Abstract
Purpose of review
Patients on maintenance dialysis commonly develop protein-energy wasting (PEW), which is associated with poor survival. There have been several advances in anabolic interventions aimed at improving PEW in these patients in recent years.
Recent findings
Oral or parenteral nutritional supplementation, especially if administered during dialysis, improves net protein anabolism in chronic hemodialysis (CHD) patients. These beneficial effects have been extended to long-term benefits in recent clinical trials. Resistance exercise, alone or combined with intradialytic oral nutrition supplementation also improves net protein balance in the acute setting although recent studies indicated a limited beneficial effect of long-term exercise alone on muscle protein accretion in CHD patients. Anabolic agents such as growth hormone and androgens have been shown to exert significant benefits on visceral protein stores, muscle mass and strength. Ghrelin, a hormone with combined orexigenic and anti-inflammatory effects, is a potential new nutritional intervention in maintenance dialysis patients.
Summary
Existing anabolic therapeutic strategies have proven to be effective in improving PEW in maintenance dialysis patients. Combined anabolic interventions and several new and established anabolic hormones represent as further promising nutritional interventions. Large-scale randomized controlled trials examining the effects of anabolic interventions on mortality and morbidity are still lacking.
Keywords: Dialysis, protein-energy wasting, nutritional supplementation, exercise, anabolic agents
Introduction
Protein-energy wasting (PEW), a state of metabolic and nutritional derangements, is closely associated with high morbidity and mortality in chronic kidney disease (CKD) patients, especially in ones on maintenance dialysis. PEW is present in approximately 20–50% of the maintenance dialysis patients and can result from multiple etiologies, including insufficient dietary nutrient intake, excessive catabolism due to dialysis, metabolic acidosis, chronic inflammation and hormonal derangements (Figure 1) [1, 2]**. Given the close association between the presence and severity of PEW and death risk in maintenance dialysis patients, it is obvious that prevention and treatment of PEW is of upmost importance. The multi-factorial origin of PEW renders this task equally challenging.
Figure 1.
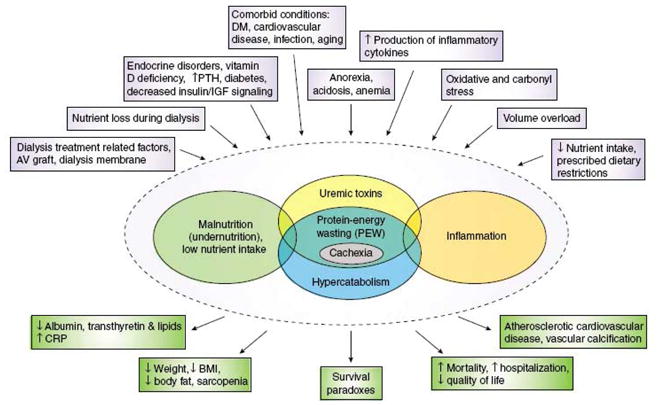
Schematic representation of the causes and manifestations of the protein–energy wasting syndrome in kidney disease. (Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008; 73: 391–398)
Total body protein content is considered to be most physiologically relevant nutritional parameter and an important determinant of PEW. Accordingly, anabolic interventions refer to a cluster of approaches focusing on improving PEW through either enhancing protein synthesis or decreasing protein catabolism, or a combination thereof to maximize total body protein stores. In this review, we will discuss the rationale and efficacy of a variety of anabolic agents, nutritional supplementation, and exercise as anabolic interventions to enhance total body protein content for the maintenance dialysis patient with or at risk of PEW with a specific emphasis on recent advancements in this area.
Nutritional supplementation
Inadequate dietary nutrient intake, especially relative to the actual needs, is one of the harbingers of PEW in maintenance dialysis patients. The etiology of this relative deficiency is multifactorial and includes hemodialysis process per se affecting both whole-body and skeletal muscle protein homeostasis[3, 4] and the increased metabolic needs during acute illness and stress conditions[5]. Recent studies indicate that oral or parenteral nutrition supplementation, especially during dialysis, could compensate for the relatively inadequate protein and energy intake.
In a series of metabolic studies, Pupim et al showed that intradialytic oral (IDON) and parenteral nutritional (IDPN) supplementation resulted in robust whole-body and skeletal muscle protein accretion rates[6]. A number of small scale studies have extended these encouraging results to more generalizable nutritional markers in the long-term, especially in the form of oral nutritional supplementation[7–13]. Caglar et al reported that IDON supplementation improved several nutritional parameters (including serum albumin and prealbumin concentrations as well as subjective global assessment) in a large group of maintenance hemodialysis (MHD) patients with PEW[7]. A significant aspect of this study was that nutritional supplementation was given during or around hemodialysis, which not only improved compliance to the treatment but also provided supplements at a time when catabolism is at its highest level in these patients. Kalantar-Zadeh et al reported in a controlled design study that in hypoalbuminemic MHD patients, a short-term (4 weeks) in-center IDON intervention was associated with a significant increase in serum albumin levels[14]. The supplementation consisted of one can of Nepro and one can of Oxepa administered during hemodialysis was also found to be practical, convenient and well-tolerated. A systematic review including 18 studies (5 randomized controlled trials [RCTs], 13 non-RCTs) suggested that enteral nutritional support (oral or tube) increased total (energy and protein) intake and increased serum albumin concentration on average by 0.23 g/dL, with no adverse effects on electrolyte status (serum phosphate and potassium)[15].
The results of a recent randomized clinical trial provided further insight to nutritional supplementation in MHD patients, albeit with certain inherent limitations. Cano et al. reported the results of the largest and arguably best executed nutritional intervention study in MHD patients with PEW. This study provided encouraging data on nutritional supplementation efforts in MHD patients with PEW. The investigators of the FINE study randomly assigned 186 MHD patients with PEW to receive one year of IDPN and oral nutritional supplementation or oral supplementation alone[16]***. After stratification by center, patients were randomized to receive IDPN and standard oral supplements providing 500 kcal/day and 25 g/day protein, or oral supplements alone for 1 year. The nutritional supplement goal was to bring patients intakes up to the recommended amounts of 30–35 kcal/kg/day and 1.2 g/kg/day, respectively. The primary outcome, two-year mortality, was similar in the two groups (39% in the control group and 43% in the IDPN group), suggesting that oral nutritional supplementation is equally effective as IDPN when oral intake is possible. Increases in serum prealbumin were associated with decreases in two-year mortality and hospitalization rate, providing the first prospective evidence of a link between response to nutritional therapy and improved outcomes.
Despite the negative primary outcome of the study, there are several important observations of the FINEs that give much cause for optimism. First, as the investigators point out, the route of administration of nutritional supplementation (i.e. oral or combined oral–parenteral) does not have any significant effect on survival in MHD patients with PEW, assuming that equal and adequate amounts of protein and calories are provided. Similarly, the route of administration does not influence the improvements in most nutritional markers that are observed following supplementation. These findings are not unexpected; several reports have shown that intradialytic oral and parenteral nutritional supplementation improve whole body and skeletal muscle protein homeostasis to a comparable extent in the short term (Figure 2)[6].
Figure 2.
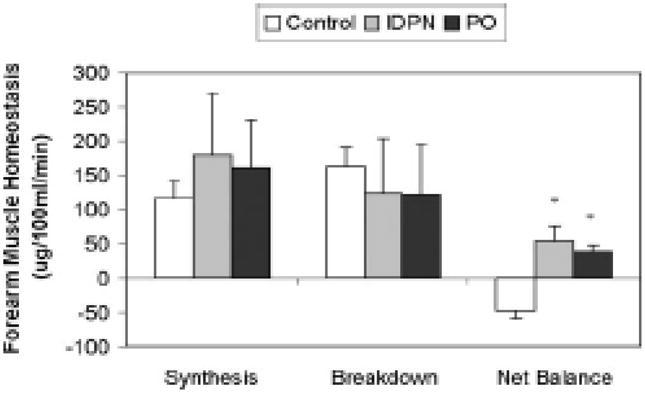
Forearm muscle protein homeostasis dynamic components during HD, comparing Control ( ), IDPN (
), IDPN ( ), and PO (
), and PO ( ) in eight CHD patients with deranged nutritional status. Skeletal muscle protein homeostasis during HD improved with both IDPN and PO versus control (P = 0.005 and 0.009 for IDPN versus control and PO versus control, respectively). PO resulted in persistent anabolic benefits in the post-HD phase for muscle protein metabolism, when anabolic benefits of IDPN dissipated (data not shown in figure). Units are ug/100ml/min. * denotes P< 0.05 versus Control. Adapted from reference 6 (Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol 2006; 17: 3149–3157) with permission.
) in eight CHD patients with deranged nutritional status. Skeletal muscle protein homeostasis during HD improved with both IDPN and PO versus control (P = 0.005 and 0.009 for IDPN versus control and PO versus control, respectively). PO resulted in persistent anabolic benefits in the post-HD phase for muscle protein metabolism, when anabolic benefits of IDPN dissipated (data not shown in figure). Units are ug/100ml/min. * denotes P< 0.05 versus Control. Adapted from reference 6 (Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol 2006; 17: 3149–3157) with permission.
Second, despite the lack of an appropriate control group, the results of the FINE study imply that nutritional supplementation does indeed improve nutritional markers in MHD patients with PEW if the targets for dietary protein and energy intake recommended by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative-KDOQI (>1.2 g/kg/day and >30 kcal/kg/day, respectively) are achieved. It is of note that the improvement in serum albumin reported by Cano et al. (~2 g/l) is highly consistent with that described in the majority of other published studies reporting the effectiveness of nutritional interventions [15]. These data also confirm the appropriateness of the KDOQI dietary protein and calorie intake guidelines[17].
Third, the results imply that nutritional interventions in general improve survival in MHD patients. This conclusion should, however, be applied with caution because the study did not include a no-intervention arm, and the nutritional improvements may just reflect a regression-to-the-mean phenomenon. Although this is a critical limitation of the study, the authors appropriately note that it would have been unethical to withhold nutritional therapy. One can, however, compare the overall 2-year mortality rate in the study (42%) with the published mortality rate obtained from European registry data, adjusted for at least one of the FINE study inclusion criteria (a serum albumin <35 g/l; 49%). This comparison indicates an approximately 15% improvement in overall mortality, a survival benefit that, if it is a treatment effect, is unmatched by any other proposed therapy for high-risk MDH patients to date. Finally, the results indicate that simple nutritional markers, such as serum prealbumin, can be used as surrogate markers not only of nutritional status but also possibly of hospitalization and survival.
Several additional caveats regarding oral or parenteral nutritional supplementation in maintenance dialysis patients include the fact that there is no study that has examined the dose, content or even the need for fat emulsions for maintenance dialysis patients with PEW, except a few small scale reports. The few published studies indicated no difference between olive oil-based fat emulsion vs. regular soybean oil-based emulsions on nutritional markers and plasma lipid, oxidative, inflammatory and immune parameters. Another important aspect of therapeutic nutritional supplementation that is usually overlooked, especially in the form of oral administration is compliance. Most, if not all of such studies are hampered by a non-compliance rate of a minimum of 25% and almost up to 40 % in some cases, despite the advantages of research conditions[18]. The landmark FINE study reported a mean compliance rate of 60% for oral supplementation group and 75% for the IDPN group. It is important to note that despite inadequate compliance in FINEs, all subjects reached a dietary protein and calorie intake of >1.2 g/kg/day and >30 kcal/kg/day, respectively and displayed significant increases in serum albumin and prealbumin.
In terms of peritoneal dialysis (PD) patients, three metabolic studies have indicated beneficial effects of amino acid (AA) dialysate on net protein balance, similar to MHD patients[19–21]*. Despite the obvious acute benefits of AA based dialysate solutions, long-term studies have not been as encouraging in PD patients with PEW[22]. While this might be simply related to study design issue such as inadequate sample size, imprecise methods used to assess nutritional status, inconsistent dietary monitoring[23], the consistency in lack of a benefit is somewhat discouraging.
Exercise
Numerous exercise regimens, including aerobic, resistance exercise or a combination of both have been suggested as nutritional interventions in ESRD population[24]. The rationale for such intervention is that exercise can induce significant physiological, functional, and psychological benefits without serious adverse events as has been shown in the healthy adults, elderly and those with frailty and/or chronic disease[25–27]. Since augmentation of skeletal muscle mass is considered to be an important aspect of nutritional management of ESRD patients, resistance training, compared to aerobic exercise, is the exercise modality of choice to improve physical performance and activity, at least theoretically[28–35].
In follow-up to earlier studies indicating type 1 and type 2 muscle fiber hypertrophy and increased peak torque of the leg extensors of the dominant leg in response to exercise in MHD patients, Johansen et al reported in a randomized clinical trial that 12 wk of moderate intensity lower body PRT can improve quadriceps muscle area measured by magnetic resonance imaging (MRI), although this was not detectable by dual energy X-ray absorptiometry (DEXA)[36]. Of note, these changes were accompanied by a significant increase body weight and fat mass. In another randomized controlled study[37], Cheema et al showed that 12-wk high-intensity PRT administered during routine HD treatment did not improve skeletal muscle quantity measured by computed tomography (CT) scan, although there were statistically significant improvements in muscle attenuation (intramuscular lipid content via attenuation evaluated by CT), muscle strength and other anthropometric data. A further analysis of 24 weeks of intervention did not result in any additional benefit either[38]. Kopple et al studied the effects of different forms of exercise training (endurance, strength, or a combination where patients underwent about one-half each of the endurance and strength training of the first two groups) on mRNA levels of genes in muscle that may contribute to increased exercise capacity[39]. While there were no significant changes in lean body mass with any of the individual exercise regimens over 6 months, it was concluded that exercise training in hemodialysis patients induces changes in skeletal muscle mRNA and increases muscle insulin growth factor-1 (IGF-I) protein, which may promote protein anabolism.
Collectively, these studies indicate that the presumed beneficial effects of resistance exercise such as improvements in muscle quality and quantity, strength and physical functioning are not consistently observed in maintenance dialysis patients. Further research is necessary to both understand the observed lack of obvious benefits and strategies to improve the exercise regimens.
Anabolic agents
Patients on maintenance dialysis have low circulating levels of certain anabolic hormones (testosterone), resistance to other anabolic hormones (growth hormone and insulin-like growth factor I) and increased levels of some catabolic hormones (cortisol). These hormonal derangements, individually or collectively, contribute to the development or worsening of PEW. It is therefore reasonable to speculate that pharmacological doses of anabolic hormones could be of potential value in the treatment of PEW of maintenance dialysis patients.
Growth hormone
Abnormalities in the physiological axis of growth hormone (GH) and IGF-I have been long-established[40]. Growth hormone is the major promoter of growth in children and exerts anabolic actions even in adults, such as enhancement of protein synthesis, reduced protein degradation, increased fat mobilization, and increased gluconeogenesis, with IGF-1 being the major mediator of these actions[1]. A few controlled studies were performed on a small number of patients for short periods of time, showing the consistent results on reducing protein catabolism, increasing LBM and visceral protein concentrations (serum albumin and transferrin)[41–44]. Most recently, a Phase-2 randomized, double-blind, placebo-controlled, 26-week proof-of-concept clinical trial in 139 adult chronic HD patients showed that GH led to statistically significant gains in LBM as compared to placebo[45]***. Statistically significant beneficial changes in other cardiovascular biomarkers of mortality (transferrin, high density lipoprotein, homocysteine) as well as quality of life were observed. There was also a trend (p = 0.06) toward increased levels of serum albumin as compared to placebo. While this promising Phase 2 trial was followed by a large-scale two-year clinical trial (OPPORTUNITY Study) testing whether GH will produce improvements in mortality, it was prematurely terminated due to slow recruitment[46]***.
Anabolic steroids (testosterone and nandrolone decanoate)
As many as 50% to 70% of CKD stage-5 men have been reported to be hypogonadal on the basis of low concentrations of total and free testosterone[47]. These disorders often worsen even after initiation of maintenance dialysis treatment[48]. Testosterone abnormalities have recently been linked to disorders in bone composition[49] and endothelial dysfunction[50] in HD patients. A recent prospective observational study showed the significant inverse correlation between testosterone levels and all-cause and cardiovascular disease-related mortality in 126 chronic HD patients during a mean 41 follow-up months[51]**. Strong inverse independent correlations were also observed between testosterone and various inflammatory markers. These observational data encourage further research into the role of testosterone as a modifiable risk factor in CKD and create a rationale for randomized controlled trials with testosterone supplementation in this patient group. Despite positive results of increased muscle mass and strength in several other clinical populations, such as elderly[52], COPD[53], and patients with HIV wasting[54], there has not been a clinic trial showing the effect of testosterone on CKD patients.
Nandrolone decanoate (ND), a non-17α-alkylated modified androgen analogue of testosterone, appears to be effective in increasing LBM and muscle strength in dialysis patients[55–58]. In a recent randomized controlled trial, Johansen et al assessed body composition, muscle strength and physical functioning while administering ND 100mg intramuscularly weekly for 6 months in 29 MHD patients[36]. ND induced a 4.5kg LBM gain and a fat loss of 2.4kg from baseline. It also demonstrated significant improvements with ND in quadriceps muscle CSA measured by MRI and physical functioning. More recently, a dose-finding study in 54 patients on HD or PD explored the efficacy and safety of low, medium or high doses of ND (50, 100 or 200 mg/week for 24 weeks, respectively, in males). The doses were halved in females[59]. The results indicated that ND increase appendicular LBM in a dose-responsive manner. It was also noted that in the majority of patients ND did not increase fluid retention in excess of that associated with protein accretion and suggested dosing of ND up to 200 mg/week in males and 50 mg/week in females should be investigated to improve body composition. On the other hand, the highest dose of ND (100 mg/week) was intolerable in females because of virilizing effects. Other potential side effects included voice change and hirsutism in women, abnormalities in prostatic markers in men, liver tests and lipid metabolism indicating that patients in future studies should be regularly followed.
Ghrelin
Ghrelin is a peptide that activates neurons of the arcuate nucleus of the hypothalamus, an area known to be important in the regulation of feeding. Ghrelin is also an endogenous ligand for the growth hormone secretagogue (GHS) receptor type 1a. Parenteral or oral administration of a ghrelin-mimetic GHS have been shown to restore levels of growth hormone and IGF-I in older persons to levels seen in young adults[60]. Ghrelin provides further benefit in muscle wasting conditions via its appetite stimulating (orexigenic) and anti-inflammatory effects[60, 61]*. A recent RCT study by Nass et all showed that a particular ghrelin mimetic given over 2 years had significant anabolic effects age-associated changes in body composition with an excellent side-effect profile.
These distinct properties along with promising data in otherwise elderly population make ghrelin and ghrelin analogues part of an attractive therapeutic strategies for the treatment of PEW in chronic disease states, such as CKD. Indeed, in an experimental study, administration of ghrelin and two synthetic ghrelin-receptor agonists (BIM-28125 and BIM-28131) increased food intake, attenuated muscle protein degradation and decreased circulating inflammatory cytokines in nephrectomized animals[62]. There are also preliminary studies of subcutaneous ghrelin administration in dialysis patients. Wynne et al administered subcutaneous ghrelin and saline placebo in a randomized, double-blind, crossover protocol to nine PD patients with mild to moderate malnutrition. Administration of subcutaneous ghrelin significantly increased short-term food intake without any significant side effects[63]. Future clinical studies exploring the efficacy and feasibility of ghrelin or ghrelin mimetics are greatly needed in this high-risk patient population.
Ccombination anabolic interventions
A potential strategy to augment the anabolic effects of nutritional supplementation is concomitant exposure to resistance exercise around the time of administration of nutritional supplementation. Short-term studies in healthy subjects and MHD patients showed that post-exercise net muscle protein accretion is increased with oral nutrition supplement when compared to exercise or oral supplement alone [64–66]. A metabolic study by Pupim et al indicated an incremental beneficial effect of GH, IDPN and exercise in MHD patients, at least in the acute setting[67]. Johansen et al showed that exercise and ND induced an additive increase in muscle CSA, albeit a small extent [36]. The next logical step is to test the hypothesis that these acute changes would translate into long-term benefits in muscle mass and strength in maintenance dialysis. Such studies are currently underway.
Economic Implications of Nutritional interventions
It is also important to assess the impact of nutritional supplements not only in terms of changes in nutritional parameters, but to extrapolate these observations to potential improvements in hospitalization, mortality, and cost-effectiveness. In a recent study, Lacson et al showed that a hypothetical increase in serum albumin concentration in the order of 2 g/L in 50% of the United States dialysis population would be associated with projections of approximately 1400 lives saved, approximately 6000 hospitalizations averted, and approximately $36 million in Medicare cost savings resulting from a reduction of approximately 20,000 hospital days over one year[68]. This is a reasonable estimation since 2 g/L increase in serum albumin is the average improvement reported in most nutritional intervention studies.
Conclusion
In summary, the available evidence suggests that the imbalance between protein synthesis and degradation in dialysis population can be compensated by various anabolic strategies. Nutritional supplementation, administered orally or parenterally, is effective in the treatment of PEW. Resistance or endurance exercise, while effective in the short term, seems to lack consistent evidence in improving LBM over long-term. Various anabolic agents are shown to increase visceral protein concentrations and muscle mass and strength simultaneously. Further larger scale randomized controlled trials of anabolic interventions, individually or combined should be performed in maintenance dialysis patients to assess their efficacy regarding quality of life, morbidity and mortality.
Acknowledgments
This work is supported in part by National Institutes of Health Grants R01-DK45604, R01-HL070938, K24-DK62849 and UL1 RR024975.
Reference List
- 1**.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50(2):343–357. doi: 10.1038/ki.1996.323. This is an important review coauthored by an expert panel convened by the International Society of Renal Nutrition and Metabolism (ISRNM) to advance a unifying and practical terminology for the protein-energy wasting syndrome that occurs in many patients with chronic kidney disease or acute kidney injury. The systematically defined nomenclature and diagnostic criteria will help to clarify thinking and communication, enhance the effectiveness of patient care, and promote more incisive research in this field. [DOI] [PubMed] [Google Scholar]
- 2.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 3.Lim VS, Ikizler TA, Raj DS, Flanigan MJ. Does hemodialysis increase protein breakdown? Dissociation between whole-body amino acid turnover and regional muscle kinetics. J Am Soc Nephrol. 2005;16(4):862–868. doi: 10.1681/ASN.2004080624. [DOI] [PubMed] [Google Scholar]
- 4.Ikizler TA, Pupim LB, Brouillette JR, Levenhagen DK, Farmer K, Hakim RM, Flakoll PJ. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282(1):E107–116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 5.Ikizler TA, Greene JH, Yenicesu M, Schulman G, Wingard RL, Hakim RM. Nitrogen balance in hospitalized chronic hemodialysis patients. Kidney Int Suppl. 1996;57:S53–56. [PubMed] [Google Scholar]
- 6.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17(11):3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 7.Caglar K, Fedje L, Dimmitt R, Hakim RM, Shyr Y, Ikizler TA. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002;62(3):1054–1059. doi: 10.1046/j.1523-1755.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- 8.Eustace JA, Coresh J, Kutchey C, Te PL, Gimenez LF, Scheel PJ, Walser M. Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int. 2000;57(6):2527–2538. doi: 10.1046/j.1523-1755.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. J Ren Nutr. 2002;12(4):229–237. doi: 10.1053/jren.2002.35300. [DOI] [PubMed] [Google Scholar]
- 10.Cano N, Labastie-Coeyrehourq J, Lacombe P, Stroumza P, di Costanzo-Dufetel J, Durbec JP, Coudray-Lucas C, Cynober L. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr. 1990;52(4):726–730. doi: 10.1093/ajcn/52.4.726. [DOI] [PubMed] [Google Scholar]
- 11.Navarro JF, Mora C, Leon C, Martin-Del Rio R, Macia ML, Gallego E, Chahin J, Mendez ML, Rivero A, Garcia J. Amino acid losses during hemodialysis with polyacrylonitrile membranes: effect of intradialytic amino acid supplementation on plasma amino acid concentrations and nutritional variables in nondiabetic patients. Am J Clin Nutr. 2000;71(3):765–773. doi: 10.1093/ajcn/71.3.765. [DOI] [PubMed] [Google Scholar]
- 12.Cano NJ, Saingra Y, Dupuy AM, Lorec-Penet AM, Portugal H, Lairon D, Cristol JP, Come A, Le Brun A, Atlan P, et al. Intradialytic parenteral nutrition: comparison of olive oil versus soybean oil-based lipid emulsions. Br J Nutr. 2006;95(1):152–159. doi: 10.1079/bjn20051595. [DOI] [PubMed] [Google Scholar]
- 13.Mortelmans AK, Duym P, Vandenbroucke J, De Smet R, Dhondt A, Lesaffer G, Verwimp H, Vanholder R. Intradialytic parenteral nutrition in malnourished hemodialysis patients: a prospective long-term study. JPEN J Parenter Enteral Nutr. 1999;23(2):90–95. doi: 10.1177/014860719902300290. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Braglia A, Chow J, Kwon O, Kuwae N, Colman S, Cockram DB, Kopple JD. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr. 2005;15(3):318–331. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Stratton RJ, Bircher G, Fouque D, Stenvinkel P, de Mutsert R, Engfer M, Elia M. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2005;46(3):387–405. doi: 10.1053/j.ajkd.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 16***.Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM. Intradialytic Parenteral Nutrition Does Not Improve Survival in Malnourished Hemodialysis Patients: A 2-Year Multicenter, Prospective, Randomized Study. J Am Soc Nephrol. 2007;18(9):2583–2591. doi: 10.1681/ASN.2007020184. This is the first study to compare the impact of two types of IDPN differing by their fatty acid content on nutritional parameters, plasma lipids and markers of oxidative stress, inflammation and immune status. The results are indicative of comparable effects between two groups. This study is one of the largest randomized clinical trials on nutritional interventions in CHD patients. [DOI] [PubMed] [Google Scholar]
- 17.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 18.Teixido-Planas J, Ortiz A, Coronel F, Montenegro J, Lopez-Menchero R, Ortiz R, Gomez C, Donate T. Oral protein-energy supplements in peritoneal dialysis: a multicenter study. Perit Dial Int. 2005;25(2):163–172. [PubMed] [Google Scholar]
- 19.Tjiong HL, van den Berg JW, Wattimena JL, Rietveld T, van Dijk LJ, van der Wiel AM, van Egmond AM, Fieren MW, Swart R. Dialysate as food: combined amino acid and glucose dialysate improves protein anabolism in renal failure patients on automated peritoneal dialysis. J Am Soc Nephrol. 2005;16(5):1486–1493. doi: 10.1681/ASN.2004050402. [DOI] [PubMed] [Google Scholar]
- 20.Tjiong HL, Rietveld T, Wattimena JL, van den Berg JW, Kahriman D, van der Steen J, Hop WC, Swart R, Fieren MW. Peritoneal dialysis with solutions containing amino acids plus glucose promotes protein synthesis during oral feeding. Clin J Am Soc Nephrol. 2007;2(1):74–80. doi: 10.2215/CJN.01370406. [DOI] [PubMed] [Google Scholar]
- 21*.Asola M, Virtanen K, Nagren K, Helin S, Taittonen M, Kastarinen H, Anderstam B, Knuuti J, Metsarinne K, Nuutila P. Amino-acid-based peritoneal dialysis solution improves amino-acid transport into skeletal muscle. Kidney Int Suppl. 2008;108:S131–136. doi: 10.1038/sj.ki.5002614. This study used Positron Emission Tomography which reflects metabolism and especially amino acid transport and showed that amino acid-containing PD solution plus glucose-based PD solutions administered for 6 weeks is associated with a significant increase in skeletal muscle amino acid uptake both in the fasting state and during insulin stimulation as compared to glucose-based PD solutions only. [DOI] [PubMed] [Google Scholar]
- 22.Li FK, Chan LY, Woo JC, Ho SK, Lo WK, Lai KN, Chan TM. A 3-year, prospective, randomized, controlled study on amino acid dialysate in patients on CAPD. Am J Kidney Dis. 2003;42(1):173–183. doi: 10.1016/s0272-6386(03)00421-9. [DOI] [PubMed] [Google Scholar]
- 23.Park MS, Choi SR, Song YS, Yoon SY, Lee SY, Han DS. New insight of amino acid-based dialysis solutions. Kidney Int Suppl. 2006;103:S110–114. doi: 10.1038/sj.ki.5001925. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18(6):1845–1854. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 25.Singh MA. Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. J Gerontol A Biol Sci Med Sci. 2002;57(5):M262–282. doi: 10.1093/gerona/57.5.m262. [DOI] [PubMed] [Google Scholar]
- 26.Cheema BS, Smith BC, Singh MA. A rationale for intradialytic exercise training as standard clinical practice in ESRD. Am J Kidney Dis. 2005;45(5):912–916. doi: 10.1053/j.ajkd.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25(4):352–364. doi: 10.1159/000087184. [DOI] [PubMed] [Google Scholar]
- 28.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 29.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63(1):291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 31.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14(9):2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 32.Desmeules S, Levesque R, Jaussent I, Leray-Moragues H, Chalabi L, Canaud B. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004;19(5):1182–1189. doi: 10.1093/ndt/gfh016. [DOI] [PubMed] [Google Scholar]
- 33.Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45(4):690–701. doi: 10.1053/j.ajkd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Painter P, Johansen KL. Improving physical functioning: time to be a part of routine care. Am J Kidney Dis. 2006;48(1):167–170. doi: 10.1053/j.ajkd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106(6):2040–2048. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- 36.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006;17(8):2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 37.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 38.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis. 2007;50(4):574–584. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W, Storer TW. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007;18(11):2975–2986. doi: 10.1681/ASN.2006070794. [DOI] [PubMed] [Google Scholar]
- 40.Mehls O, Tonshoff B, Blum WF, Heinrich U, Seidel C. Growth hormone and insulin-like growth factor I in chronic renal failure--pathophysiology and rationale for growth hormone treatment. Acta Paediatr Scand Suppl. 1990;370:28–34. doi: 10.1111/j.1651-2227.1990.tb11666.x. discussion 35. [DOI] [PubMed] [Google Scholar]
- 41.Iglesias P, Diez JJ, Fernandez-Reyes MJ, Aguilera A, Burgues S, Martinez-Ara J, Miguel JL, Gomez-Pan A, Selgas R. Recombinant human growth hormone therapy in malnourished dialysis patients: a randomized controlled study. Am J Kidney Dis. 1998;32(3):454–463. doi: 10.1053/ajkd.1998.v32.pm9740162. [DOI] [PubMed] [Google Scholar]
- 42.Johannsson G, Bengtsson BA, Ahlmen J. Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: anabolic effect and functional improvement. Am J Kidney Dis. 1999;33(4):709–717. doi: 10.1016/s0272-6386(99)70223-4. [DOI] [PubMed] [Google Scholar]
- 43.Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, Pedersen FB. Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clin Nephrol. 2000;53(2):99–107. [PubMed] [Google Scholar]
- 44.Kotzmann H, Yilmaz N, Lercher P, Riedl M, Schmidt A, Schuster E, Kreuzer S, Geyer G, Frisch H, Horl WH, et al. Differential effects of growth hormone therapy in malnourished hemodialysis patients. Kidney Int. 2001;60(4):1578–1585. doi: 10.1046/j.1523-1755.2001.00971.x. [DOI] [PubMed] [Google Scholar]
- 45***.Feldt-Rasmussen B, Lange M, Sulowicz W, Gafter U, Lai KN, Wiedemann J, Christiansen JS, El Nahas M. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol. 2007;18(7):2161–2171. doi: 10.1681/ASN.2006111207. This is the largest prospective, randomized study in adult HD patients to show a significant beneficial effect of hGH on lean body mass. A dose-dependent reduction in body fat mass in hGH-treated patients was observed. hGH treatment had a favorable impact on cardiovascular risk markers such serum transferrin, serum HDL, and plasma homocysteine. [DOI] [PubMed] [Google Scholar]
- 46***.Kopple JD, Cheung AK, Christiansen JS, Djurhuus CB, El Nahas M, Feldt-Rasmussen B, Lange M, Mitch WE, Wanner C, Wiedemann J, et al. OPPORTUNITY: a randomized clinical trial of growth hormone on outcome in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(6):1741–1751. doi: 10.2215/CJN.02760608. This study was designed to asses the effects of GH on mortality in a larger group of MHD patients but was terminated due to slow recruitment. No increased risk with GH was observed. The early termination of this study attests to the complicated nature of the CHD patients and complexity of executing RCTs in this patient population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albaaj F, Sivalingham M, Haynes P, McKinnon G, Foley RN, Waldek S, O’Donoghue DJ, Kalra PA. Prevalence of hypogonadism in male patients with renal failure. Postgrad Med J. 2006;82(972):693–696. doi: 10.1136/pgmj.2006.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries CP, Gooren LJ, Oe PL. Haemodialysis and testicular function. Int J Androl. 1984;7(2):97–103. doi: 10.1111/j.1365-2605.1984.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 49.Doumouchtsis KK, Kostakis AI, Doumouchtsis SK, Grapsa EI, Passalidou IA, Tziamalis MP, Poulakou MV, Vlachos IS, Perrea DN. The effect of sexual hormone abnormalities on proximal femur bone mineral density in hemodialysis patients and the possible role of RANKL. Hemodial Int. 2008;12(1):100–107. doi: 10.1111/j.1542-4758.2008.00249.x. [DOI] [PubMed] [Google Scholar]
- 50.Karakitsos D, Patrianakos AP, De Groot E, Boletis J, Karabinis A, Kyriazis J, Samonis G, Parthenakis FI, Vardas PE, Daphnis E. Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol. 2006;26(6):536–543. doi: 10.1159/000097816. [DOI] [PubMed] [Google Scholar]
- 51**.Carrero JJ, Qureshi AR, Parini P, Arver S, Lindholm B, Barany P, Heimburger O, Stenvinkel P. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol. 2009;20(3):613–620. doi: 10.1681/ASN.2008060664. This is the first report to show that testosterone concentrations are inversely related to mortality due to both CVD and all causes in male HD patients. This association was attenuated after adjustment for s-creatinine. Strong inverse independent correlations were observed between testosterone and various inflammatory markers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 53.Svartberg J, Aasebo U, Hjalmarsen A, Sundsfjord J, Jorde R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir Med. 2004;98(9):906–913. doi: 10.1016/j.rmed.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Kong A, Edmonds P. Testosterone therapy in HIV wasting syndrome: systematic review and meta-analysis. Lancet Infect Dis. 2002;2(11):692–699. doi: 10.1016/s1473-3099(02)00441-3. [DOI] [PubMed] [Google Scholar]
- 55.Barton Pai A, Chretien C, Lau AH. The effects of nandrolone decanoate on nutritional parameters in hemodialysis patients. Clin Nephrol. 2002;58(1):38–46. doi: 10.5414/cnp58038. [DOI] [PubMed] [Google Scholar]
- 56.Dombros NV, Digenis GE, Soliman G, Oreopoulos DG. Anabolic steroids in the treatment of malnourished CAPD patients: a retrospective study. Perit Dial Int. 1994;14(4):344–347. [PubMed] [Google Scholar]
- 57.Gascon A, Belvis JJ, Berisa F, Iglesias E, Estopinan V, Teruel JL. Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis. Geriatr Nephrol Urol. 1999;9(2):67–72. doi: 10.1023/a:1008306301255. [DOI] [PubMed] [Google Scholar]
- 58.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. 1999;281(14):1275–1281. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 59.Macdonald JH, Marcora SM, Jibani MM, Kumwenda MJ, Ahmed W, Lemmey AB. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin Pract. 2007;106(3):c125–135. doi: 10.1159/000103000. [DOI] [PubMed] [Google Scholar]
- 60.Kamiji MM, Inui A. The role of ghrelin and ghrelin analogues in wasting disease. Curr Opin Clin Nutr Metab Care. 2008;11(4):443–451. doi: 10.1097/MCO.0b013e328303dee4. [DOI] [PubMed] [Google Scholar]
- 61*.Granado M, Martin AI, Lopez-Menduina M, Lopez-Calderon A, Villanua MA. GH-releasing peptide-2 administration prevents liver inflammatory response in endotoxemia. Am J Physiol Endocrinol Metab. 2008;294(1):E131–141. doi: 10.1152/ajpendo.00308.2007. This comprehensive review highlights recent developments in research with ghrelin in wasting conditions such as cancer, diabetes mellitus, malabsorptive diseases, chronic obstructive pulmonary disease, anorexia nervosa, renal failure, liver failure, and chronic heart failure. [DOI] [PubMed] [Google Scholar]
- 62.Deboer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, Mitch WE, Taylor JE, Halem HA, Dong JZ, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149(2):827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, Brown EA, Bloom SR, Choi P. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16(7):2111–2118. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 64.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 65.Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol. 1998;274(2 Pt 1):C406–414. doi: 10.1152/ajpcell.1998.274.2.C406. [DOI] [PubMed] [Google Scholar]
- 66*.Majchrzak KM, Pupim LB, Flakoll PJ, Ikizler TA. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant. 2008;23(4):1362–1369. doi: 10.1093/ndt/gfm773. This metabolic study shows the additive beneficial effect of resistance exercise on protein accretion obtained by nutritional supplementation alone and provides rationale for future long-term studies using this combined nutritional intervention strategy. [DOI] [PubMed] [Google Scholar]
- 67.Pupim LB, Flakoll PJ, Yu C, Ikizler TA. Recombinant human growth hormone improves muscle amino acid uptake and whole-body protein metabolism in chronic hemodialysis patients. Am J Clin Nutr. 2005;82(6):1235–1243. doi: 10.1093/ajcn/82.6.1235. [DOI] [PubMed] [Google Scholar]
- 68.Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, Hakim RM. Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr. 2007;17(6):363–371. doi: 10.1053/j.jrn.2007.08.009. [DOI] [PubMed] [Google Scholar]


