Abstract
Under normal conditions, iron is taken up by the cells through the transferrin-mediated pathway. However, in hereditary hemochromatosis, a common iron-overloading disorder associated with mutations in the HFE gene, iron in plasma exceeds transferrin-binding capacity, and non–transferrin-bound iron (NTBI) appears in the circulation of patients with iron overload. NTBI can be taken up by hepatocytes through a transferrin-independent pathway. Lipocalin 2 (Lcn2), a secreted protein of the lipocalin family, has emerged as the mediator of an alternative, transferrin-independent pathway for cellular iron delivery. To evaluate the importance of Lcn2 in the pathogenesis of hepatic iron loading in Hfe knockout mice, we generated HfeLcn2 double-deficient mice. Our studies revealed that deletion of Lcn2 in Hfe-knockout mice does not influence hepatic iron accumulation in Hfe−/− mice, or their response to iron loading, as the phenotype of HfeLcn2−/− mice remained indistinguishable from that of Hfe−/− mice.
Conclusion
Lcn2 is not essential for iron delivery to hepatocytes in hemochromatosis.
Under normal conditions, the major pathway for cellular iron uptake is through internalization of the complex of iron-bound transferrin and the transferrin receptor. Thereafter, iron is released from transferrin as the result of acidic pH in endosomes and is transported to the cytosol by divalent metal ion transporter 1 (also known as SLC11A2).1,2 However, there is convincing evidence that, in situations of disrupted iron homeostasis, iron can also be delivered to cells by alternative, transferrin-independent mechanisms. For example, mice and humans lacking transferrin, while anemic, develop iron overload in the liver, suggesting that iron is being delivered to hepatocytes by transferrin-independent pathways.3,4 Likewise, in HFE-related hereditary hemochromatosis (HH), a common disorder of iron homeostasis characterized by excessive iron deposition principally in the liver,5,6 iron overload exceeds the iron-binding capacity of serum transferrin,7,8 and this non–transferrin-bound iron (NTBI) is cleared from the circulation and deposited into hepatocytes.9 This alternate iron uptake pathway is further brought into evidence by studies in Dmt1−/− knockout mice.10 In fact, despite the presence of a disrupted transferrin cycle, Dmt1−/− mice have abnormally high iron liver stores. Moreover, iron loading with iron dextran leads to significant accumulation of iron in Dmt1−/− hepatocytes, further indicating that the liver has alternate mechanisms than the transferrin cycle for iron uptake.10 The identification of the components of NTBI, however, remains elusive.
Lipocalin 2 (Lcn2), also called neutrophil gelatinase-associated lipocalin, or 24p3,11 has been proposed to be a mediator of the transferrin-independent iron delivery pathway12 after it was found that Lcn2 can bind bacterial ferric siderophores,13 and that Lcn2–siderophore–iron complexes can transport iron into cells during kidney development.14
Lcn2 has been found to exert a broad range of biological activities, which seems to depend on whether Lcn2 is bound to iron-laden siderophores or not, implicating iron-loaded Lcn2-siderophore as an iron donor and, conversely, iron-free Lcn2-siderophore as an iron chelator.15,16 As such, iron-loaded Lcn2-siderophore (Holo-Lcn2) has been shown to be required for mesenchymal–epithelial transition of embryonic kidney14 and oncogene Ras-transformed epithelial cells,17 as well as for kidney protection from renal failure,18,19 and delivery of ferric ion to mouse spermatozoa.20 In contrast, the Lcn2–siderophore complex without iron21 and Lcn2 (Apo-Lcn2)22 chelates iron from cells and, through iron deprivation, can induce apoptosis of pro-B cells22 and inhibit bacterial growth13,23,24 and erythropoiesis.25,26 Additional evidence that Lcn2 may assist cellular iron trafficking is provided by the demonstration that iron delivered through Lcn2 has been shown to regulate iron-sensitive genes21 and the identification of one of its receptors as being megalin.27 Megalin is also known to bind another iron-binding protein, namely lactoferrin.28,29 Thus, Lcn2 has emerged as a possible candidate involved in NTBI uptake under iron overload conditions and, as such, is possibly implicated in the pathophysiology of HH.12, 30
In this study, we investigated whether Lcn2, as a component of an alternative iron delivery system, may contribute to the pathophysiology of HH. For this purpose, we generated and then characterized iron metabolism in HfeLcn2 double mutant mice.
Materials and Methods
Animals
All procedures were performed in accordance with Canadian Council on Animal Care guidelines and approved by our institution’s Animal Care Committee. Control, wild-type mice were C57BL/6 female mice purchased from Charles River Laboratories, Inc. (Wilmington, MA). Hfe−/− mice were kindly provided by Dr. Nancy C. Andrews, Howard Hughes Medical Institute and Harvard Medical School, Children’s Hospital (Boston, MA)31 in the 129/SvEvTac background and were back-crossed onto the C57BL/6 (B6) background for 10 generations.32 Lcn2−/− mice generated in the C57BL/6 (B6) background have been described.23 Compound mutants (HfeLcn2−/−) were obtained by interbreeding Hfe−/− and Lcn2−/− mice and were genotyped via polymerase chain reaction (PCR) (Supplementary Fig. 1A,B). The following primers were used: Hfe, 5′-AGTTGG-GAGTGGTGTCCGA-3′, 5′-TGGCTACAGTGTGAG-AGGC-3′, and 5′CTAGCTTCGGCCGTGACG-3′; Lcn2, 5′-CCTCAAGGACGACAACATCA-3′, 5′-ACCCATT-CAGTTGTCAATGC-3′, 5′-TTGGGT- GGAGAGGC-TATTC-3′, and 5′-AGGTGAGATGACAGGAGATC-3′. All mice used in the experiments were genotyped via PCR assay performed on DNA prepared from mouse tails. Absence of Lcn2 expression in Lcn2−/− and HfeLcn2−/− mice was confirmed via western blotting using a monoclonal antimouse Lcn2/neutrophil gelatinase-associated lipocalin antibody (R&D Systems, Inc., Minneapolis, MN) (Supplementary Fig. 1C).
Diets
Mice were given a commercial diet (Harlan Teklad, Madison, WI) or, when indicated, an iron-supplemented diet containing 2.5% (wt/wt) carbonyl iron (Sigma Immunochemicals, St. Louis, MO).
Measurement of Serum Iron, Transferrin Saturation, and Tissue Iron Concentration
Serum iron, total iron-binding capacity, and transferrin saturation were assessed via colorimetric assay.33 Iron levels in the liver, spleen, heart, and kidneys were measured via acid digestion of tissue samples followed by iron quantification via atomic absorption spectroscopy.33
Quantitative Reverse-Transcription PCR
Total RNA was isolated with Trizol reagent (Invitrogen, Burlington, Ontario, Canada), and reverse-transcription was performed with the Thermoscript RT-PCR system (Invitrogen). Hepcidin 1 (Hamp1) and β-actin messenger RNA levels were measured via real-time PCR using a Rotor Gene 3000™ Real Time DNA Detection System (Montreal Biotech, Inc., Kirkland, Quebec, Canada) with the QuantiTect SYBRGreen I PCR kit (Qiagen, Mississauga, Ontario, Canada) as described,33 and expression levels of hepcidin were normalized to the housekeeping gene β-actin. The primers employed were as follows: β-actin, 5′-TGTTACCAACTGGGACGACA-3′ and 5′-GGTGTTGAAGGTCTCAAA-3′; Hamp 1, 5′AGAGC-TGCAGCCTTTGCAC3′ and 5′GAAGATGCAGATG-GGGAAGT3′.
Statistical Analysis
All statistics were calculated using SigmaStat 3.1 (Systat Software, Richmond, CA). All values are expressed as the mean ± standard deviation. Multiple comparisons were evaluated statistically via one-way analysis of variance, followed by the Bonferroni multiple comparison test.
Results
Hfe−/− mice develop iron overload characterized by high circulating iron levels and deposition of excess iron in the liver, but with resistance to iron loading in the spleen due to deficient iron storage in macrophages.31 To determine whether Lcn2 participates in iron delivery to the liver in Hfe-deficient mice, we interbred Hfe−/− and Lcn2−/− mice. Circulating iron levels, assessed by measuring serum iron and transferrin saturation as well as iron deposition in the liver and spleen, were analyzed at 10 and 20 weeks of age (Fig. 1). Iron parameters in Lcn2 single knockout mice were similar to those in wild-type mice (B6), whereas Hfe−/− and HfeLcn2 double mutants had higher amounts of circulating and liver iron than B6 mice (≈1.5- to 2-fold higher [P < 0.05]) (Fig. 1A–C). In contrast, spleen iron content, though slightly increased with age, was lower in 10-week-old Hfe−/− and HfeLcn2−/− mice compared with B6 (31% lower [P < 0.05]) or Lcn2−/− mice (Fig. 1D). No significant differences were found in heart iron (Fig. 1E), whereas iron levels in kidneys were slightly increased in both Hfe−/− and HfeLcn2−/− mice compared with B6 mice (Fig. 1F). Thus, we found no significant differences between Hfe−/− and HfeLcn2−/− mice regarding iron parameters. These results indicate that iron accumulation in the livers of Hfe−/− mice did not improve in the absence of Lcn2, suggesting that Lcn2-mediated cellular iron delivery is not essential in iron uptake by the liver in Hfe−/− mice.
Fig. 1.
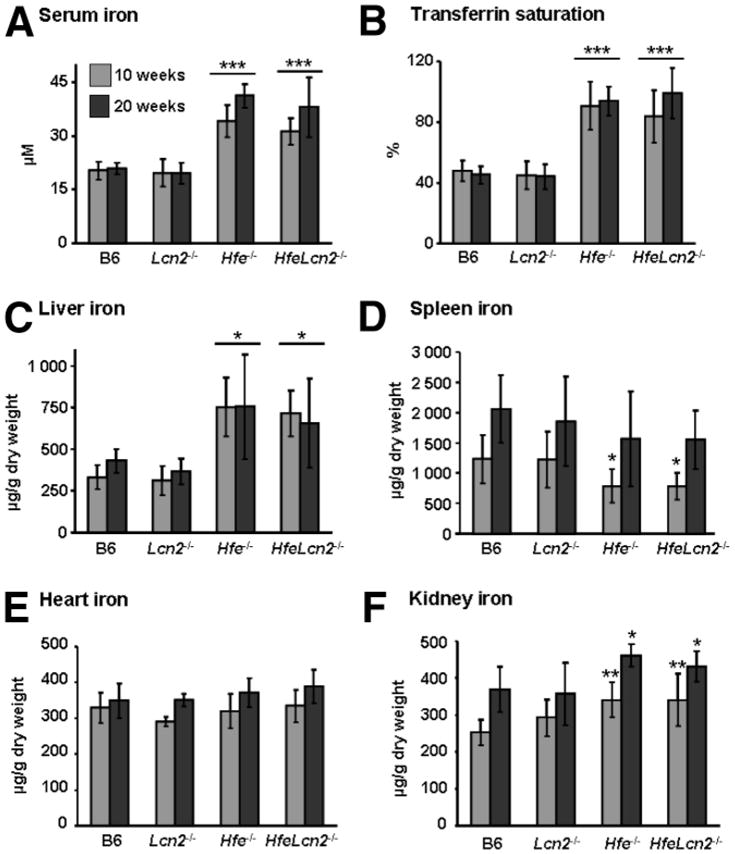
Iron parameters in 10- and 20-week-old wild-type (B6) and mutant mice. (A) Serum iron. (B) Transferrin saturation. (C–F) Iron concentration in the (C) liver, (D) spleen, (E) heart, and (F) kidney. The results are expressed as the mean ± standard deviation (n = 6 to 8 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 (mutant mice versus B6 mice of the same age).
To further investigate the responses to dietary iron loading in compound mutants, we challenged the mice with a 2.5% wt/wt carbonyl iron-supplemented diet for 2 weeks (Fig. 2). B6 and Lcn2−/− mice fed the iron-enriched diet showed significant increments of serum iron, transferrin saturation (>50% increase [P < 0.01]), hepatic iron content (>4-fold rise [P < 0.001]) and spleen iron content (>2.5-fold elevation [P < 0.001]) compared with mice on the control diet (Fig. 2A–C). Whereas Hfe−/− and HfeLcn2−/− double knockout mice also manifested heightened liver iron content over the already-elevated levels observed on the control diet in response to dietary iron loading (>2-fold rise; P < 0.001), the increase in iron loading of the spleen was considerably more modest than what was seen in B6 (P < 0.01) or Lcn2−/− mice on the iron-supplemented diet (Fig. 2D).
Fig. 2.
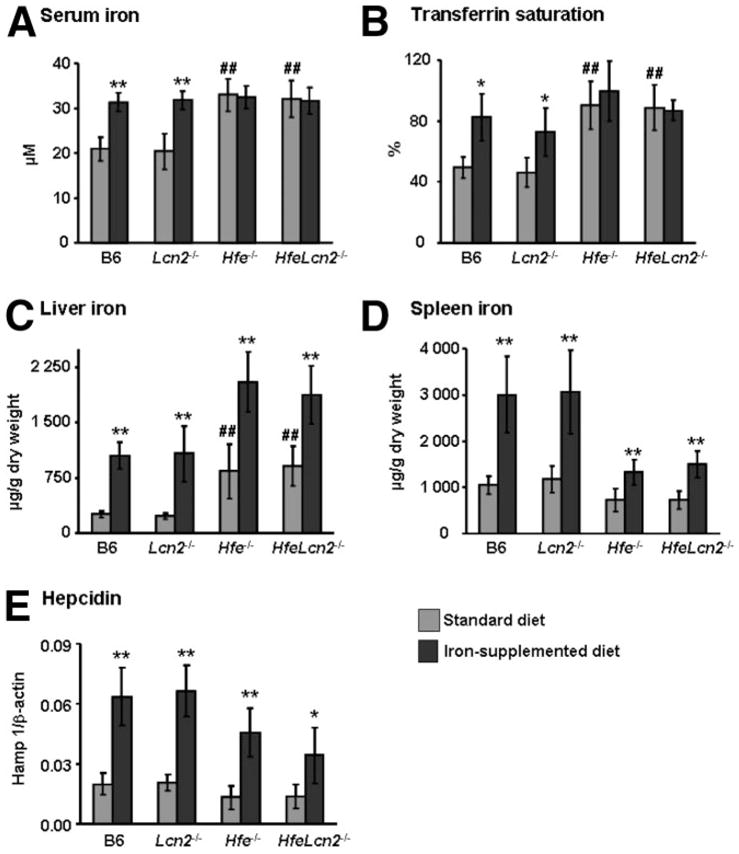
Response to iron-loading in wild-type (B6) and mutant mice. (A) Serum iron. (B) Transferrin saturation. (C) Iron concentration in the liver. (D) Iron concentration in the spleen. (E) Hepatic hepcidin 1 messenger RNA expression (Hamp 1) in mice on a standard diet (gray bars) and mice on a diet supplemented with 2.5% carbonyl iron for 2 weeks (black bars). The results are expressed as the mean ± standard deviation (n = 6 to 8 mice per group). ##P < 0.001 (mutant mice versus B6 mice). *P < 0.01, **P < 0.001 (iron-supplemented versus standard diet).
Because augmented iron absorption in Hfe−/− mice has been related to inappropriate expression levels of hepcidin,34 the principal regulator of systemic iron homeostasis, we also measured hepatic hepcidin 1 messenger RNA. As illustrated in Fig. 2E, we found lower amounts of hepcidin in both Hfe−/− and HfeLcn2 double mutants than in B6 and Lcn2−/− mice on the control diet (≈30% decrease). Hepcidin levels rose approximately 2.5- to 3.5-fold in response to iron loading in all mouse strains (P < 0.01) but remained significantly lower in Hfe−/− and HfeLcn2−/− mice compared with B6 (P < 0.05) or Lcn2−/− mice. Because the response to iron loading was indistinguishable between Hfe−/− and HfeLcn2−/− mice, these results further confirm that Lcn2 is not essential for iron delivery to the liver and the regulation of hepcidin in HH.
Discussion
Although under normal conditions hepatocytes acquire iron mostly through the transferrin-receptor pathway, the existence of uptake of NTBI in HH is now well established. Several candidates have been proposed, including Lcn2, but clear demonstration of its participation and importance in HH has not been yet provided.
We set out to determine whether Lcn2 represents a physiologically relevant mechanism of iron uptake by the liver in HH, an iron-overloading disease. For this purpose, we generated and characterized iron metabolism in HfeLcn2 double knockout mice. The Hfe−/− mice have increased plasma NTBI levels and, importantly, hepatocytes from Hfe−/− mice have been shown to uptake significantly more NTBI than control mice.8 We found that basal iron status and iron metabolism changes induced by oral iron supplementation were indistinguishable between Hfe single knockout and HfeLcn2 double knockout mice. Our results indicate that Lcn2 is dispensable for NTBI uptake by hepatocytes in HH. However, they do not exclude that the Lcn2-mediated iron delivery pathway may be involved in other pathologies.
The identification of the components of NTBI delivery pathways remains important for the understanding of the patophysiology of HH and other iron overload diseases such as hypotransferrinemia and thalassemia, because in these situations plasma iron exceeds the binding capacity of transferrin and uptake of NTBI significantly contributes to iron accumulation in the liver, pancreas, and heart.35
NTBI uptake mechanisms have been described in a variety of cell lines,36,37 including hepatocytes.38 In hepatocytes, this system requires the reduction of Fe3+ to Fe2+.38 Iron salts (such as iron ascorbate, citrate, and nitrilotriacetate) have been suggested as candidates for low molecular-weight NTBI carriers, but the transporters of NTBI have not yet been identified. In addition to Lcn2, which the present study suggests is redundant, potential candidates include L-type voltage-dependent Ca2+ channels (LVDCCs)39 and Zip14.40
LVDCCs have been identified as key transporters of iron into cardiomyocytes and neuronal cells under iron overload conditions.39, 41 Further support for a role for cardiac LVDCCs in myocardial NTBI uptake under conditions of iron overload comes from the demonstration that LVDCC blockers are protective and able to attenuate myocardial iron accumulation in iron-overloaded mice.42
Zip14 is a zinc transporter and member of the SLC39A metal ion transporter family43 that is highly expressed in hepatocytes. Recent studies have shown that mouse Zip14 transports both iron and zinc in cultured hepatocytes.40
In conclusion, the work presented here shows that Lcn2 is dispensable for iron delivery to hepatocytes in the context of Hfe deficiency. Further studies will be necessary to establish whether other candidates—namely LVDCCs and Zip14 —are involved in the uptake of NTBI by hepatocytes under iron overload conditions such as HH.
Acknowledgments
We thank Ovid Da Silva for editorial assistance.
Supported by grants from the Canadian Institutes of Health Research (MOP44045) and the Natural Sciences and Engineering Research Council of Canada (298518-06). M. M. S. is the recipient of a Research Scholarship–Junior 2 award from the Fonds de la Recherche en Santé du Québec.
Abbreviations
- HH
hereditary hemochromatosis
- Lcn2
lipocalin 2
- LVDCC
L-type voltage-dependent Ca2+ channel
- NTBI
non–transferrin-bound iron
- PCR
polymerase chain reaction
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 2.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci U S A. 1987;84:3457–3461. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheth S, Brittenham GM. Genetic disorders affecting proteins of iron metabolism: clinical implications. Ann Rev Med. 2000;51:443–464. doi: 10.1146/annurev.med.51.1.443. [DOI] [PubMed] [Google Scholar]
- 5.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 6.Pietrangelo A. Hereditary hemochromatosis—a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 7.Loreal O, Gosriwatana I, Guyader D, Porter J, Brissot P, Hider RC. Determination of non-transferrin-bound iron in genetic hemochromatosis using a new HPLC-based method. J Hepatol. 2000;32:727–733. doi: 10.1016/s0168-8278(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 8.Chua AC, Olynyk JK, Leedman PJ, Trinder D. Nontransferrin-bound iron uptake by hepatocytes is increased in the Hfe knockout mouse model of hereditary hemochromatosis. Blood. 2004;104:1519–1525. doi: 10.1182/blood-2003-11-3872. [DOI] [PubMed] [Google Scholar]
- 9.Brissot P, Wright TL, Ma WL, Weisiger RA. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985;76:1463–1470. doi: 10.1172/JCI112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunshin H, Fujiwara Y, Custodio AO, DiRenzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 12.Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–606. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- 13.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 16.Devarajan P. Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle. Cancer Ther. 2007;5:463–470. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanai J, Mammoto T, Seth P, Mori K, Karumanchi SA, Barasch J, et al. Lipocalin 2 diminishes invasiveness and metastasis of Ras-transformed cells. J Biol Chem. 2005;280:13641–13647. doi: 10.1074/jbc.M413047200. [DOI] [PubMed] [Google Scholar]
- 18.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 19.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elangovan N, Lee Y-C, Tzeng W-F, Chu S-T. Delivery of ferric ion to mouse spermatozoa is mediated by lipocalin internalization. Biochem Biophys Res Commun. 2004;319:1096–1104. doi: 10.1016/j.bbrc.2004.05.091. [DOI] [PubMed] [Google Scholar]
- 21.Li J-Y, Ram G, Gast K, Chen X, Barasch K, Mori K, et al. Detection of intracellular iron by its regulatory effect. Am J Physiol Cell Physiol. 2004;287:C1547–C1559. doi: 10.1152/ajpcell.00260.2004. [DOI] [PubMed] [Google Scholar]
- 22.Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 23.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 24.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2008;215:526–537. doi: 10.1002/jcp.21334. [DOI] [PubMed] [Google Scholar]
- 26.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005;19:1881–1883. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- 27.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Willnow TE, Goldstein JL, Orth K, Brown MS, Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem. 1992;267:26172–26180. [PubMed] [Google Scholar]
- 29.Meilinger M, Haumer M, Szakmary KA, Steinböck F, Scheiber B, Goldenberg H, et al. Removal of lactoferrin from plasma is mediated by binding to low density lipoprotein receptor-related protein/α2-macroglobulin receptor and transport to endosomes. FEBS Lett. 1995;360:70–74. doi: 10.1016/0014-5793(95)00082-k. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 31.Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11. [PubMed] [Google Scholar]
- 32.Constante M, Jiang W, Wang D, Raymond V-A, Bilodeau M, Santos MM. Distinct requirements for Hfe in basal and induced hepcidin levels in iron overload and inflammation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G229–G237. doi: 10.1152/ajpgi.00092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makui H, Soares RJ, Jiang W, Constante M, Santos MM. Contribution of Hfe expression in macrophages to the regulation of hepatic hepcidin levels and iron loading. Blood. 2005;106:2189–2195. doi: 10.1182/blood-2005-02-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, et al. Decreased liver hepcidin expression in the hfe knockout mouse. Blood Cells Mol Dis. 2002;29:361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 35.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23:185–192. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan J, Jordan I, Sturrock A. Regulation of the transferrin-independent iron transport system in cultured cells. J Biol Chem. 1991;266:2997–3004. [PubMed] [Google Scholar]
- 37.Inman RS, Wessling-Resnick M. Characterization of transferrin-independent iron transport in K562 cells. Unique properties provide evidence for multiple pathways of iron uptake. J Biol Chem. 1993;268:8521–8528. [PubMed] [Google Scholar]
- 38.Randell EW, Parkes JG, Olivieri NF, Templeton DM. Uptake of non-transferrin-bound iron by both reductive and nonreductive processes is modulated by intracellular iron. J Biol Chem. 1994;269:16046–16053. [PubMed] [Google Scholar]
- 39.Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-Type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 40.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaasch J, Geldenhuys W, Lockman P, Allen D, Van der Schyf C. Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem Res. 2007;32:1686–1693. doi: 10.1007/s11064-007-9313-1. [DOI] [PubMed] [Google Scholar]
- 42.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 43.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Letters. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]


