Abstract
Background
Current strategies to overcome the global problem of antimicrobial resistance include research in finding new and innovative antimicrobials from plants. This study was carried out to determine the antibacterial activity of plant extracts of Olea africana stem-bark, Psidium guajava leaves, Vernonia amygdalina leaves, Lantana camara leaves and Mangifera indica leaves which are used in folklore medicine to treat infections of microbial origin in Longisa region of Bomet District, Kenya.
Methods
Methanol extracts were derived and screened. Standard cultures of Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 25923 were used in the study. The antibacterial tests used were the agar well diffusion assays at concentration 1gm/ml. Minimum Inhibition Concentration (MIC) was determined in the plant extract that showed some efficacy against the tested microorganisms. Gentamicin (10µg) was used as a positive control.
Results
The methanol extracts showed weak antibacterial activity against the study organisms compared to Gentamicin. All extracts exhibited a significant bactericidal activity against S. aureus while L. camara and V. amygdalina lacked efficacy against P.aeruginosa and E. coli. O. africana and P. guajava presented the lowest MIC against S.aureus (62.5 mg/ml and 250 mg/ml respectively P.guajava and M. indica showed analogous MICs against P.aeruginosa (250 mg/ml). P.guajava exhibited a better MIC against E.coli (500 mg/ml).
Conclusions
This in-vitro study corroborated the antimicrobial activity of the selected plants used in folklore medicine. The plants could be potential sources of new antimicrobial agent.
Key words: Medicinal Plant extracts, antibacterial activity, MIC
Introduction
Many works have been done which aim at knowing the different antimicrobial and phytochemical constituents of medicinal plants and using them for the treatment of microbial as possible alternatives to chemically synthetic drugs to which many infectious microorganisms have become resistant. Moreover, antibacterial pharmaceuticals are not accessible to the majority of the communities in the developing countries1. Increase in resistance calls for new antibacterial drugs, one source of which are traditional medicinal plants. Plants may provide natural source of antimicrobial drugs that will/or provide novel or lead compounds that may be employed in controlling some infections globally.
Olea africana (Oleaceae) is a tree of about 3–15 m in height, may assume bushy habit if stunted. Leaf or Stem bark are used to treat sore throat, kidney problems, backache, colic or urinary tract infections. Olea africana has been demonstrated scientifically possessing; antimicrobial activity, antiarrhythmic activity, diuretic activity, anti-inflammatory activity and hepatic activity 2. Phytochemical tests in laboratories indicated the presence of alkaloids, saponins and tannins, but not of cardiac or cyanogenic glycosides. Psidium guajava L (Myrtaceae) is a native plant of tropical America. Different parts of the plants are used in treatment of various human aliments such as wounds, ulcers, bowels and cholera. Pharmacological investigations indicated that its bark, fruit, and leaves possess, hypoglycemic, anti-inflammatory, analgesic, antipyretic, spasmolytic, CNS depressant and antimicrobial activities. The leaves of P. guajava contain an essential oil rich in cineol, tannins, triterpenes and flavonoids3–5. Vernonia amygdalina (Compositae) is a shrub, of 2–5 m tall with petiolate green leaves of about 6 mm diameter. The roots and the leaves are used in ethno medicine to treat fever, hiccups, kidney problems, stomach discomfort and bacterial infections6. The active components of the plant have been shown to be mainly sesquiterpene lactones like vernodalin and vernoamygdalin and steroid glycosides like vernonioside B1 and vernoniol B7. Lantana camara Linn. (Verbenaceae) is a rugged evergreen strong-smelling herb, native of tropical America, but now naturalized in many parts of Africa. All the parts of this plant have been used traditionally for several ailments throughout the world. The leaves are used as a bechic, antitumoral, antibacterial and antihypertensive agent8. The root of this plant is used for the treatment of malaria, rheumatism and skin rashes9. Mangifera indica (Anacardiaceae) is a large evergreen tree, with a heavy, dome-shaped crown. It is found all over the tropical regions of the world where it is used as a horticultural and medicinal plant. The use of leaf extracts as antimicrobial in the treatment of burns, scalds, sores, wounds, abscesses and other infections in humans and animals has been reported in a number of ethnobotanical surveys10. The leaves have been reported to contain saponins, glycosides, unsaturated sterols, polyphenols, euxanthin acid, mangiferine, mangin, gallic tannins11. This study looks into the in vitro antibacterial activity of these plants against three pathogenic microorganisms (Staphylococus aureus, Pseudomonas aeruginosa and Escherichia coli) that cause the most common cases of infectious diseases of poverished communities in Bomet District. Qualitative phytochemical screening of alkaloids, tannins, flavonoids, anthraquinones, saponins, glycoside or cardiac glycosides was carried out using standard methods 12, 13.
Materials and method
Plant collection and Identification
Ethno pharmacological information of several medicinal plants was sourced from literatures14, 15 and through consultation from elderly people in age bracket of above 50 years from Siwot Village, Longisa division in Bomet district, Kenya, during the month of January 2008. Plants selected for study was obtained by ranking basing on diseases they treated, frequency of their use and their availability. Taxonomic identity of voucher specimen was done by comparing with those of known identity in the Makerere University herbarium in Kampala.
Processing and Extraction of Plant Material
The plant parts were chopped and shade-dried at room temperature for 2 weeks then grounded using metallic mortar and pestle to a fine powder for ease of extraction of active compounds. The grounded samples were then transported for extraction process at the Pharmacology Laboratory of Faculty of Vet medicine, Makerere University. The grounded powder were weighed on Satorius balance type BA 610, soaked in known volumes of 95 % Analytical Methanol and allowed to stand for two days with intermittent shaking. Filtration through cotton wool was done to remove coarse particles and finely through filter paper (Whatman® No.1, England) in Buchner funnel. This was followed by concentration on Rotavapour type Buchi-R-Switzerland at 50 °C to recover the solvent used. Extracts were dissolved in small drops of Dimethyl sulfoxide (DMSO) and toped up with Physiological saline and the stock solution kept at 4 °C.
Preparation of the test micro-organisms
This process followed the previously established procedures for testing antimicrobial agents. Standard cultures of bacteria from the American Type Culture Collection were obtained from the Microbiology Laboratory,Faculty of Veterinary Medicine of Makerere University. A Gram positive bacterium; Staphylococcus aureus ATCC 25923, was used as a wound/skin pathogen and Gram negative bacteria; Escherichia coli ATCC 25922, was used to represent pathogens that cause gastro enteritis while Pseudomonas aeruginosa ATCC 27853, was used as an environmental pathogen16. Standardized bacterial suspension was prepared by picking a colony of respective bacteria using sterile wire loop and suspending it in 5 ml Brain heart infusion liquid media. The dilutions formed the bacterial stock solutions for use in the agar-well diffusion assays.
Preparation of culture media
Mueller Hinton agar (Becton Dickinson ® M.D USA) was used for direct sensitivity testing. The media was prepared and treated according to manufacturer's guidelines. Thirty five (35) g medium was mixed with one litre of distilled water, enclosed in a screw cap container and autoclaved at 121 °C for 15 minutes. The medium was later dispensed into 90 mm sterile agar plates and left to set. The agar plates were incubated for 24 hours at 37 °C to confirm their sterility. When no growth occurred after 24 hours, the plates were considered sterile.
Agar-Well Diffusion Assay
A concentration of 1 g/ml of the plant extracts was designed from the stock solution for agar well diffusion assay. Cultures of S.aureus, E.coli and P.aeruginosa were inoculated separately on the surface of Mueller Hinton agar plates by surface spreading using a sterile cotton swab and each bacterium evenly spread over the entire surface of agar plate to obtain a uniform inoculum. The sensitivity testing of the plant extracts was done using the agar well diffusion method17 whereby, wells of 6 mm diameter and 5 mm depth were made on the solid agar using a sterile glass borer. About 50 µl of the methanol extract, of the concentration 1 g/ml, was dispensed into respective wells and 10 µg Gentamycin was used as a positive control since it is a broad spectrum antibiotic. Physiological saline/Dimethyl sulfoxide (DMSO) was used as negative control. All the tests were run in triplicates for quality results. The set up was incubated for 24 hours at 37 °C. Twenty four (24) hours later, the zones of inhibition were measured using a ruler (AIM®) and a pair of divider then results reported in millimeters (mm).
Minimum Inhibition Concentration (MIC) Evaluation
The MIC was evaluated on plant extracts that showed antibacterial activity in the agar well diffusion assay on any organism. This test was performed at five concentration of each extract (500 mg/ml, 250 mg/ml, 125 mg/ml, 62.5 mg/ml and 31.25 mg/ml) employing doubling dilutions of plant extract in Brain heart infusion broth up to the fifth dilution. One (1) ml of the resultant broth was put in test tube and equal amounts of the extracts (1 ml) were added to the first test tube and serial dilution done with the last 1 ml being discarded. To complete the test, each organism was separately suspended in 5 ml of Brain heart infusion broth and incubated overnight, after which 0.1 ml was added to all the test tubes and preparation incubated at 37 °C for 18 hours. After incubation, a loop full from each tube was sub cultured on nutrient agar to see if bacteria growth was inhibited (Minimum Bactericidal Activity). Growth of bacteria on solid media indicated that particular concentration of the extract was unable to inhibit the bacteria. The MIC was defined as the lowest concentration of an antimicrobial that inhibited the visible growth of a microorganism after overnight incubation 18.
Qualitative Phytochemical analysis of the plant extract
This was a qualitative analysis done at the department of Botany Laboratory, Faculty of Science, Makerere University. A small portion of the extract was used for phytochemical screening test. Alkaloids, Saponins, Tannins, Flavonoids, Glycosides, Cardiac glycosides and Anthraquinones was carried out using standard methods12,13
Data Analysis
Microsoft Excel® was used to enter and capture data. Various graphs and tables were extracted from this data. Data was then exported to SPSS for further analysis. The MIC for each microorganism was analyzed using one-way analysis of variance (ANOVA). P value < 0.05 was considered as significant. SPSS 15.0 was employed for statistical analysis.
Results and discussion
Qualitative phytochemical screening indicated that the methanol plant extracts contained classes of compounds as shown in Table 1. All plants exhibited different kinds of secondary metabolites. This probably contributed to their antibacterial activity. All extracts showed presence of Tannins and Flavonoids as seen in Table 1. Mangifera indica Contained all screened metabolites except Cardiac Glycosides. This result is in accord with former studies executed on the M. indica ethanol extract of its leaves 10. The bioactive ingredients could be responsible for antibacterial activity of the plant extracts. Tannins have been found to form irreversible complexes with proline-rich proteins resulting in the inhibition of the cell protein synthesis. This activity was exhibited by Mangifera indica and Psidium guajava against test organisms19. Previous studies show that secondary plant metabolites constitute an important source of microbicides, pesticides and many pharmaceutical drugs20, 21.
Table 1.
Classes of Bioactive compounds identified in the Methanol plant extract
| Compound | O. africana | P.guajava | V.amygdalina | L.camara | M.indica |
| Alkaloids | ++ | − | ++ | + | ++ |
| Tannins | + | ++ | + | + | + |
| Saponinns | − | + | − | + | + |
| Flavonoids | + | ++ | + | + | ++ |
| Anthraquinones | − | NT | − | − | + |
| Glycosides | − | + | − | − | ++ |
| Cardiacglycosides | NT | − | − | + | − |
Key: NT; Not tested, ++; strongly positive, +; positive, −; Negative
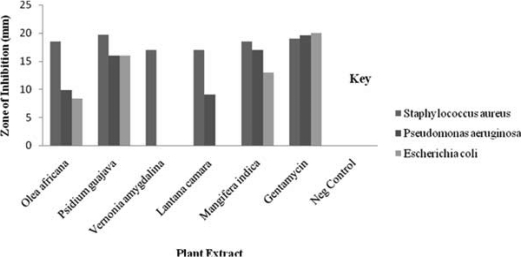
The results showed that plant extracts demonstrated antibacterial activity against the S.aureus, E.coli and P.aeruginosa. It can be noted from Figure 1 that Staphylococcus aureus was the most susceptible of the three organisms and E.coli the least. All the plant species showed activity against Staphylococcus aureus (Fig 1). Psidium guajava had the highest zone of inhibition to the other extracts (19.7 mm) against S.aureus, while Olea africana had the least MIC (62.5 mg/ml) against S.aureus signifying higher activity. From the three methanol extracts with activities against E.coli, Psidium guajava was the most active with a Zone of inhibition of 16 mm and an MIC of 500 mg/ml. This was followed by Mangifera indica (13 mm), with an MIC of 1000 mg/ml and lastly Olea africana with a zone of inhibition of 8.3 mm and an MIC of 1000 mg/ml. The most active methanol plant extract from the study against Pseudomonas aeruginosa was Mangifera indica with a Zone of Inhibition of 17 mm and an MIC of 250 mg/ml, followed by P.guajava (16 mm) with an MIC of 250 mg/ml. This was followed by Olea africana with a Zone of Inhibition of 9.8 mm and an MIC of 1000 mg/ml. The most active methanol plant extract on Staphylococcus aureus was Psidium guajava with a zone of inhibition of 19.7 mm and an MIC of 250 mg/ml, followed by Olea africana and Mangifera indica both with analogous zone of inhibition (18.5 mm) and a better MIC of 62.5 mg/ml and 500 mg/ml respectively. Lastly were Vernonia amygdalina and Lantana camara both with zone of inhibition of 17 mm and an MIC of 1000 mg/ml. Escherichia coli showed resistance to Vernonia amygdalina and Lantana camara while Pseudomonas aeruginosa was resistant to Vernonia amygdalina. According to statistical analysis results of one way ANOVA of Staphylococcus aureus the P-value was 0.306, F- calculated was 2.5 and F-critical was 19.2, one way ANOVA for Pseudomonas aeruginosa; P-value was 0.808, F-calculated was 0.225 and F-critical was 6.94. Lastly one way ANOVA for Escherichia coli P-value was 0.530, F-calculated 0.455 and F-critical 6.61. Since P-values were greater than 0.05 (P >0.05). Hence, we fail to reject the null hypothesis and conclude that there is no significant variation in the MIC at 95% confidence Interval for each of the test organisms.
Fig 1.
Chart showing the Zone of inhibition of Plant Methanol Extract
The zones of inhibition produced by the test organisms indicated their susceptibility to the plant extracts; it was observed that the zones of inhibition varied from one organism to another and from one plant part extract to another. According to Prescott22 the effect of an agent varies with target species. The effective use of P.guajava in diarrhea, dysentery and gastroenteritis can be related to guava's documented antibacterial properties. Bark and leaf extracts have shown to have in vitro toxic action against numerous bacteria. In several studies guava showed significant antibacterial activity against such common diarrhea-causing bacteria as Staphylococcus, Shigella, Salmonella, Bacillus, E. coli, Clostridium, and Pseudomonas (3–5). Olea africana had the least MIC (62.5 mg/ml) against S.aureus signifying higher activity in this study. The plant showed broad spectrum activity against the tested organisms. This justified the use of plant in treatment of infections of microbial origin2. Vernonia amygdalina lacked efficacy against P. aeruginosa. Also, Vernonia amygdalina and Lantana camara extracts did not have measurable activity against E.coli. These observations are likely to be the result of the differences in the cell wall structure between gram-negatives and gram-positive bacteria, with gram-negative outer membrane acting as a barrier to many environmental substances, including antibiotics23.
Vernonia amygdalina showed no activity against E.coli and P.aeruginosa thus indicating its narrow spectrum of activity according to the study. A similar study was carried out to determine the antibacterial potential of V. amygdalina using a panel multidrug resistant gram-negative and gram-positive bacteria and standard strains: E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Aqueous extract of V. amygdalina leaves was found to produce growth inhibitory zones of 15.6 to 16.1 mm for E. coli and 12.1–12.3 mm for S.aureus while two out of the eight Pseudomonas aeruginosa isolates tested showed susceptibility (inhibitory zone diameter of 6.7+0.2 mm) to V. amygdalina by agar well diffusion only. This result was in contrary to our results and indicated that water could be the ideal solvent for extraction24. Lantana camara showed activity against Staphylococcus aureus and was inactive against E.coli. Related studies on Lantana camara root-bark prepared with solvents of different polarity, was evaluated by the agar-well diffusion method. Twelve bacteria, six each of gram-positive and gram-negative strains, were used in the study. The activity of the chloroform and methanol extracts of L. camara was found to be more specific towards the gram-positive strains, although gram-negative P. aeruginosa was also inhibited by the methanol extracts of the plant in a dose dependent manner. The water extracts of L. camara was found to be inactive25.
In conclusion Methanol extracts of the plant parts showed antibacterial activity against disease-causing organisms and this suggest that constituents of the plants could be useful in chemotherapy. From the findings od this study, the following recommendations could be made; Firstly, there is a need to further isolate the active antibacterial agent (s) and secondly, it is necessary to determine toxicity of the active constituents, their side effects and pharmacokinetics effects.
Acknowledgement
I would like to acknowledge my supervisors, Associate prof. Deo Olila and Dr. John Kateregga, for their professional guidance. Thanks go to my parents, Mr & Mrs. Mutai, for their support always during research work and all Laboratory technicians and Technologist for their technical support.
References
- 1.World Health Organisation, author. Global strategy for containment of antibiotic resistance. Geneva: WHO; 2001. p. 99. [Google Scholar]
- 2.Anesini C, Perez C. Screening of plants used in Argentinian folk medicine for antimicrobial activity. Journal of Ethnopharmacology. 1993;39(2):119–128. doi: 10.1016/0378-8741(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 3.Olajide O A, Awe S O, Makinde J M. Pharmacological studies on the leaf of Psidium guajava. Fitoterapia. 1999;70:25–31. [Google Scholar]
- 4.Begum S, Hassan S I, Siddiqui B S, Shaheen F, Ghayur M N, Gilani A H. Triperpenoids from the leaves from Psidium guajava. Phytochemistry. 2002;61:399–403. doi: 10.1016/s0031-9422(02)00190-5. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves FA, Andrade Neto M, Bezerra JN, Macrae A, Sousa OV, Fonteles-Filho AA, et al. Antibacterial activity of GUAVA, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyer (Heller) Rev Inst Med Trop Sao Paulo. 2008;50(1):11–15. doi: 10.1590/s0036-46652008000100003. [DOI] [PubMed] [Google Scholar]
- 6.Banso A, Adeyemo SO, Jeremiah P. Antimicrobial properties of Vernonia amygdalina extract. J Applied Sci Magt. 1999;3:9–11. [Google Scholar]
- 7.Kupcham SM, Hernichway RJ, Wermer D. Vernodalin and Vernomygdin. Two new sesquiterpene lactones from Vernonia amygdalina del. J Org Chem. 1969;3:3908–3909. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 8.Taoubi K, Fauvel MT, Gleye J, Moulis C. Phenylpropanoid glycosides from Lantana camara and Lippia multiflora. Planta Med. 1997;63:192–193. doi: 10.1055/s-2006-957647. [DOI] [PubMed] [Google Scholar]
- 9.Chharba SC, Mahunnah RLA, Mshiu EN. Plants used in traditional medicine in eastern Tanzania. J Ethnopharmacol. 1993;39:83–103. doi: 10.1016/0378-8741(93)90024-y. [DOI] [PubMed] [Google Scholar]
- 10.Odyek O, Bbosa G S, Waako P, Kyegombe D B, Bukenya-Ziraba R, Ogwal-Okeng J. Antibacterial activity of Mangifera indica (L.) African Journal of Ecology, Afr J Ecol. 2007;45(Suppl 1):13–16. [Google Scholar]
- 11.Ngo T. Contribution to researches on mango leaves raw materials in northern Vietnam. Science Technological Publish. 2001:563–565. [Google Scholar]
- 12.Harbone JB. Phytochemical Methods. A Guide to Modern Techniques of plant Analysis. 3rd edition. Newyork: Chapman and Hall; 1998. pp. 1–198. [Google Scholar]
- 13.Evans WC. Trease and Evans Pharmacognosy. 14th edition. WB Saunders Company Limited; 1998. pp. 15–16. [Google Scholar]
- 14.Iwu MM. Handbook of African Medicinal Plants. Boca Raton: CRC Press Inc.; 1993. pp. 223–225. [Google Scholar]
- 15.Dweck AC. Article for cosmetics & toiletries magazine ethnobotanical plants from Africa. Iltshire, UK: Black Medicare Ltd; 2001. Available from: URL: http://www.dweckdata.co.uk/ [Google Scholar]
- 16.Carter G R, Cole J R. Diagnostic Procedures in Vet. Bacteriology and Mycology. 5th Edn. London and Newyork: Academic Press, Inc; 1990. [Google Scholar]
- 17.Irobi ON, Moo-Young M, Daramola SO. In J Pharmacognosy. 1996;34(2):87–90. [Google Scholar]
- 18.Andrew JM. Determination of MIC. Journal of Antimicrobial Chemotherapy. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 19.Subhuti Dharmananda. Gallnuts and the Uses of Tannins in Chinese Medicine. 2003. A paper delivered at Institute for Traditional Medicine, Portland, Oregon. [Google Scholar]
- 20.Ibrahim MB, Owonubi MO, Onaopo JA. Antibacterial effect of extract of leaf, stem and roof bark of Anogeissus leiocarpus on some bacterial organisms. J Pharm Res Dev. 1997;2(1):20–23. [Google Scholar]
- 21.Kolapo AL, Ogundiya MO, Okunade MB. Antimicrobial activities of some Nigerian chewing stick. 2007. Available from: URL: http://www.siu.odu/webl/leaflets/ogun diya hfm. [Google Scholar]
- 22.Prescott LMH, arley JP, Klein D. Microbiology (International Edition) Fifth edition. Mc Graw Hill Book Company; 2002. pp. 809–819. [Google Scholar]
- 23.Tortora GJ, Funke BR, Case CL. Microbiology: An introduction. San Franscisco: Benjamin Cummings; 2001. [Google Scholar]
- 24.Iwalokun B A, Bamiro S B, Durojaiye O O. An antimicrobial evaluation of Vernonia amygdalina (Compositae) against gram-positive and gram-negative bacteria from Lagos, Nigeria. West African Journal of Pharmacology and Drug Research. 2004;19 [Google Scholar]
- 25.Basu Subhalakshmi, Ghosh Abhijit, Hazra Banasri. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: isolation of emodin and physcion as active antibacterial agents. Calcutta: Department of Pharmaceutical Technology, Jadavpur University; 2002. 700 032, India. [DOI] [PubMed] [Google Scholar]