Abstract
The present study examined the overloading of ion-exchange membrane adsorbers, a form of frontal chromatography, as the final purification step in the production of mAbs (monoclonal antibodies) produced from CHO (Chinese-hamster ovary) cells. Preferential binding of impurities over antibody product was exploited using commercially available cation- and anion-exchange membranes. Three different antibody feedstreams previously purified over Protein A and ion-exchange column chromatography were tested. Feedstream conductivity and pH were adjusted to induce product and impurity adsorption. Membranes were then overloaded in a normal flow mode, resulting in retention of impurities and breakthrough of purified antibody. Although some amount of the product also binds to the membranes (usually ≤30 g mAb/l membrane), yields of ≥99% were achieved by marginalizing the losses, typically by loading more than 3 kg mAb/l membrane. Analyses of the purified pools show consistent removal of impurities despite strong mAb–ligand interactions and high membrane loadings. The clearance of host cell proteins was affected by pH and conductivity, but was unaffected by flow rate, membrane properties or scale. The importance of the present study lies in our demonstration of an alternative use of ion-exchange membranes for fast, effective and high yielding purification of mAbs.
Keywords: competitive adsorption, displacement chromatography, flow-through chromatography, ion-exchange membrane, monoclonal antibody (mAb), overload chromatography
Abbreviations: CEX, cation exchange; CHO, Chinese-hamster ovary; CHOP, CHO protein; HRP, horseradish peroxidase; LOQ, limit of quantification; mAb, monoclonal antibody; MV, membrane volume; PPM, parts per million; SEC, size-exclusion chromatography
Introduction
The advantages of membrane chromatography over traditional column-based separations are well established. Column chromatography methods are robust and reliable but generally have low throughput due to pore diffusion limitations within the resin beads. Membranes have shorter diffusion times and therefore separation efficiencies can be maintained at high flow rates [1–5]. Membranes are also more convenient because they do not require column hardware or packing [6], they reduce buffer usage and floor space requirements and they generally improve manufacturing flexibility [7].
Despite having many advantages, membrane chromatography did not acquire the success anticipated almost two decades ago. The inability of membrane chromatography to gain industrial acceptance has been attributed to the reticence among users in applying new technologies [8]. Technical, operational and regulatory implications of new technologies and the investment in existing equipment have also been described as chief barriers to change [9]. Additionally, membranes are expensive and their binding capacities for mAbs (monoclonal antibodies) are relatively low compared with modern resins. Consequently, their breadth of use is limited because they are not a good medium for performing industrial-scale elution chromatography (bind and elute chromatography) [10].
However, the views on the use of membranes for mAb downstream processing may be changing as the biopharmaceutical industry has been evolving. Zhou and Tressel [11] reviewed the findings of multiple researchers who have shown that anion-exchange membranes operated in flow-through mode are effective downstream of Protein A purification. Membranes are ideal in this ‘polishing’ position, because they have a distinct flow rate advantage and sufficient capacity within a relatively small footprint for binding trace impurities and contaminants [6,12,13]. Additionally, advancements in process knowledge and improved Protein A resins are raising the acceptance level for membrane usage by decreasing the amount of impurities passed downstream, and there is also an increasingly tangible need for high-throughput technologies that can handle bigger batches. Bioreactor titres for mAbs are increasing [9] and batches greater than 100 kg may be difficult to purify using traditional column chromatography [14]. Membrane usage is likely to increase in the future as drug producers seek greater speed and efficiency and effective cost of goods.
One application of membranes that has received little attention from mAb purification researchers is frontal chromatography [15], where the mobile phase conditions (load conditions) promote both impurity and product adsorption. For clarity, henceforth we call this technique overload chromatography, so as not to be confused with another form of frontal chromatography where the product does not bind, called flow-through chromatography. These terms are informal and are defined here for discussion purposes only.
Overload chromatography exploits the differential binding between the product and impurities. In the examples studied in the present paper, the impurities appear to bind stronger than the product to ion-exchange membranes, adsorbing tightly while the product desorbs and flows into the membrane effluent. Because some antibody stays bound to the membranes, the economic viability of the step hinges on the collection of a disproportionately large amount of the product in the membrane effluent.
The inherent yield loss associated with this approach may be one of the reasons why it has not been seriously studied by mAb purification researchers until now. Historically, flow-through chromatography has been preferred for its high yield and generally straightforward operation, and these benefits were believed to end at the operating limits where the product begins to bind. However, this is not always the case, and theoretically, operating under conditions where the product and impurities compete may be advantageous.
In the present study, we investigate overload chromatography for the unique circumstances encountered in mAb downstream processing. The platform use of Protein A followed by ion-exchange column chromatography produces a relatively consistent set of conditions that includes large-volume pools with only trace impurities. Such conditions are ideal for using membranes as the third and final polishing step, whether it be in flow-through or overload mode. However, it is only the overload mode that is uniquely advantageous to membranes, as the large pool volumes and potential for appreciable yield loss discourage the use of traditional packed resin beds. A more detailed explanation for this is provided in the Results and discussion section.
Applying overload chromatography to the purification of Genentech mAb feedstreams, we observed that impurities that are commonly present downstream of Protein A bind to ion-exchange membranes despite the presence of strong mAb–ligand interactions. Our evidence suggests that it is possible to leverage this phenomenon to purify antibodies, and we believe that it can be an effective and potentially economical means of purification.
In the present study, overload chromatography on ion-exchange membrane adsorbers was examined as the final polishing step for mAb purification. Three different feedstreams produced at Genentech, previously processed over Protein A and ion-exchange column chromatography, were tested using commercially available cation- and anion-exchange membranes. Our analysis begins with mAb 1 where we demonstrate proof of concept. It then expands to include mAbs 2 and 3, where we investigate the impact of pH and conductivity on cation- and anion-exchange membranes. We then return to mAb 1 and use it as a model (i) to explore the differences between cation-exchange membranes produced by two commercial vendors, (ii) to evaluate the effect of flow rate and the impact of scale and (iii) to study the mechanism of impurity removal.
Materials and methods
Feedstock
Feedstocks were selected from industrial, pilot or small-scale cell culture batches at Genentech (South San Francisco, CA, U.S.A.), and each was partially purified, meaning that the cells were separated and the clarified fluid was purified over Protein A followed by ion-exchange chromatography (Table 1). Multiple batches of mAb 1 were used, resulting in small differences in CHOP [CHO (Chinese-hamster ovary) protein] levels. These differences are likely a result of assay variability and possibly lot differences. The Protein A pool was used to investigate the mechanism of impurity clearance. Table 1 shows feedstock characteristics for each mAb used in the present study.
Table 1. Summary of feedstream characteristics.
| Product | Upstream process | Nomenclature | pH | Conductivity (mS/cm) | Concentration (g/l) | IgG type | pIa |
|---|---|---|---|---|---|---|---|
| mAb 1 | Protein A followed by flow-through anion exchangeb | Anion exchange pool | 5.5 | 6.0 | 4.8 | 1 | 8.9 |
| Protein Ab | Protein A pool | 5.5 | 4.4 | 5.9 | 1 | ||
| mAb 2 | Protein A followed by flow-through anion exchange | Anion-exchange pool | 8.0 | 5.0 | 5.4 | 1 | 9.3 |
| mAb 3 | Protein A followed by bind and elute cation exchange | Cation-exchange pool | 5.5 | 9.0 | 4.1 | 1 | 7.7 |
aCalculated pI based on the amino acid sequence.
bPool pH and conductivity have been previously adjusted to ensure adequate product stability.
mAb quantification
The concentration of antibody was determined via absorbance (A) at 280 and 320 nm using a UV–visible spectrophotometer (8453 model G1103A; Agilent Technologies; Santa Clara, CA, U.S.A.) or NanoDrop 1000 model ND-1000 (Thermo Fisher Scientific; Waltham, MA, U.S.A.). Species other than antibody (i.e. impurities) were too low in concentration to have an appreciable effect on UV absorbance. As needed, samples were diluted with an appropriate non-interfering diluent in the range of 0.1–1.0 absorbance unit. Sample preparation and UV measurements were performed in duplicate and the average value was recorded. The mAb absorption coefficients ranged from 1.45 to 1.70 mg−1·ml−1·cm−1.
CHO host cell protein (CHOP) quantification
An ELISA was used to quantify the levels of the host cell protein called CHOP. Anti-CHOP antibodies were immobilized on microtitre plate wells. Dilutions of the samples containing CHOP, standards and controls were incubated in the wells, followed by incubation with anti-CHOP antibodies conjugated with HRP (horseradish peroxidase). The HRP enzymatic activity was detected with o-phenylenediamine, and the CHOP was quantified by reading absorbance at 490 nm in a microtitre plate reader. Based on the principles of sandwich ELISA, the concentration of peroxidase corresponded to the CHOP concentration. The assay range for the ELISA was typically 10–320 ng/ml with intra-assay variability ≤ 10%. CHOP values were reported in units of ng/ml. Alternatively, they were divided by the mAb concentration and the results were reported in units of PPM (parts per million; ng of CHOP/mg of mAb). The CHOP ELISA is a generic assay capable of quantifying total CHOP levels but not the concentration of individual proteins.
Filtrate samples exhibiting CHOP levels below the LOQ (limit of quantification) were concentrated in an effort to obtain quantifiable results. Samples were concentrated approx. 10-fold using an Amicon® Ultra-15 centrifugal 10 kDa NMWCO (nominal molecular-mass cut-off) filter produced by Millipore (Billerica, MA, U.S.A.) centrifuged on an Eppendorf 5810R centrifuge (Eppendorf, Hamburg, Germany) at 5–25°C and 3000–4700 g for 10–20 min.
Gentamicin quantification
Gentamicin levels were determined using a competition ELISA. A polyclonal antibody directed to gentamicin and a second synthesized form of gentamicin was immobilized on microtitre plate wells. Gentamicin competes with the synthesized form for binding to the antibody. The amount of bound synthesized gentamicin was detected using HRP–streptavidin and o-phenylenediamine dihydrochloride substrate. Gentamicin was detected by reading the absorbance at 490 nm in a microtitre plate reader. The assay range for the ELISA was typically 3–90 ng/ml. Gentamicin values were reported in units of ng/ml. Alternatively, they were divided by the mAb concentration and the results are reported in units of PPM (ng of gentamicin/mg of mAb).
SEC (size-exclusion chromatography)
A TSK G3000SWXL SEC column (diameter=7.8 mm, height=300 mm; part number 08541) manufactured by Tosoh Bioscience (Tokyo, Japan) was operated at ambient temperature (approx. 25°C) on a 1200 series HPLC instrument (Agilent Technologies) and used to determine the relative levels of mAb monomer for the collected samples. Each sample was diluted to approx. 0.5 g/l antibody using a mobile phase containing a 200 mM potassium phosphate/250 mM potassium chloride buffer at pH 6.2. Runs were 30 min with a 0.5 ml/min flow rate and 50 μl injections. If protein concentrations were near 0.5 g/l in the initial samples, no dilution was performed prior to operation. Additionally, if the initial concentration was ≤0.25 g/l, then a 100 μl injection was used to try to normalize for the mass loaded on to the column. UV 280 nm absorbance was recorded and peaks were analysed manually using ChemStation software (Agilent Technologies).
Membranes
Membranes Mustang® S and Q and Sartobind® S were purchased from Pall Corporation (East Hills, NY, U.S.A.) and Sartorius-Stedim (Aubagne, France) respectively. MV (membrane volume) is the total physical volume of the membrane (solids and voids) and is reported in units of millilitres or litres. Table 2 lists the relevant information for each membrane used in the present study.
Table 2. Summary of membrane characteristics.
| Membrane | Device | Part number | Membrane thickness (cm) | Layers number | MV (ml) | Pore diameter (μm) |
|---|---|---|---|---|---|---|
| Mustang® S | 25 mm Acrodisc® | MSTG25S6 | 0.01375 | 6 | 0.18 | 0.8 |
| Mustang® S | Capsule | CLM05MSTGSP1 | 0.01375 | 16 | 10 | 0.8 |
| Sartobind® S | 25 mm MA5 | S5F | 0.0275 | 1 | 0.14 | 3–5 |
| Mustang® Q | Coin | MSTG18Q16 | 0.01375 | 16 | 0.35 | 0.8 |
Filtration systems
Small- and pilot-scale tests were performed using an AKTA Explorer 100 or AKTA Pilot (GE Healthcare, Fairfield, CT, U.S.A.). Small-scale tests were also performed using a manual system consisting of a Masterflex® L/S® digital economy drive peristaltic pump (Cole Parmer, Vernon Hills, IL, U.S.A.), in-line DTX™ Plus TNF-R pressure sensor (Becton Dickinson, Franklin Lakes, NJ, U.S.A.) and an AND EK-1200i balance (A&D, Tokyo, Japan). The balance was used to monitor the flow rate of the pump by measuring the mass accumulation. Mass was converted to volume assuming a feedstream density of 1.0 g/ml. Pressure from the in-line transducers and mass from the balance were continuously monitored using a NetDAQ™ 2640A/41A network data acquisition system (Fluke, Everett, WA, U.S.A.), which was linked to a computer running the software Trendlink™ version 3.1.1 (Canary Labs, Martinsburg, PA, U.S.A.) and RsCom version 2.40 (A&D).
Experimental
Feedstocks were removed from cold storage (2–8°C or ≤–70°C) and allowed to equilibrate to room temperature (approx. 22°C). Subsequently, they were pH and/or conductivity adjusted as necessary from the conditions shown in Table 1 using a titrating agent (1.5 M Tris base or 1 M citric acid) or diluent (purified water or 5 M sodium chloride). To minimize adsorber fouling, all feedstocks were 0.2 μm filtered as a precautionary measure using a Millipak-20 (Millipore), AcroPak™ 20 (Pall Corporation) or 1000 ml vacuum filter (Thermo Fisher Scientific, Rochester, NY, U.S.A.).
The filtration system was rinsed with purified water or a buffer (typically 20 mM acetate, pH 5.5) and then the membrane was placed in-line and flushed with 50–500 MV of purified water or equilibration buffer (20 mM acetate, pH 5.5). Feed was directed to the membrane at a constant flow rate of 333–2667 MV/h until the target amount of antibody was loaded and then the membrane was washed with a buffer to remove any unbound species. The wash buffer was selected to maintain retention of the mAb and impurities and was thus similar in pH as the feed but was lower in conductivity than the feed. For testing the effects of scale, the wash and equilibration buffers were purified water and the membrane adsorber was eluted into a high-salt buffer (20 mM acetate and 350 mM sodium chloride, pH 5.5, or 25 mM Tris and 250 mM sodium chloride, pH 8.0) at a similar flow rate to the load and wash phases.
Results and discussion
Overloading membranes in mAb downstream processing
For mAb purification, a unique aspect of overload chromatography is that it does not lend itself to being efficiently performed on traditional packed resin beds. This is because of the conditions typically encountered downstream of Protein A. Most commercial mAb processes start with Protein A and are followed by ion-exchange column chromatography, resulting in large pools with trace impurities. To further purify a pool like this using an overloaded column, the ideal dimensions would be very thin and wide to accommodate short process times (high flow rates) with minimal binding capacity required. This configuration is impractical due to a variety of flow distribution and column packing limitations. Flow distribution issues require operating a smaller diameter column with increased bed height. Again, in overload mode, the column is operated under conditions where the mAb can bind to the resin; thus increasing the bed height will reduce the yield and increase the flow resistance. To overcome these obstacles requires operating small columns at longer process times (low flow rates). By comparison, membranes offer a unique opportunity. A small membrane is capable of avoiding the flow distribution and resistance issues encountered with a column, while allowing for ample removal of trace impurities, minimal loss of product and short process times.
Before proceeding, it should be noted that overload chromatography has been studied for many years on columns [16–19], and it is also worth noting that if the nature of the multilateral binding were reversed, causing antibody to bind tighter than impurities, then packed bed resins would be favoured over membranes because of their superior binding capacity and lower costs.
Proof of concept
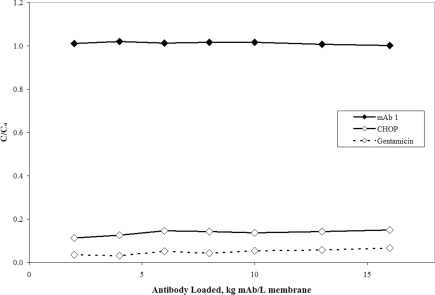
Figure 1 shows the antibody, CHOP and gentamicin breakthrough curves for mAb 1 anion-exchange pool on a small-scale Mustang® S membrane at pH 5.5 and at a conductivity of 6.0 mS/cm. As shown in Table 1, this feedstock was previously processed over Protein A followed by flow-through anion-exchange column chromatography. In the load conditions described, mAb 1 with a pI of 8.9 is positively charged and readily binds to the negatively charged membrane along with CHOP and gentamicin. Loading beyond the point of mAb breakthrough (where C/Co is approx. 1) revealed greater than 80% of the CHOP and 90% of the gentamicin bound to the membrane, as evidenced by C/Co values substantially less than 1. The respective levels of CHOP and gentamicin were reduced from 38 and 7 PPM in the feed to 5.7 and 0.5 PPM by the end of the experiment at approx. 16 kg mAb/l membrane. The results of this experiment show that trace levels of CHOP and gentamicin can bind to a cation-exchange membrane in the presence of a relatively high concentration of positively charged mAb.
Figure 1. Breakthrough curves for mAb 1, CHOP and gentamicin over cation-exchange membrane Mustang® S.
C/Co is the ratio of CHOP, gentamicin or mAb in the membrane effluent to that in the feed. The mAb 1 (pI 8.9) anion-exchange pool at pH 5.5 and 6.0 mS/cm was loaded on to 16 kg mAb/l membrane (2.2 kg mAb/m2 membrane) over a small-scale Acrodisc® membrane (six-layer device, 0.18 ml MV) at 667 MV/h (55 cm/h). The feed concentrations for mAb, CHOP and gentamicin were 4.8 mg/ml, 181 ng/ml (38 PPM) and 34 ng/ml (7.1 PPM) respectively.
Although not depicted in Figure 1, our experience with IgG adsorption on Mustang® S has shown a binding capacity upper limit of approx. 30 g mAb/l membrane, which when loaded to 16 kg mAb/l membrane translates to a minimum theoretical yield of approx. 99.8%. The yield for this experiment was measured at approx. 100% after loading only 5 kg mAb/l membrane. These findings are consistent with theory and illustrate how product losses can be marginalized by extensive overloading. For some readers, it may be desirable to establish a minimum membrane loading based on an acceptable low limit for yield. We believe the binding capacity is a good starting point for this analysis, as it appears to be consistent between small and pilot scales; however, it should be noted that it is likely to be dependent on the specific antibody, the type and brand of ion-exchange membrane and the solution pH and conductivity. Therefore minimum loadings should be established using experimentally determined binding capacities.
Although the mechanism of adsorption has not been elucidated, a likely explanation for the fractional binding in Figure 1 is that the heterogeneous CHOP population has subpopulations that bind to the membrane tightly, and other subpopulations with Limited to no binding. This feedstream was processed through Protein A and anion-exchange flow-through column chromatography and therefore the remaining CHOP would tend to be more basic and thus prone to strong binding on a CEX (cation exchange) membrane in the conditions tested. However, given the heterogeneity of the CHOP, it would be difficult to remove all in a single step or even multiple steps, because each operating condition results in different binding strengths. Therefore one might always expect some degree of multilateral binding, breakthrough and leaking profiles depending on the operating conditions. Gentamicin also contains a heterogeneous population of components [20–22], but far less so than CHOP, and at the pH tested, all isomers should be positively charged and thus bind to the membrane. Additional research is necessary in order to better explain the observed behaviour.
Impact of pH and conductivity
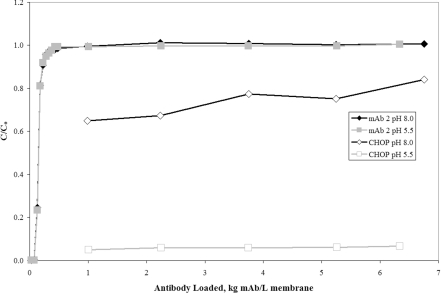
The mAb 2 source material at pH 8.0 and 5.0 mS/cm (Table 1) was split into two separate pools; the first pool was not adjusted and the second was titrated to pH 5.5 and 6.4 mS/cm using 1 M citric acid. Figure 2 shows the antibody and CHOP breakthrough curves for both pools processed over a small-scale Mustang® S cation-exchange membrane. For the two different pH conditions, similar yield (measured at >96%) and antibody breakthrough were observed. However, the CHOP breakthrough was very different, indicating pH dependence consistent with electrostatic adsorption. The difference in CHOP binding is possibly due to an increase in net charge caused by the change in pH from 8.0 to 5.5. Such results would be expected given the upstream process. Similar to mAb 1, the mAb 2 feedstream was previously processed through Protein A and anion-exchange flow-through column chromatography, which should have produced a more basic CHOP population that binds more tightly to a CEX membrane at low pH.
Figure 2. Breakthrough curves for CHOP and mAb 2 over the cation-exchange membrane Mustang® S at pH 8.0 and 5.5.
C/Co is the ratio of CHOP or mAb in the membrane effluent to that in the feed. The mAb 2 (pI 9.3) anion-exchange pool was split into two separate pools: the first pool was maintained at the initial conditions of pH 8.0 and 5.0 mS/cm and the second pool was pH adjusted to 5.5 and 6.4 mS/cm using 1 M citric acid. Both pools were loaded on to approx. 6.5 kg mAb/l membrane (0.89 kg mAb/m2 membrane) over a small-scale Acrodisc® cation-exchange membrane (six-layer device, 0.18 ml MV) at 667 MV/h (55 cm/h). The feed concentration of mAb 2 was 5.4 mg/ml and the concentration of CHOP was 314 and 274 ng/ml (58 and 51 PPM) at pH 8.0 and 5.5 respectively.
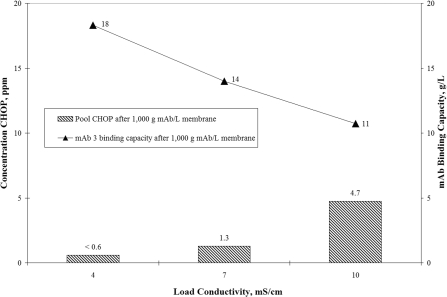
Conductivity dependencies on an anion-exchange membrane are shown in Figure 3. mAb 3 was selected because its lower pI (pI 7.7) facilitated operation at a pH above the pI without significant risk of product degradation. The primary y-axis shows the pool CHOP levels in PPM after loading mAb 3 cation-exchange pool to 1 kg mAb/l membrane over a small-scale Mustang® Q at pH 8.0 and conductivities 4, 7 and 10 mS/cm. Overlaid with these data on the secondary y-axis are the corresponding mAb 3 binding capacities determined after loading to 1 kg mAb/l membrane (>100% breakthrough). The results show an increase in pool CHOP from <0.6 PPM (<LOQ) at 4 mS/cm to 4.7 PPM at 10 mS/cm. At the same time, the mAb 3 binding capacity decreased by 42% from approx. 18 to 11 g mAb/l membrane. Binding of CHOP appears to decrease with increasing conductivity, which is consistent with previous observations made for anion-exchange membranes used in flow-through mode [7]. Despite the decrease in purification, the anion-exchange membrane does an impressive job, reducing CHOP at least 38-fold from the feedstream value of approx. 180 PPM. This reduction is significantly higher than for the previous studies using an anion-exchange flow-through pool run over a cation-exchange membrane (approx. 5- and 13-fold for mAb 1 and mAb 2 respectively). The exact reason for the enhanced separation is not known but it could be due to the nature of the feedstock and the upstream process (Table 1). It has been reported in the literature that around neutral pH and at low conductivity, a large percentage of CHO host cell proteins are negatively charged [11]. It would appear that many of these acidic species bound to the upstream cation-exchange column and were co-eluted with the antibody at pH 5.5.
Figure 3. Effect of conductivity on CHOP clearance and mAb 3 binding capacity for the anion-exchange membrane Mustang® Q.
CHOP concentration (shown by bars) is plotted on the primary y-axis and mAb 3 binding capacity (solid triangles) is plotted on the secondary y-axis. The mAb 3 (pI 7.3) cation-exchange pool at pH 5.5 and 9 mS/cm was pH adjusted to 8.0 using 1.5 M Tris base. The feedstock was then split into three separate pools and the conductivity was adjusted using purified water. Each pool was processed over a small-scale Coin anion-exchange membrane (16-layer device, 0.35 ml MV) at 600 MV/h (131 cm/h) to 1 kg mAb/l membrane (0.14 kg mAb/m2 membrane). The respective mAb and CHOP feed concentrations at 4, 7 and 10 mS/cm were 1.6, 3.0 and 4.2 mg/ml for mAb 3 and 280, 530 and 740 ng/ml (approx. 180 PPM) for CHOP. CHOP levels in the pool at 4 mS/cm were below the LOQ despite a greater than 10 times concentration on an Amicon® UF device. The LOQ of the CHOP assay was 10 ng/ml.
Reviewing the results from Figures 1–3, it is clear that this approach is not mAb dependent and can be performed reasonably well on both cation- and anion-exchange membranes. It is also clear that impurity and mAb binding are based on electrostatic adsorption and are therefore subject to the factors that affect charge interactions including pH and conductivity.
Brand comparison
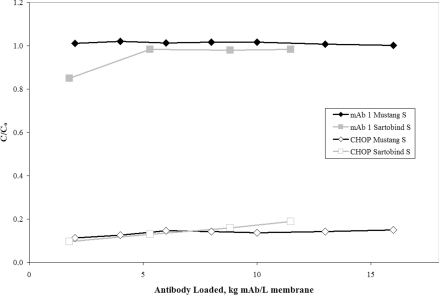
Figure 4 shows the mAb 1 and CHOP breakthrough curves for the Sartobind® S overlaid with the previous set of Mustang® S data shown in Figure 1. For the Sartobind® S, the batch of mAb 1 anion-exchange pool contained slightly lower levels of CHOP, but was processed over the small-scale membrane at the same pH and conductivity as the previous experiment (pH 5.5 and 6 mS/cm). The Sartobind® S reduced CHOP from 29 PPM in the feed to an initial value of 3.3 PPM at 1.8 kg mAb/l membrane, and by the end of the experiment at 11.5 kg mAb/l membrane, the levels increased slightly to 5.6 PPM. Although the CHOP appears to break through slightly faster for the Sartobind® S, we suspect this may be attributed to the fact that there is only a single membrane layer inside the MA5 device. Alternatively, it could also be explained by a difference in CHOP caused by feedstock variability. A repeat test comparing the devices on the same feedstock using a more representative, multi-layer Sartobind device is necessary before any significant distinctions can be made. At a minimum, the data demonstrate reasonable agreement in CHOP clearance between the Sartobind® and Mustang® S membranes. More importantly for biopharmaceutical manufacturers, this approach does not appear to be specific to a single membrane supplier.
Figure 4. Breakthrough curves for CHOP and mAb 1 over the cation-exchange membranes Mustang® S and Sartobind® S.
C/Co is the ratio of CHOP or mAb in the membrane effluent to that in the feed. The mAb 1 (pI 8.9) anion-exchange pool at pH 5.5 and 6.0 mS/cm was loaded on to approx. 11.5 kg mAb/l membrane (3.16 kg mAb/m2 membrane) over a small-scale Sartobind® S MA5 (one-layer device, 0.14 ml MV) at 857 MV/h (24 cm/h). The results are overlaid with the small-scale Mustang® S Acrodisc® data shown previously in Figure 1. The feed concentrations for mAb and CHOP were 4.8 mg/ml and 181 ng/ml (38 PPM) for the Mustang® S and 4.9 mg/ml and 143 ng/ml (29 PPM) for Sartobind® S.
Effect of flow rate
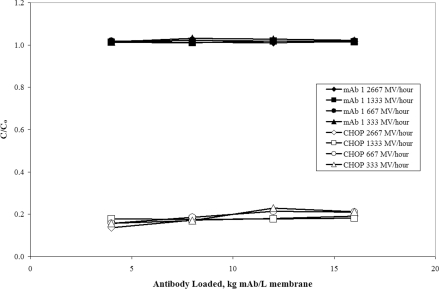
To test the effect of flow rate on CHOP breakthrough, the Mustang® S was studied over a broad range of conditions. Previous publications have shown that flow rate has little to no effect on membrane separations [2–5,10], but it was not immediately clear whether this would hold true in this case. To ascertain the effect of flow rate, mAb 1 anion-exchange pool at pH 5.5 and 6 mS/cm was processed over small-scale Mustang® S membranes at 333, 667, 1333 and 2667 MV/h (27, 55, 110 and 220 cm/h). The mAb 1 and CHOP breakthrough curves at each flow rate are overlaid in Figure 5. The Mustang® S initially reduced CHOP from 45 to approx. 6.9 PPM, increasing to an average of approx. 8.7 PPM by 16 kg mAb/l membrane. The mAb and CHOP breakthrough curves are similar, indicating that like other types of membrane separations, the performance of overload chromatography is independent of flow rate.
Figure 5. Breakthrough curves for CHOP and mAb 1 at flow rates 333, 667, 1333 and 2667 MV/h over cation-exchange membrane Mustang® S.
C/Co is the ratio of CHOP or mAb in the membrane effluent to that in the feed. The mAb 1 (pI 8.9) anion-exchange pool at pH 5.5 and 6.0 mS/cm was loaded on to 16 kg mAb/l membrane (2.2 kg mAb/m2 membrane) over small-scale Acrodisc® membranes (six-layer device, 0.18 ml MV) at flow rates ranging from 333 to 2667 MV/h (27–220 cm/h). The feed concentrations for mAb and CHOP were 4.7 mg/ml and 211 ng/ml (45 PPM) respectively.
Impact of scale
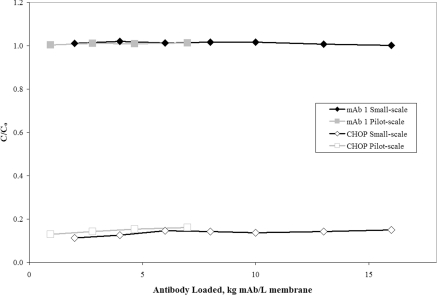
To determine the impact of scale, a larger Mustang® S membrane representing a 56-fold increase in scale was tested using mAb 1 and compared with previous results. The pilot-scale device was 10 ml and had 16 layers of membrane compared with 0.18 ml and 6 layers at small scale. A separate batch of mAb 1 anion-exchange pool at pH 5.5 and 6 mS/cm was used for the present study. After the load phase, the membrane was washed and eluted. Figure 6 shows the mAb 1 and CHOP breakthrough curves overlaid with previous small-scale Mustang® S data shown in Figure 1. The CHOP breakthrough at pilot scale shows excellent agreement with small scale. The 10 ml pilot-scale device reduced CHOP from 42 PPM in the feed to a final value of 4.9 PPM at 7 kg mAb/l membrane. These results are similar to small scale (Figure 1), where the Acrodisc® reduced CHOP from 38 PPM in the feed to 5.3 PPM at approx. 8 kg mAb/l membrane.
Figure 6. Effect of scale on CHOP and mAb 1 breakthrough for the cation-exchange membrane Mustang® S.
C/Co is the ratio of CHOP or mAb in the membrane effluent to that in the feed. The mAb 1 (pI 8.9) anion-exchange pool at pH 5.5 and 6.0 mS/cm was loaded to 7 kg mAb/l membrane over the pilot-scale Mustang® S capsule (16-layer device, 10 ml MV) at 555 MV/h (124 cm/h). The pilot-scale results are overlaid with the small-scale Mustang® S Acrodisc® data shown previously in Figure 1. The feed concentration of mAb 1 was 4.7 mg/ml and the concentration of CHOP was 194 ng/ml (41 PPM).
Analysis performed on the high-salt membrane elution showed a substantial enrichment of CHOP (results not shown), which supports the conclusions drawn from Figures 2 and 3 that impurities bind to the membrane due to electrostatic forces. Additionally, the yield was 98%, which closely matches the previous small-scale result of approx. 100%. We consider the pilot device as representative of industrial scale because the number of membrane layers, pleating and device assembly are similar to much larger cartridges and capsules that could be used for mAb production. Based on these findings, it appears that scaled-down flat sheet membrane devices are capable of predicting the performance of much larger pleated devices.
To reduce costs, it may be desirable to reuse the membranes. Our experience suggests Limited reuse may be possible, as the pilot-scale experiment shown in Figure 6 was repeated a second time on the same membrane with similar results obtained. However, we also have evidence that suggests that cleaning membranes loaded to high throughputs may be problematic, necessitating the use of aggressive regeneration solutions such as 6 M guanidinium chloride. These findings are preliminary and a more thorough evaluation is needed to determine the feasibility and value of membrane reuse.
Preliminary evidence for the mechanism of impurity removal
Although the mechanism of impurity removal is not known, our leading hypothesis is competitive binding. To test this hypothesis, an experiment was performed using mAb 1 Protein A pool at pH 5.5 and 4.4 mS/cm. In general, the approach used was similar to that employed by Veeraragavan et al. [18] in their analysis of overload chromatography with ovalbumin on a packed anion-exchange column.
Four small-scale Mustang® S membrane devices were loaded with mAb 1 to different levels (1, 5, 10 and 15 kg mAb/l membrane) and then chased with a buffer to a UV baseline of zero. The membranes were then eluted in a linear gradient from 0 to 100% buffered 2 M sodium chloride. Grab samples were collected during overloading and fractions were collected during the gradient and measured offline for total protein concentration, CHOP, gentamicin and SEC.
The Protein A pool was used as the feedstream because it contained a 10-fold higher CHOP concentration compared with the previously used anion-exchange pool. Using the Protein A pool introduced some uncertainty with respect to impurity binding because of the more diverse CHOP population, but the higher levels were viewed as advantageous because the feedstream was more aggressive and in theory could help to reduce the antibody loading and amplify any purification effects. Changes to antibody and gentamicin binding were not expected because of the similarities in concentration and solution conditions between the two pools.
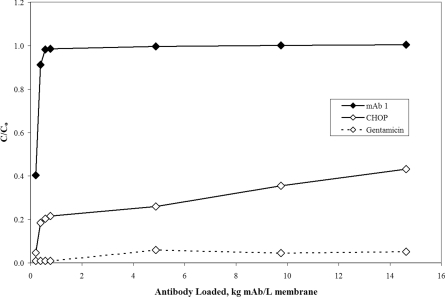
Figure 7 shows the breakthrough curves for antibody, CHOP and gentamicin taken from the highest mAb loading conditions (15 kg mAb/l membrane). These data reflect the grab samples taken from the membrane effluent during the overload phase of the experiment. The breakthrough of antibody and gentamicin is similar to results previously reported in Figure 1, while the breakthrough of CHOP shows signs of increased multilateral binding and leaking. These results are consistent with expectations and likely reflect increased levels of acidic CHOP. Again, the anion-exchange flow-through column is responsible for the removal of acidic species. Without the aid of this upstream step, the load would be expected to contain more host cell proteins that have trouble binding to a cation-exchange membrane in the conditions tested. Although the CHOP population is more diverse than the anion-exchange pool, fundamentally this should not be a problem for testing our hypothesis because most of the impurities bind to the membrane. Overall, it appears that the Protein A pool is an acceptable, more aggressive model for testing our hypothesis.
Figure 7. mAb 1, CHOP and gentamicin breakthrough curves for Protein A pool.
C/Co is the ratio of mAb 1, CHOP or gentamicin in the membrane effluent to that in the feed. The mAb 1 (pI 8.9) Protein A pool at pH 5.5 and 4.4 mS/cm was loaded on to approx. 15 kg mAb/l membrane (2.1 kg mAb/m2 membrane) over a small-scale Mustang® S Acrodisc® membrane (six-layer device, 0.18 ml MV) at 1333 MV/h (110 cm/h). The feed concentrations for mAb, CHOP and gentamicin were 5.9 mg/ml, 2400 ng/ml (410 PPM) and 41 ng/ml (6.9 PPM) respectively.
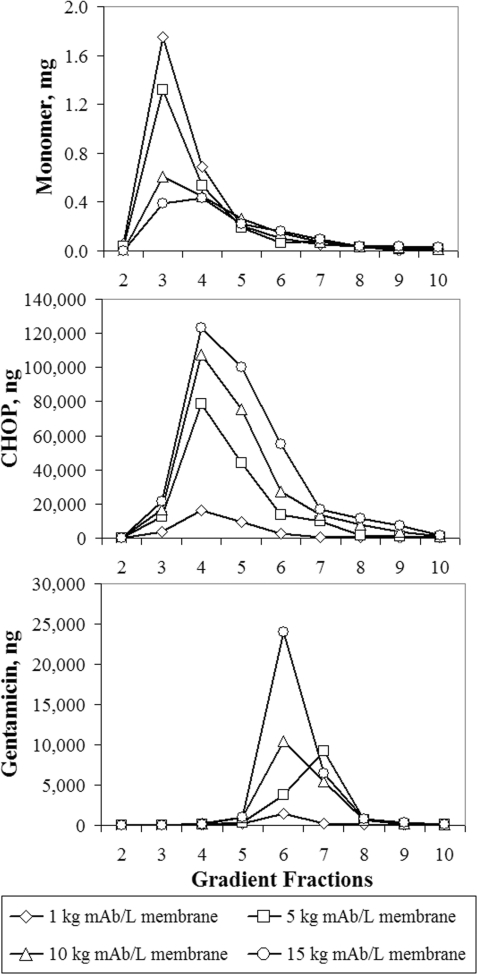
Figure 8 shows the mass of mAb monomer, CHOP and gentamicin in each of the gradient elution fractions. The relative position of the peak maxima shows that monomers elute first, followed by CHOP and then gentamicin. Because the gradient moves from low to high salt concentration, the position in the gradient elution provides some insight into the strength of binding. The results suggest that gentamicin binds stronger than CHOP, which binds stronger than monomers. Additionally, as mAb loading increased from 1 to 15 kg mAb/l membrane, the levels of monomer decreased, while CHOP and gentamicin increased. One possible explanation for this behaviour is that CHOP and gentamicin displace monomers on the membrane surface.
Figure 8. Mass of immobilized mAb 1 monomer, CHOP and gentamicin collected in each fraction during gradient elution of the Mustang® S membrane after loading 1, 5, 10 and 15 kg mAb/l membrane (0.14, 0.69, 1.4 and 2.1 kg mAb/m2 membrane).
The mAb 1 (pI 8.9) Protein A pool at pH 5.5 and 4.4 mS/cm was processed over small-scale Mustang® S Acrodisc® membranes (six-layer device, 0.18 ml MV) at 1333 MV/h (110 cm/h), washed using 20 mM acetate (pH 5.5) and then eluted in a linear gradient from 0 to 100% into 2 M NaCl and 20 mM acetate (pH 5.1) over 20 ml. Gradient fractions were collected every 2 ml and pooling was initiated based on volume. The first fraction was omitted because it contained only buffer. The feed concentrations for mAb, CHOP and gentamicin were 5.9 mg/ml, 2400 ng/ml (410 PPM) and 41 ng/ml (6.9 PPM) respectively.
For gentamicin, there are some published data that suggest that this species may have strong adsorbtive properties. Kundu et al. [23] showed that it is possible to displace large, tightly bound proteins from a cation-exchange column using the low-molecular-mass antibiotics neomycin B and streptomycin A. Like the displacers used by Kundu et al. [23], gentamicin is an aminoglycoside antibiotic [20,21] with a large net positive charge and thus would be expected to bind tightly to a cation-exchange membrane at the conditions tested.
A study of adsorption kinetics and generation of single- and multi-component adsorption isotherms is necessary to know for sure whether the observations are due to competitive binding.
Conclusions
Overloading ion-exchange membrane adsorbers, a form of frontal chromatography, was examined as the final step in the purification of mAbs. We call this technique overload chromatography. For three mAb feedstreams previously purified over Protein A and ion-exchange column chromatography, CHOP levels < 10 PPM and yields of usually >99% were achieved with commercially available membrane adsorbers Mustang® S and Q and Sartobind® S. Results show that impurity clearance is consistent with the factors that influence electrostatic adsorption. CHOP purification decreases with changes in pH and conductivity that reduce net charge and increase ionic shielding. A comparison between membrane brands shows similar performance between the Mustang® and Sartobind® membranes. Experimental results at flow rates ranging from 333 to 2667 MV/h were consistent with the theory and literature claims that membrane performance is independent of flow rate. Scale-up studies representing a 56-fold increase in MV confirmed that small, bench-top devices are capable of predicting impurity clearance for much larger pleated membranes.
The trace impurities found downstream of Protein A are difficult to remove and the preferred method of clearance is often a combination of column chromatography steps. Overload chromatography using membranes is significant because it provides an alternative approach for dealing with some of these problematic species. It also opens the door to further exploration for process streamlining, perhaps via the direct linking of column and membrane steps. Overload chromatography is an effective application of a purification technology that is uniquely advantageous to membranes, as it lends itself to the large volume pools typically encountered downstream of Protein A. Overall, the results show that this approach can be used as a rapid, high-yielding, final purification step for the production of mAbs.
Acknowledgements
We thank Ryan Renslow, Luke Barbara and Stefan Yohe for their significant contributions to this paper.
References
- 1.Gerstner J. A., Hamilton R., Cramer S. M. J. Chromatogr. A. 1992;596:173–180. [Google Scholar]
- 2.Reif O. W., Freitag R. J. Chromatogr. A. 1993;654:29–41. doi: 10.1016/0021-9673(93)83062-W. [DOI] [PubMed] [Google Scholar]
- 3.Tennikova T. B., Svec F. J. Chromatogr. A. 1993;646:279–288. [Google Scholar]
- 4.Kubota N., Konno Y., Miura S., Saito K., Sugita K., Watanabe K., Sugo T. Biotechnol. Prog. 1996;12:869–872. [Google Scholar]
- 5.Camperi S. A., Navarro del Cañizo A. A., Wolman F. J., Smolko E. E., Cascone O., Grasselli R. Biotechnol. Prog. 1999;15:500–505. doi: 10.1021/bp990054g. [DOI] [PubMed] [Google Scholar]
- 6.Boi C. J. Chromatogr. B. 2007;848:19–27. doi: 10.1016/j.jchromb.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Phillips M., Cormier J., Ferrence J., Dowd C., Kiss R., Lutz H., Carter J. J. Chromatogr. A. 2005;1078:74–82. doi: 10.1016/j.chroma.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Charcosset C. Biotechnol. Adv. 2006;24:482–492. doi: 10.1016/j.biotechadv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Low D., O'Leary R., Pujar N. S. J. Chromatogr. B. 2007;848:48–63. doi: 10.1016/j.jchromb.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen H. L., Fahrner R. L., Xu Y., Norling L. A., Blank G. S. J. Chromatogr. A. 2001;907:145–154. doi: 10.1016/s0021-9673(00)01041-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J. X., Tressel T. Biotechnol. Prog. 2006;22:341–349. doi: 10.1021/bp050425v. [DOI] [PubMed] [Google Scholar]
- 12.van Reis R., Zydney A. Curr. Opin. Biotechnol. 2001;12:208–211. doi: 10.1016/s0958-1669(00)00201-9. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh R. J. Chromatogr. A. 2002;952:13–27. doi: 10.1016/s0021-9673(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 14.Thömmes J., Etzel M. Biotechnol. Prog. 2007;23:42–45. doi: 10.1021/bp0603661. [DOI] [PubMed] [Google Scholar]
- 15.McNaught A.D., Wilkinson A. IUPAC Compendium of Chemical Terminology. Oxford: Blackwell; 1997. [Google Scholar]
- 16.Mazza C., Kundu A., Cramer C. Biotechnol. Tech. 1998;12:137–141. [Google Scholar]
- 17.Graber M., Condoret J. S. J. Chromatogr. 1992;584:115–120. doi: 10.1016/0378-4347(92)80016-j. [DOI] [PubMed] [Google Scholar]
- 18.Veeraragavan K., Bernier A., Braendli E. J. Chromatogr. 1991;541:207–220. [Google Scholar]
- 19.Gallant S. R., Vunnum S., Cramer S. M. J. Chromatogr. A. 1996;725:295–314. [Google Scholar]
- 20.Lee S. B., Ryu D. D. Y. Biotechnol. Bioeng. 1979;21:2045–2059. doi: 10.1002/bit.260211212. [DOI] [PubMed] [Google Scholar]
- 21.Seidl G., Nerad H. P. Chromatographia. 1988;25:169–171. [Google Scholar]
- 22.McDonald P., Victa C., Carter-Franklin J. N., Fahrner R. Biotechnol. Bioeng. 2009;102:1141–1151. doi: 10.1002/bit.22127. [DOI] [PubMed] [Google Scholar]
- 23.Kundu A., Vunnum S., Cramer S. M. J. Chromatogr. A. 1995;707:57–67. [Google Scholar]