Abstract
Objective
The second-generation antipsychotics are associated with metabolic abnormalities in patients with schizophrenia. Elderly patients with Alzheimer’s disease are frequently treated with these antipsychotics but there is little data available on their metabolic effects.
Methods
We assessed 186 male and 235 female Alzheimer’s disease outpatients from the Clinical Antipsychotic Trials of Intervention Effectiveness–Alzheimer’s Disease (CATIE-AD) for changes in weight, waist circumference, blood pressure, fasting glucose, and lipids in relation to duration of second-generation antipsychotics (i.e., olanzapine, quetiapine, and risperidone) use throughout the 36-week trial, using logistic regression and mixed-effects models.
Results
Females showed significant weight gain of 0.14 lb per week of use (p = 0.006) while change was nonsignificant in males. The odds ratios of significant weight gain (i.e., ≥ 7% of body weight) compared to patients who did not use antipsychotics were 1.56 (95% CI 0.53 to 4.58), 2.89 (95% CI 0.97 to 8.64), and 3.38 (95% CI 1.24 to 9.23) among patients with antipsychotics use ≤ 12 weeks, > 12 to 24 weeks, and > 24 weeks during the trial, respectively. Olanzapine and quetiapine treatments were significantly associated with weight gain (0.12 and 0.14 pounds/week, respectively). In addition, olanzapine was significantly associated with decreases in HDL cholesterol (−0.19mG/dL/week), and increased girth (0.07 inches/week) relative to the placebo group. No treatment effects were noted for changes in blood pressure, glucose, and triglycerides.
Conclusion
Second-generation antipsychotics use was associated with weight gain in females, with olanzapine and quetiapine in particular, and with unfavorable change in HDL cholesterol and girth with olanzapine. The potential consequences of these effects suggest that patients with Alzheimer’s disease treated with second-generation antipsychotics should be monitored closely.
Introduction
Neuropsychiatric symptoms are highly prevalent among patients with Alzheimer’s disease 1 and second-generation antipsychotics have been frequently used for their treatment. 2 second-generation antipsychotics, however, especially olanzapine, are associated with metabolic abnormalities, including weight gain, increased risk for diabetes mellitus, and worsening lipid profile in patients with schizophrenia. 3-6
The data regarding adverse metabolic effects of second-generation antipsychotics has been in younger or middle-aged adults and patients with Alzheimer’s disease have not been systematically assessed. The several randomized, placebo-controlled trials of second-generation antipsychotics in Alzheimer’s disease and dementia patients, mainly residing in nursing homes, have not assessed potential metabolic changes and they were not outcomes of interest. 7, 8 Considering that epidemiology studies indicate that weight, diabetes mellitus, and dyslipidemia are risk factors for both cardiovascular disease and Alzheimer’s disease, 9, 10 and the established relationships of second-generation antipsychotics use with cerebrovascular adverse events 11 and deaths 12 in patients with dementia, it is important to know whether antopsychotic treatment worsens cardiometabolic risks in patients with Alzheimer’s disease.
The Clinical Antipsychotic Trials of Intervention Effectiveness–Alzheimer’s Disease (CATIE-AD) employed a unique design, reflecting decisions made in clinical practice, to compare the effectiveness of three second-generation antipsychotics, olanzapine, quetiapine, and risperidone, and placebo over 36 weeks for treating delusions and aggression in outpatients with Alzheimer’s disease and provided well-controlled longitudinal data to generate hypotheses for future testing. 13 To assess metabolic effects of second-generation antipsychotics in these patients, we conducted a prospective analysis of CATIE-AD interventions.
Methods
CATIE-AD study design
CATIE-AD was a 42-site, multi-center, randomized, double-blind, placebo-controlled trial designed to examine the effectiveness of second-generation antipsychotics over the course of 36 weeks in treating psychosis and agitation occurring in outpatients with Alzheimer’s disease. 13 Briefly, four phases (phase 1, 2, 3, and open-choice phase) were designed to reflect clinical practice and decisions in prescribing second-generation antipsychotics among patients. In phase 1, 2, or 3, if a patient did not exhibit efficacy or tolerability at a time after 2 weeks of treatment, study physicians could move the patient to the subsequent phase to receive a study medication (not placebo) differing from his/her previous treatments.
In phase 1, a total of 421 eligible patients were randomized in a double-blind fashion to the olanzapine, quetiapine, risperidone, or placebo groups in a 2:2:2:3 ratio. After randomization, clinic visits occurred at weeks 2, 4, 8, 12, 24, and 36. Patients doing well on assigned treatment continued on phase 1 treatment throughout the trial. Patients who discontinued their phase 1 treatments were double-blindly randomized to one of the antipsychotics or the antidepressant citalopram in phase 2. Patients who discontinued from phase 2 and entered phase 3 received randomly assigned, open-label study medications that were not previously prescribed. A patient could also directly shift from any of the three phases to the open-choice phase to receive non-study medications of the study physician’s choice. The length of phases 1, 2, 3 and open-choice could range from 2-36, 2-34, 2-32, and 2-34 weeks, respectively. The primary study end point was the time until discontinuation of treatment for any reason in phase 1. 14 The Institutional Review Board of each site approved the study and all subjects or their legally authorized representatives provided written informed consent.
Current study
The current study assessed the effects of second-generation antipsychotics on metabolic factors by a prospective analysis of the CATIE-AD trial. A total of 421 subjects (186 men, 235 women) who had a baseline and at least one post-baseline measure of metabolic factors were included. Changes in weight and other metabolic measures from baseline to the last observation were outcomes in the analysis (see below). 3 The duration of each second-generation antipsychotic treatment and the total duration of any second-generation antipsychotic treatment during follow-up were the exposures of interest. Analyses were conducted regardless of phase and the order of treatments unless otherwise specified.
Clinical data collection
Participants’ age, gender, ethnicity, and years of education were obtained. Height, weight, blood pressure, waist, and hip circumference were measured at baseline and at each clinic visit during the trial. Body mass index (BMI, calculated as weight (kilograms)/height (meters2)) and waist-hip-ratio were derived from these data. Blood pressure was measured once in a seated position at each visit.
Laboratory measurements
Blood samples were obtained at baseline and at weeks 12, 24, and 36 during the trial (regardless of phases). Serum glucose assays and plasma total cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol assays were performed by Quintiles central laboratory using standard methods, and low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald equation. The time from the last meal to the blood draw varied among patients and we defined those blood samples obtained at least 8 hours since the last meal as fasting samples. 15
Duration of study medication use
The start and end dates of each second-generation antipsychotic and placebo treatment were recorded for every patient. The duration of olanzapine, quetiapine, risperidone, or placebo use was calculated as the time interval between the start and end dates regardless of phase. For example, if a patient reached phase 3 at the end of the trial then the patient had switched treatment twice after the initial randomized assignment. Thus, the patient could have three duration variables to reflect the length of exposure to each second-generation antipsychotic or placebo administered during the trial. The period during which a patient received placebo, citalopram, or open-choice antipsychotic medications other than the second-generation antipsychotics did not contribute to the duration calculations for antipsychotic use.
Non-study medication use
The use of non-study medications including antihypertensive, lipid-modifying, and antidiabetic medications was assessed at each visit and recorded on medication record forms that included dose, frequency and dates of use.
Statistical analysis
Analysis of change in metabolic measures from baseline to the last observation was performed for all subjects who had baseline and at least one follow-up metabolic measure. Separate general linear regression models were fitted for each metabolic factor including weight, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL cholesterol, fasting glucose, and fasting triglycerides. The change in each metabolic factor from baseline to last observation was the dependent variable. Cumulative duration of any second-generation antipsychotics use was the primary independent variable. The regression coefficient associated with duration represents the change in the metabolic factor per week of antipsychotic use. Differences in this duration regression coefficient by gender, age group (≥ 80 vs < 80 y), and baseline BMI (BMI < 25 kg/m2, 25 ≤ BMI < 30 kg/m2, BMI ≥ 30 kg/m2) were tested for significance by including duration × gender, duration × age group, and duration × baseline BMI interaction terms.
Logistic regression was used to examine whether longer cumulative duration of second-generation antipsychotics use increases the likelihood of having clinically significant weight gain, as commonly defined as at least 7% increase from baseline. The binary dependent variable was categorized percentage weight gain (< 7% and ≥ 7%). The independent variable was categorized cumulative duration of antipsychotic use (none, 1 day to 12 weeks, > 12 to 24 weeks, and > 24 to 36 weeks). Age and gender were included in the model as covariates.
With regard to the effects of individual antipsychotic treatment on metabolic measures, we evaluated weekly rate of change in metabolic measures by initial randomized groups (the olanzapine, quetiapine, risperidone, or placebo groups). All available follow-up visits in phase 1 were included for each subject in order to assess the effects of each individual medication treatment. Analyses of triglyceride and glucose were restricted to fasting samples and subjects whose glucose concentrations were less than 100 mg/dL with unknown fasting status. In a mixed-effects model, a metabolic factor at a given follow-up time was regressed on time since randomization (random effect), and the randomized group was modeled as a fixed effect. The main effect of treatment group examined the group difference of each metabolic measure at baseline, while the group × time interaction term tested whether the weekly rate of change in each metabolic measure differed across groups. Age and gender were included as covariates to control for potential confounding effects.
Study site was not used as a covariate because an unconditional means models for each metabolic factor 16 indicated that the changes in metabolic factors did not significantly cluster by sites. (The proportion of the total co-variance between sites ranged from 0.004 to 0.039 compared to the sum of between- and within-site covariance).
All analyses were conducted using Statistical Analysis System version 9.1 software (SAS Institute, Cary, NC). Statistical significance was accepted at two-sided p < 0.05.
Results
Baseline sample characteristics
A total of 421 patients with Alzheimer’s disease were randomized to four groups (olanzapine, quetiapine, risperidone, or placebo). We summarized the demographic, cognitive, and metabolic characteristics at randomization in Table 1. There were no significant differences in baseline characteristics among randomized groups except for mean systolic blood pressure (p = 0.02). The mean age at randomization was 77.9 years (range 51 to 103 years). Fifty-six percent were female, 21% were non-Caucasian, and 59% were married. On average, patients were overweight and had elevated SBP. Almost half of the subjects (46%) were using antihypertensive agents, 60% cholinesterase inhibitor or memantine anti-dementia medications, 24% cholesterol and triglyceride reducers, and 10% oral blood glucose lowering medications at randomization. Table 1 also includes the number of subjects reaching specific trial phases by weeks 12, 24, and 36.
Table 1. Baseline Patient Characteristics.
| Total sample (N = 421) |
Olanzapine (N = 100) |
Quetiapine (N = 94) |
Risperidone (N = 85) |
Placebo (N = 142) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Demographic | ||||||||||
| Age (years) | 77.9 | 7.5 | 78.8 | 7.3 | 77.3 | 8.7 | 78.4 | 7.1 | 77.3 | 7.1 |
| Education (years) | 12.3 | 3.4 | 11.8 | 3.6 | 12.6 | 3.3 | 12.8 | 3.1 | 12 | 3.3 |
| Cognitive | ||||||||||
| MMSE score | 15 | 5.8 | 15 | 5.4 | 14.9 | 6.1 | 15.7 | 6.1 | 14.7 | 5.8 |
| ADAS-cog score 1 | 34.6 | 13.3 | 34.6 | 12.7 | 36.1 | 13.6 | 31.1 | 13.6 | 35.7 | 13.2 |
| BPRS total score | 27.8 | 12.3 | 27 | 11.8 | 28 | 12.3 | 27.7 | 13.6 | 28.2 | 12 |
| Metabolic | ||||||||||
| Weight (pounds) | 150.3 | 31.4 | 148.9 | 36.6 | 153.3 | 30.5 | 151.7 | 31.4 | 148.3 | 28 |
| BMI (kg/m2) | 25.4 | 4.6 | 25.3 | 5.7 | 25.5 | 3.9 | 25.9 | 4.8 | 25.2 | 4 |
| Waist circumference (inches) |
35.6 | 5 | 35.3 | 5.6 | 36.3 | 4.8 | 35.9 | 5.4 | 35.2 | 4.6 |
| Systolic BP (mmHg) | 136.5 | 18.4 | 132.4 | 18.1 | 135.6 | 17 | 136.8 | 19.1 | 139.7 | 18.8 |
| Diastolic BP (mmHg) | 75.7 | 10.4 | 75 | 11.4 | 75.8 | 10.3 | 75.7 | 9.4 | 76.1 | 10.3 |
| HDL (mg/dL) | 53.4 | 15.7 | 52.3 | 15.8 | 52.9 | 14 | 55.6 | 18.3 | 53.3 | 15.1 |
| Triglyceride (mg/dL) 2 | 151.5 | 91.4 | 155.3 | 93.3 | 151.6 | 99.3 | 150.7 | 83.8 | 149.4 | 90.1 |
| Glucose (mg/dL) 2 | 89.2 | 19.2 | 88 | 9.3 | 88.4 | 20.5 | 89 | 10.2 | 90.4 | 25.7 |
| Demographic | N | % | N | % | N | % | N | % | N | % |
| Female | 235 | 56 | 55 | 55 | 50 | 53 | 49 | 58 | 81 | 57 |
| Race | ||||||||||
| White | 331 | 79 | 80 | 81 | 76 | 81 | 68 | 80 | 107 | 76 |
| Black | 75 | 18 | 14 | 14 | 15 | 16 | 15 | 18 | 31 | 22 |
| Other | 13 | 3 | 5 | 5 | 3 | 3 | 2 | 2 | 3 | 2 |
| Married | 249 | 59 | 63 | 63 | 56 | 60 | 49 | 58 | 81 | 57 |
| Number of subjects reaching specific trial period | ||||||||||
| Week 12 | ||||||||||
| Total 3 | 369 4 | 66 | 67 | 67 | 49 | |||||
| Phase 1 | 155 | 41 | 31 | 34 | 49 | |||||
| Week 24 | ||||||||||
| Total | 337 | 49 | 58 | 53 | 24 | |||||
| Phase 1 | 94 | 24 | 23 | 23 | 24 | |||||
| Week 36 | ||||||||||
| Total | 315 | 43 | 45 | 38 | 21 | |||||
| Phase 1 | 78 | 20 | 18 | 19 | 21 | |||||
Alzheimer’s Disease Assessment Scale-cognitive subscale score
Fasting triglyceride and glucose measures were available among 286 patients with 64, 65, 56, and 101 in the olanzapine, quetiapine, risperidone, and placebo groups, respectively
Number of patients reaching trial follow-up regardless of phase
Including patients on olanzapine, quetiapine, risperidone, placebo, citalopram, or open-choice medications
Cumulative duration of total second-generation antipsychotic use
During the trial, 349 (83%) subjects received second-generation antipsychotics for at least one day. Seventy-two subjects (17%) out of a total sample of 421 had no exposure to second-generation antipsychotics because they received placebo, citalopram, or open-choice antipsychotic medications other than the study medications. The cumulative duration of antipsychotic use ranged from 0 to 46 weeks with a median of 12.1 weeks. Among subjects who received antipsychotics for at least one day (N = 349), 73 (21%), 63 (18%), and 61 (17%) subjects were on olanzapine, quetiapine, and risperidone alone, respectively. Forty-three (12%) subjects had olanzapine and quetiapine, 42 (12%) olanzapine and risperidone, 43 (12%) quetiapine and risperidone, and 24 (7%) had all three second-generation antipsychotics at some time during the trial follow-up.
Weight gain and duration of antipsychotic use
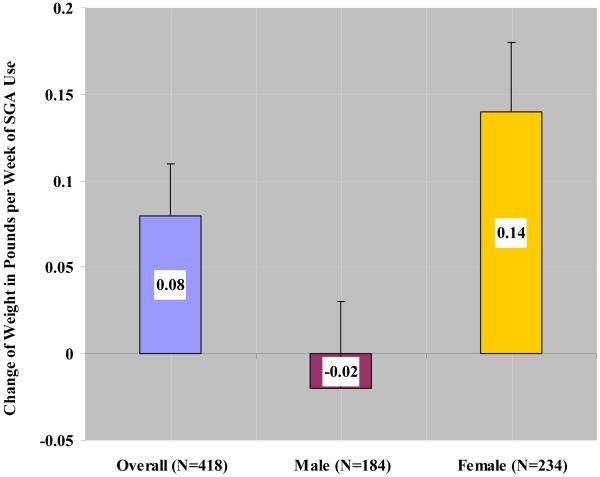
Among 418 subjects with weight measurements available at both randomization and end of trial, duration of antipsychotic use was statistically significantly associated with weight gain after adjustment for age (p = 0.02) (Figure 1). As the primary independent variable in the general linear regression model, 71 of 418 subjects had no exposure (cumulative duration of zero) to second generation antipsychotics. These subjects with no SGA exposure were included in the model as the reference group. The regression coefficient associated with duration represents the additional weight change per week of antipsychotic use beyond the weight change experienced in the unexposed group. A significant interaction between duration of antipsychotic use and gender was found (p = 0.008). In the overall sample, average weight gain was 0.08 pounds per week of antipsychotic use (SE = 0.03). Female subjects showed a statistically significant weight gain of 0.14 pounds per week of antipsychotic use (SE = 0.04, p = 0.006) while the change in weight in males was −0.02 pounds per week of antipsychotic use (SE = 0.04, p = 0.64). No significant interaction between duration of antipsychotic use and age group (≥ 80 vs < 80 yrs) was noted (p = 0.45).
Figure 1.
Age-adjusted weight change (in pounds, +/− one standard error) per week of cumulative second-generation antipsychotic (SGA) use beyond that experienced in the no SGA use group. Data are presented in the overall sample and stratified by gender (N = 418). An additional average weight gain of 0.08 (SE = 0.03) pounds per week of SGA use was observed in the overall sample (p = 0.02). There was a significant interaction between duration of SGA use and gender (p = 0.008).
Similarly, duration of antipsychotic use was associated with increase in BMI after adjustment for age (0.02 kg/m2 per week of antipsychotic use, p = 0.006), with a significant interaction between duration of antipsychotic use and gender (p = 0.005). Females showed a significant increase in BMI of 0.03 kg/m2 per week of antipsychotic use (p = 0.004) while males did not (−0.003 kg/m2 per week of antipsychotic use, p = 0.64).
To examine whether the association between weight gain and duration of antipsychotic use was influenced by baseline BMI, we tested the interaction term duration × baseline BMI. Because only 14 patients (5 men and 9 women) were underweight at baseline (i.e., BMI < 20 kg/m2) we created three categories of baseline BMI: under + normal weight (BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obese (BMI ≥ 30 kg/m2). There were 125, 69, 38 females, and 81, 75, and 24 males in each of the categories, respectively. No evidence of effect modification by baseline BMI was found (duration × baseline BMI, p = 0.93), nor when we further tested whether baseline BMI modifies the influence of gender on weight gain per week of antipsychotic use using a 3-way interaction term (duration × gender × baseline BMI) (p = 0.43)
Clinically significant weight gain and duration of second-generation antipsychotic use
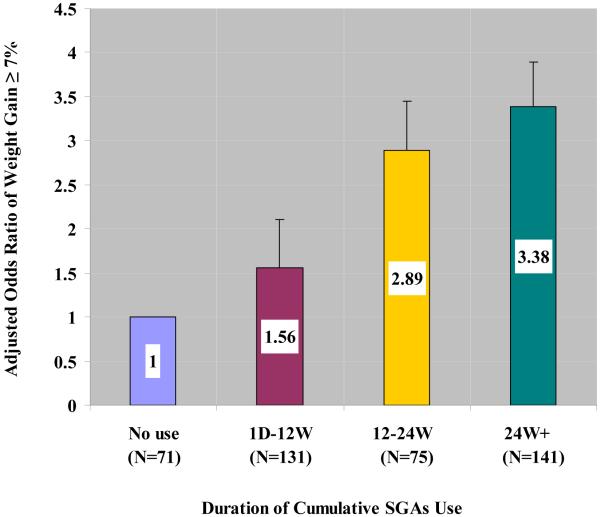
The duration-dependent association between antipsychotic use and clinically significant weight gain is presented in Figure 2. Compared to subjects with no use, subjects with at least 24 weeks of antipsychotic use were more likely to have clinically significant weight gain defined as at least 7% increase from baseline (age- and gender-adjusted odds ratio = 3.38, 95% CI 1.24 to 9.23, p = 0.02). Female gender had a marginally increased likelihood for significant weight gain than males (Odds Ratio = 1.79, 95%CI (0.998, 3.211), p = 0.051) with no significant interaction between duration of antipsychotic use and gender (p = 0.89).
Figure 2.
Odds ratio (+/− one standard error) of clinically significant weight gain (i.e., ≥ 7%) by cumulative duration of second-generation antipsychotics (SGA) use with adjustment for age at randomization and gender (N = 418). SGA use is associated with increased risk for clinically significant weight gain (≥ 7%) in a duration-dependent manner (p for trend = 0.03). Compared to patients with no SGA use, the odds ratios were: 1.56 (95% CI 0.53 to 4.58, p = 0.42) among patients with SGA use ≤ 12 weeks; 2.89 (95% CI 0.97 to 8.64, p = 0.06) among patients with SGA use between 12 to 24 weeks; 3.38 (95% CI 1.24 to 9.23, p = 0.02) among patients with SGA use more than 24 weeks during the trial. The prevalence of clinically significant weight gain was 7%, 10%, 17%, and 20% among patients with no SGA use, SGA use ≤ 12, 12-24, and > 24 weeks, respectively.
Second-generation antipsychotic treatment interruption
We modeled duration of any second-generation antipsychotic treatment as a continuous exposure in the above analysis. For patients who remained in phase 1 throughout the trial, or those who switched from one antipsychotic to another across phases, the duration variable reflects the treatment exposure without interruption. When a patient was randomly assigned to a second-generation antipsychotic in phase 1, then to citalopram in phase 2, and switched to another antipsychotic in phase 3, a gap between antipsychotic treatments occurred that might dilute the effects of treatment. Only 22 participants of a total of 83 randomized to citalopram in phase 2 experienced gaps between second-generation antipsychotic treatments, i.e. received one second-generation antipsychotic in phase 1, citalopram in phase 2, and another second-generation antipsychotic in phase 3. The mean (SD) duration of citalopram use among these 22 participants was 9.7 (6.0) weeks, ranging from 1.1 to 21.4 weeks. To rule out the effect of treatment interruption, we further tested the association between weight gain and duration of any second-generation antipsychotic excluding these 22 participants and the results were not altered.
Effects of individual second-generation antipsychotic treatment
The effects of each antipsychotic treatment on the weekly rate of changes in metabolic measures are summarized in Table 2. For each participant, all available follow-up visits in phase 1 were included in this analysis. Multivariate mixed effects models were fitted to evaluate the weekly rate of change of each metabolic measure in relation to the treatment groups with adjustment for age and gender. At randomization, none of the metabolic measures differed among groups. Longitudinally, when compared to the placebo group, significant average weekly weight gain was observed in the olanzapine and quetiapine groups (0.12 pounds/week, SE = 0.06, p = 0.03 and 0.14 pounds/week, SE = 0.06, p = 0.02), and was marginally significant in the risperidone group (0.10 pounds/week, SE = 0.06, p = 0.07). Similar patterns of BMI change were apparent among the three antipsychotic treatments. In addition, olanzapine treatment was significantly associated with increased waist circumference (0.07 inches/week, SE = 0.02, p = 0.004) and decreased HDL cholesterol (−0.19 mG/dL/week, SE = 0.07, p = 0.004). No effects of antipsychotic treatment were noted for changes in systolic and diastolic BP, glucose, and triglycerides.
Table 2. Change in Metabolic Measures per Week of Treatment by Randomized Group in Phase 1.
| Metabolic Measures | Randomized Group |
N 1 | Rate of Change 2 |
SE | P Value 3 |
|---|---|---|---|---|---|
| Weight (pounds) | Olanzapine | 99 | 0.12 | 0.06 | 0.032 |
| Quetiapine | 94 | 0.14 | 0.06 | 0.019 | |
| Risperidone | 85 | 0.10 | 0.06 | 0.07 | |
| BMI (kg/m2) | Olanzapine | 97 | 0.02 | 0.01 | 0.039 |
| Quetiapine | 92 | 0.03 | 0.01 | 0.014 | |
| Risperidone | 84 | 0.02 | 0.01 | 0.07 | |
|
Waist circumference
(inches) |
Olanzapine | 96 | 0.07 | 0.02 | 0.004 |
| Quetiapine | 92 | 0.01 | 0.03 | 0.61 | |
| Risperidone | 80 | 0.01 | 0.03 | 0.72 | |
| Systolic BP (mmHg) | Olanzapine | 99 | −0.01 | 0.12 | 0.95 |
| Quetiapine | 94 | 0.17 | 0.12 | 0.16 | |
| Risperidone | 85 | −0.11 | 0.12 | 0.36 | |
| Diastolic BP (mmHg) | Olanzapine | 99 | −0.05 | 0.07 | 0.43 |
| Quetiapine | 94 | 0.09 | 0.07 | 0.23 | |
| Risperidone | 85 | −0.13 | 0.07 | 0.06 | |
|
HDL cholesterol
(mg/dL) |
Olanzapine | 99 | −0.19 | 0.07 | 0.004 |
| Quetiapine | 93 | −0.09 | 0.07 | 0.19 | |
| Risperidone | 83 | 0.03 | 0.07 | 0.68 | |
| Triglyceride (mg/dL) | Olanzapine | 75 | 0.42 | 0.59 | 0.48 |
| Quetiapine | 73 | 0.55 | 0.63 | 0.38 | |
| Risperidone | 67 | 0.43 | 0.65 | 0.51 | |
| Glucose (mg/dL) | Olanzapine | 77 | 0.10 | 0.11 | 0.37 |
| Quetiapine | 72 | 0.12 | 0.12 | 0.32 | |
| Risperidone | 67 | 0.24 | 0.13 | 0.06 |
All available follow-up visits in phase 1 were included for each subject in the longitudinal data analysis. Analysis on triglyceride and glucose were performed among fasting samples and patients whose glucose < 100mg/dL with unknown fasting status
Adjusted for age at randomization and gender
P-value obtained from mixed effects model evaluating the weekly rate of change in each second-generation antipsychotic treatment group relative to the placebo group
Discussion
Clinically significant weight gain, increasing with duration of use, is associated with atypical antipsychotic treatment among outpatients with Alzheimer’s disease. Additionally, an increase in waist circumference and decrease in HDL cholesterol is associated with olanzapine. The relationship of the weight gain to duration of treatment and a gender interaction makes this observation particularly compelling.
The rate of weight gain was 0.10 - 0.14 pounds/week and the magnitude of the gain was 1.4, 1.7, and 1.2 pounds after 12 weeks for olanzapine, quetiapine, and risperidone, respectively. Weight gain in older people cannot be viewed as a positive event unless it is due to increased lean muscle mass which was unlikely to have occurred in this study that did not include a physical training intervention and observed increased waist girth. Visceral adiposity is associated with greater risks of insulin resistance, dyslipidemia, and glucose intolerance, and must be considered as an adverse effect rather than a benefit in these individuals.
Most data regarding adverse metabolic effects of second-generation antipsychotics has been in younger or middle-aged adults. The magnitude of the weight gain, increase in waist circumference, and change in HDL cholesterol reported in Alzheimer’s disease patients is similar to that reported from a large randomized trial in younger people with schizophrenia (mean age 41 years, 74% male, and mean BMI 30 kg/m2), 5, 6 although Alzheimer’s disease patients had substantially lower BMI, narrower girths, and higher HDL cholesterol levels at baseline.
In the few randomized, placebo-controlled trials in Alzheimer’s disease and other dementia patients, the associations between antipsychotic use and weight change were inconsistent or not adequately reported. Although olanzapine was associated with about a 1.0 kg increase and a higher incidence of significant weight gain compared to risperidone and placebo in one 10-week outpatient trial, 17 in a 26-week trial in outpatients with mild to moderate Alzheimer’s disease but without agitation or psychotic symptoms, a mean 1.4 kg increase in weight was not significantly greater than the placebo group. 18 Similarly, increased weight of about 1.0 kg was associated with olanzapine in one nursing home trial 19 but not in another. 20 Two 10-week long quetiapine trials in dementia patients in nursing homes did not report significant differences in weight change between the treatment and placebo groups, 21, 22 Three of four risperidone trials 7 did not address 23 or did not report changes in weight, BMI, or other metabolic measures. 24, 25 The one trial that stated there was a significant increase in weight with risperidone compared to placebo provided no details. 26 By comparison, we found significantly greater weight gain in olanzapine- and quetiapine-treated patients compared to placebo and a statistical trend for risperidone.
Females showed significant weight gain associated with antipsychotic treatment, while males did not. Females were older than males (mean age 78.6 (7.8) vs. 76.8 (6.9) years, respectively, p = 0.015) and had higher HDL cholesterol (58.5 (15.2) vs. 47.1 (14.2) mg/dL, p < 0.0001). At baseline, males and females were similar with regard to cognitive function, years of education, BMI, blood pressure, fasting glucose and triglyceride levels. Because elderly females proportionally have more fat mass and less lean body mass than elderly males, they may be more susceptible to second-generation antipsychotic-associated weight gain. The difference in weight changes between females and males observed here was not reported in other studies among Alzheimer’s disease patients or the elderly and warrants further examination.
Importantly, we observed an increasing difference in actual weight gain with continuing antipsychotic treatment over the 36-week trial. The prevalence of significant weight gain was 10%, 17%, and 20% among patients with less than 12 weeks, 12-24 weeks, and greater than 24 weeks of use compared to 7% with no use. This is particularly concerning given the association of weight gain with both cerebrovascular disease morbidity and mortality, both of which appear independently associated with antipsychotic use in dementia patients and are listed as warnings in the drugs’ labeling. Increased risk of second-generation antipsychotic-associated cerebrovascular adverse events is evident among patients with dementia. 11 We previously reported increased risks for death with second-generation antipsychotics compared to placebo treatment in 15 randomized, placebo-controlled trials. 12 Although some patients in the current trial may have benefited clinically from antipsychotic use in terms of particular symptom reduction (e.g., anger, aggression, and paranoid ideas), they did not benefit in performance of activities of daily living, care needs, or quality of life. 27 Considered together, the risks of adverse events associated with longer term use of second-generation antipsychotics must be carefully balanced against their benefits. 7, 8, 12, 14, 27
There are several limitations to the present study. First, the design of CATIE-AD did not allow for a parallel group comparison over 36 weeks of all patients randomized to their original (phase 1) treatment. The number of patients treated continuously with one antipsychotic over the entire trial was small (N = 78), representing persons who were responding to and tolerating their initially randomized medication or placebo. Secondly, incomplete laboratory data due to non-fasting blood samples and 12-week assessment intervals decreased the sample size and power to assess changes in fasting glucose and triglycerides. Thirdly, we were not able to address a potential dose effect due to the flexible dosing design. Average doses, however, in both phases 1 and 2 were from 5.5 to 5.6, 56.5 to 61.1, and 1.0 to 1.1 mg/day for olanzapine, quetiapine, and risperidone, respectively. 14 These doses were generally lower than other Alzheimer’s disease trials and considerably lower than doses used to treat schizophrenia or in the CATIE-Schizophrenia trial.5
In summary, about 20% of outpatients with Alzheimer’s disease developed significant weight gain increasing over 36 weeks with antipsychotic treatment. Patients treated with olanzapine in particular showed unfavorable changes in HDL cholesterol and abdominal girth. These are additional risks to consider when initiating and continuing antipsychotic therapy in individuals with Alzheimer’s disease. Clinical trials of the atypical antipsychotics have shown modest efficacy compared to placebo in improving behavioral symptoms but adverse effects limit their overall effectiveness. 14, 27 Alzheimer’s disease patients treated with second-generation antipsychotics should be monitored closely.
Acknowledgements and Disclosures
The CATIE–AD Study Group includes the following members:
Advisors and protocol committee — G. Alexopoulos, C.E. Davis, K.L. Davis, S. Davis, J.K. Hsiao, D.V. Jeste, I.R. Katz, K.R. Krishnan, G. Koch, B. Lebowitz, J.A. Lieberman, C.G. Lyketsos, D. Marin, B.G. Pollock, P.V. Rabins, R.A. Rosenheck, M. Sano, S. Schultz, G.W. Small, D. Sultzer; Trial center — K. Dagerman, M.S. Ismail, L.S. Schneider, P.N. Tariot;
NIMH staff — J. Baum, B. Bowers, D. Eskenazi, P. Gibbons, J. Gonzalez, W.R. Harlan, J.K. Hsiao, S. Hyman, T. Insel, B.D., Lebowitz, G.S. Norquist, J.T. Olin, L. Ritz, J.B. Severe;
University of North Carolina (UNC), Chapel Hill, and Duke University, Durham — J.A. Lieberman (formerly at UNC, currently at Columbia University) and C.E. Davis (principal investigators); R. Keefe (Duke University),
I. Rojas, S. Stroup (University of North Carolina at Chapel Hill), M. Swartz (Duke University);
Quintiles — S. Abbott, P.J. Butler, M. DiJohn, J. Ho, B. LaPlante, I. Amara, S. Davis, S. Kavanagh; VA New England MIRECC: Jennifer Cahill, Haiqun Lin PhD.
Study investigators — L. Adler, Clinical Insights, Baltimore; I. Ahmed, University of Hawaii, Honolulu; P. Aupperle, University of Medicine and Dentistry of New Jersey, Piscataway; S. Bartels, Dartmouth–Hitchcock Medical Center, Lebanon, NH; A. Baskys, Veterans Affairs Medical Center, Long Beach, CA; J. Beyer and M. Doraiswamy, Duke University Medical Center, Durham, NC; L. Blake, Northwestern University Medical School, Chicago; C.T. Cohen, SUNY Brooklyn, Brooklyn, NY; S. Colon, Veterans Affairs Medical Center, Tuscaloosa, AL; D. Devanand, Columbia University, New York; M. Dysken, Veterans Affairs Medical Center, Minneapolis; A. Freeman III, Louisiana State University Health Sciences Center, Shreveport; G.T. Grossberg, Saint Louis University, St. Louis; H. Grossman, Mount Sinai School of Medicine, New York; S. Gupta, Global Research and Consulting, Olean, NY; G. Haefner, Berman Research Group, Hialeah, FL; D. Jeste, University of California San Diego and Veterans Affairs Medical Center, San Diego; B. Jones and D. Johnston, Wake Forest University, Winston-Salem, NC; I. Katz and D. Weintraub, University of Pennsylvania and Veterans Affairs Medical Center, Coatesville; Z. Lebedeva and M. Parsa, Northeast Ohio Health Center, Columbus, OH; M.T. Levy, Staten Island University Hospital, Staten Island, NY; C.G. Lyketsos, Johns Hopkins University, Baltimore; D. McManus, Southern Illinois University, Springfield; J.E. Mintzer, Medical University of South Carolina, North Charleston; R. Ownby, University of Miami, Miami; E. Palmer, Lahey Clinic, Burlington, MA; E. Pfeiffer, University of South Florida Suncoast Gerontology Center, Tampa; N. Pomara, Nathan S. Kline Institute, Orangeburg, NY; S. Potkin, University of California Irvine, Irvine; J.M. Ryan, Monroe Community Hospital, Rochester, NY; C. Sadowsky, Palm Beach Neurology, West Palm Beach, FL; B. Saltz, Mental Health Advocates, Boca Raton, FL; S. Scheinthal, University of Medicine and Dentistry of New Jersey, Stratford; L.S. Schneider and S. Pawluczyk, University of Southern California, Los Angeles; S. Schultz, University of Iowa College of Medicine, Iowa City; J. Sheikh, Stanford University School of Medicine, Stanford, CA; F. Sicuro, Millennium Psychiatric Associates, St. Louis; P. Solomon, Southwestern Vermont Medical Center, Bennington, VT; D. Sultzer, Veterans Affairs Greater Los Angeles Healthcare System, University of California, Los Angeles, Los Angeles; L. Tune, Wesley Woods Health Center, Atlanta; C.H. van Dyck, Yale University School of Medicine, New Haven, CT; M. Weiner, University of Texas Southwestern Medical Center, Dallas.
Disclosures:
Dr. Zheng, Dr. Mack, Ms. Dagerman, Dr. Hsiao, Dr. Lebowitz, Dr. Mack and Dr.Vigen, report no competing interests.
Dr. Lyketsos has received research funding from Forest, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Eli Lilly, Ortho-McNeil, Bristol-Myers Squibb, Novartis, NIMH, the National Institute on Aging, and the Associated Jewish Federation of Baltimore, Weinberg Foundation; has received lecture honoraria or travel support from Pfizer, Forest, GlaxoSmithKline, and Health Monitor; and has served as consultant/adviser to AstraZeneca, GlaxoSmithKline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, and Merz.
Dr. Stroup has served as a consultant for Janssen, Lilly, AstraZeneca, and Solvay. He has received honoraria for speaking at events sponsored by Lilly and Lundbeck.
Dr. Sultzer has received research funding from Forest and Pfizer, has received lecture honoraria from Forest, and has served as a consultant to Eli Lilly and AstraZeneca.
Dr. Tariot has received consulting fees from AC Immune, AstraZeneca, Avid, Baxter Healthcare, Eisai, Epix, Forest, Memory, Myriad, Pfizer, and Sanofi-Aventis; research support from Elan, Mitsubishi Pharma, Neurochem, Ono, the National Institute on Aging, NIMH, the Alzheimer’s Association, the Arizona Department of Health Services, and the Institute for Mental Health Research; consulting fees and research support from Abbott, GlaxoSmithKline, Merck, Merz, Takeda, and Wyeth; and educational fees from Lundbeck; he is also a contributor to the patent “Biomarkers of Alzheimer’s Disease.”
Dr. Schneider has been a consultant for Pfizer, Eli Lilly, Johnson & Johnson, AstraZeneca, and Bristol-Myers Squibb, all manufacturers of antipsychotic medication, and to Forest Pharmaceuticals and Lundbeck, manufacturers of citalopram. He has provided expert testimony in litigation involving Eli Lilly and Lundbeck, manufacturers of drugs used in this study.
The content of this article does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
This research was supported by NIMH research grant N01 MH-9001. AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica (Johnson & Johnson), and Eli Lilly provided medications for this study.
Drs. Zheng, Mack, Vigen, and Schneider had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
A poster presentation of these results will be made in the “Hot Topics” session of the International Congress on Alzheimer’s Disease meeting, July 30, 2008, in Chicago.
Footnotes
ClinicalTrials.gov number: NCT00015548
Reference
- 1.Tariot PN, Mack JL, Patterson MB, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995 Sep;152(9):1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(Suppl 2):5–99. discussion 100-102; quiz 103-104. [PubMed] [Google Scholar]
- 3.Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004 Feb;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 4.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(Suppl 7):22–31. [PubMed] [Google Scholar]
- 5.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 6.Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008 Apr;101(1-3):273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006 Mar;14(3):191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 8.Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD003476.pub2. CD003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006 Sep;260(3):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer’s disease. Neurol Res. 2006 Sep;28(6):612–620. doi: 10.1179/016164106X130407. [DOI] [PubMed] [Google Scholar]
- 11.Wooltorton E. Olanzapine (Zyprexa): increased incidence of cerebrovascular events in dementia trials. Cmaj. 2004 Apr 27;170(9):1395. doi: 10.1503/cmaj.1040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. Jama. 2005 Oct 19;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 13.Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry. 2001;9(4):346–360. Fall. [PubMed] [Google Scholar]
- 14.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006 Oct 12;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 15.Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. Jama. 2000 Dec 27;284(24):3157–3159. doi: 10.1001/jama.284.24.3157. [DOI] [PubMed] [Google Scholar]
- 16.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–355. [Google Scholar]
- 17.Deberdt WG, Dysken MW, Rappaport SA, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry. 2005 Aug;13(8):722–730. doi: 10.1176/appi.ajgp.13.8.722. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy J, Deberdt W, Siegal A, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry. 2005 Nov;20(11):1020–1027. doi: 10.1002/gps.1397. [DOI] [PubMed] [Google Scholar]
- 19.De Deyn PP, Carrasco MM, Deberdt W, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2004 Feb;19(2):115–126. doi: 10.1002/gps.1032. [DOI] [PubMed] [Google Scholar]
- 20.Street JS, Clark WS, Gannon KS, et al. The HGEU Study Group Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2000 Oct;57(10):968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- 21.Tariot PN, Schneider L, Katz IR, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry. 2006 Sep;14(9):767–776. doi: 10.1097/01.JGP.0000196628.12010.35. [DOI] [PubMed] [Google Scholar]
- 22.Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007 Feb;4(1):81–93. doi: 10.2174/156720507779939805. [DOI] [PubMed] [Google Scholar]
- 23.Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M, Risperidone Study Group Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry. 1999 Feb;60(2):107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- 24.Mintzer J, Greenspan A, Caers I, et al. Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. Am J Geriatr Psychiatry. 2006 Mar;14(3):280–291. doi: 10.1097/01.JGP.0000194643.63245.8c. [DOI] [PubMed] [Google Scholar]
- 25.De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999 Sep 22;53(5):946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- 26.Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003 Feb;64(2):134–143. doi: 10.4088/jcp.v64n0205. [DOI] [PubMed] [Google Scholar]
- 27.Sultzer DL, Davis SM, Tariot PN, et al. Clinical Symptom Responses to Atypical Antipsychotic Medications in Alzheimer’s Disease: Phase 1 Outcomes From the CATIE-AD Effectiveness Trial. Am J Psychiatry. 2008;AiA:1–11. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]