992 Jan;66(1):37–45. doi: 10.1128/jvi.66.1.37-45.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. L., Standring D. N. Production of hepatitis B virus nucleocapsidlike core particles in Xenopus oocytes: assembly occurs mainly in the cytoplasm and does not require the nucleus. J Virol. 1991 Oct;65(10):5457–5464. doi: 10.1128/jvi.65.10.5457-5464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yang S. Q., Standring D. N. Characterization of hepatitis B virus capsid particle assembly in Xenopus oocytes. J Virol. 1992 May;66(5):3086–3092. doi: 10.1128/jvi.66.5.3086-3092.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]