Abstract
Primary sensory cortices have been assumed to serve as stimulus analyzers while cognitive functions such as learning and memory have been allocated to “higher” cortical areas. However, the primary auditory cortex (A1) is now known to encode the acquired significance of sound as indicated by associatively-induced specific shifts of tuning to the frequencies of conditioned stimuli (CS) and gains in area of CS representations. Rewarding brain stimulation can be a very powerful motivator and brain reward systems have been implicated in addictive behavior. Therefore, it is possible that a cue for brain reward will gain cortical territory and perhaps thereby increase its control of subsequent behavior. To investigate the effect of brain reward on cortical organization, adult male rats (n = 11) were first tested with varying amounts of stimulation of the ventral tegmental area (VTAstm) to generate sigmoidal psychometric functions of nose poke (NP) rates as a function of reward magnitude (duration). Next, we attempted to accomplish tone control of NPs by maintaining intertrial NPs using a low reward duration and presenting a 20 s tone (2.0 kHz, 70 dB) which signaled an increase in reward to a high magnitude 10 s after tone onset. Tone control was demonstrated by a significant increase in the rate of NPs during the first 10 s of tone presentation, which anticipated the delivery of the high magnitude of reward. Tone control was achieved in seven of 11 subjects. This was accompanied by a highly specific and significant gain in representational area, specifically for the half-octave range centered on the CS frequency. However, this plasticity developed only in tone-controlled (TC) animals. The auditory cortex of non-tone-controlled subjects (n = 4) did not differ from that of naïve controls (n = 9) although their VTAstm was as rewarding as for the TC group. These findings reveal that auditory instrumental behavior can be controlled by rewarding VTAstm and that such control appears necessary for the highly specific recruitment of cortical cells to increase the representation of a sound that acquires behavioral importance.
Keywords: Association, Frequency, Plasticity, Tonotopic map, Ventral tegmental area
1. Introduction
The functional organization of the cerebral cortex traditionally has been assumed to involve serial processing of environmental information from “lower” to “higher” areas. Thus, the primary auditory, somatosensory and visual fields have been assigned the role of “stimulus analyzers”, performing only sensory/perceptual tasks, whereas their output to adjacent, associative fields is supposed to initiate “cognitive processing”, including learning and memory, in these higher regions. As a result of a heritage of 19th century advances in neuroscience, which conceived of nervous system organization largely in sensory–motor terms for all behavior (Young, 1970), sensory/perceptual processes were explicitly cleaved from learning/memory purely on anatomical grounds. An influential cytoarchitectonic monograph held that primary auditory cortex was “auditory sensory” while neighboring areas were “auditory psychic” (Campbell, 1905). This was a codification of long-held assumptions, agreeing with introspection, that separated sensation and perception on the one hand from the interpretation of meaning and thought on the other (Diamond, 1985).
Although many studies during the period 1956–1984 validated learning-induced plasticity in the primary auditory cortex (A1) (reviewed in Weinberger & Diamond, 1987), more recent studies on the effects of associative processes on auditory frequency receptive fields have revealed that learning actually changes the tuning of neurons in A1. More specifically, it shifts tuning from a pre-training “best frequency” toward or to the frequency of a tone that serves as an effective signal for reward or punishment (e.g., Bakin & Weinberger, 1990; Gao & Suga, 2000; Kisley & Gerstein, 2001; Morris, Friston, & Dolan, 1998). This receptive field (RF) plasticity has key attributes of associative memory that make it an attractive candidate for the storage of behaviorally relevant auditory information. In addition to being associative, it is highly specific to the frequency of the conditioned stimulus, discriminative, develops rapidly, consolidates over hours and days, and retained for weeks or months. Moreover, specific RF plasticity develops in all tasks studied (e.g., classical and instrumental one-tone and two-tone discrimination learning), for both appetitive and aversive reinforcers, and in all taxa studied (bat, cat, guinea pig, rat and human) (reviewed in Edeline, 1999; Weinberger, 2007). Insofar as tonotopic maps are comprised of the best tuning of myriads of cells, learning-induced tuning shifts should increase the number of cells that respond best to tones that gain behavioral importance through learning. This has been found in both the owl monkey (Recanzone, Schreiner, & Merzenich, 1993) and the rat (Rutkowski & Weinberger, 2005). In the latter case, the degree of behavioral significance was highly correlated with the amount of gain in cortical area, suggesting that the latter may serve as a “memory code” for the degree of learned importance of stimuli.
Important environmental stimuli may exert strong control of behavior because of their association with powerful reinforcers, such as addictive agents. Neurobiological studies of addictive behavior have naturally focused on the neural systems and cellular mechanisms of these reinforcers (e.g., Day & Carelli, 2007; Schultz, 2001; Solinas, Goldberg, & Piomelli, 2008). The present experiment is concerned with the sensory stimulus aspect of behavioral control. We asked whether a tone could gain behavioral control by serving as a cue for a marked increase in the magnitude of a powerful reward. If so, would such tone control be reflected in a significant increase in its cortical area of representation? Our approach was to use intracranial self-stimulation of the ventral tegmental area and attempt to exert tone control of instrumental behavior that anticipated delivery of a substantial increase in the reward value of brain stimulation.
2. Methods
2.1. Subjects
Adult male Sprague–Dawley rats (n = 20, 376.5 ± 56.8 g at time of first handling) from Charles River Laboratories (Wilmington, MA) were individually housed in a temperature-controlled (22 °C) vivarium on a standard 12/12 h light–dark cycle (lights on at 06:00 h PST). Experimental subjects (n = 11) and naïve animals (n = 9) had access to food and water ad libitum. All subjects were handled for at least 3 days before surgery. Experimental procedures were carried out in accordance with the University of California, Irvine's Institutional Animal Care and Use Committee and National Institutes of Health's Office of Laboratory Animal Welfare guidelines.
2.2. Surgical preparation
Experimental subjects were implanted with stimulating electrodes under sodium pentobarbital anesthesia (50 mg/kg, i.p., Nembutal sodium solution, Abbott Laboratories, North Chicago, IL). Bronchial secretions were reduced by treatment with atropine sulfate (0.25 mg/kg, i.m., Phoenix Pharmaceutical, St. Joseph, MO) prior to surgery. Core body temperature was maintained at 37 °C via a homeothermic heating blanket system (Harvard Apparatus, Kent, UK). The animal was placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with blunt ear bars, the scalp was injected with a local anesthetic (Xylocaine 2% Injection, Astra-Zeneca, Wilmington, DE) before being resected and six anchoring stainless steel screws (#0–80, 0.125″ length) were inserted into the calvarium. An insulated monopolar stainless steel electrode with a threaded plastic connector (0.25 mm bare diameter, model MS303/1-AIU, Plastics One, Roanoke, VA) was implanted into the left ventral tegmental area (VTA) of the medial forebrain bundle (anterior–posterior [AP] = −4.8 mm, medial–lateral [ML] = 0.7 mm, dorsal–ventral [DV] = −9.0 mm (Paxinos & Watson, 2007) and the ground wire was attached to an anchoring screw. The electrode was secured to the calvarium and screws by a pedestal of methyl methacrylate (Teets denture material, co-Oral-Ite Dental Mfg. Co., Diamond Springs, CA) leaving the plastic connector exposed for connection to the stimulator. The incision was sutured around the base of the pedestal and the subject was given a topical local anesthetic (Xylocaine 2% Jelly, AstraZeneca) and antibiotic (Animax ointment, Pharmaderm, Melville, NY). Animals were recovered in an incubator before being returned to the vivarium.
2.3. Behavioral training
Experimental subjects were allowed at least 7 days for recovery before training. All testing and training were conducted in an operant test chamber (model H10-11R-TC, Coulbourn Instruments [CI], Whitehall, PA) enclosed within a sound-attenuated acoustic chamber (Industrial Acoustics Co. [IAC], Bronx, NY). The test chamber was equipped with a nose poke operandum (1″ diameter, CI model H21-10R) located at the center of one wall with a speaker (8 Ω,CI model H12-01M) and houselight above it. Nose pokes (NP) were detected by an infrared photo beam inside the entrance of the nose poke device. The implanted electrode was connected to an isolated programmable stimulator (CI model A13–65) through a suspended cable that allowed free movement. Intracranial self-stimulation (ICSS) consisted of a train of monophasic (cathodal) rectangular pulses (400 μA, 0.1 ms) that were immediately given after each NP. Train duration and pulse frequency varied according to the individual subject and the phases of the behavioral training as described below.
In the first phase of the experiment, subjects were tested and trained to NP consistently and reproducibly for ICSS on a continuous reinforcement schedule (1:1 fixed ratio of NP to stimulation). Train duration was maintained constant at 500 ms, while pulse frequency was increased incrementally until the subject demonstrated consistent and sustainable self-stimulation. Subjects were shaped under the same testing protocol for 2–3 days. None of the subjects had stimulation-induced motor responses or had NP for ICSS less than 1000 times during the last 30 min training session.
In the second phase of training, reward magnitude was varied by the duration of the stimulation train (Mark & Gallistel, 1993). A minimum of two consistent sigmoidal psychometric functions was obtained for each subject by determining the rate of NPs across 10 different durations of the stimulation train (see Fig. 1). Each psychometric session began with a 2 min warm up period in which the train duration was 700 ms. Psychometric data were then gathered while animals received 10 different train durations (100–1000 ms in 100 ms increments) each lasting 30 s, presented in random order until six repetitions of each duration had been presented. The rate of NPs varied as a function of the duration of stimulation. A Weibull function relating NP rate vs. train duration was calculated for each session:
where y is NP rate estimates derived from the fitted function, x correspond to the train durations, L and U are the lower and upper asymptotes of the fitted equation, T is the train duration when y is midway between L and U (i.e., threshold), and S is a shape parameter. If the function was not sigmoidal, then the pulse frequency was adjusted. The pulse frequency that produced 2 days of consistent sigmoidal functions was chosen for each subject. The “high” reward train duration was defined as the train duration that elicited the maximum rate of NPs. The “low” reward train duration was defined as the train duration that produced a response rate at, or close to, 50% of the maximum response.
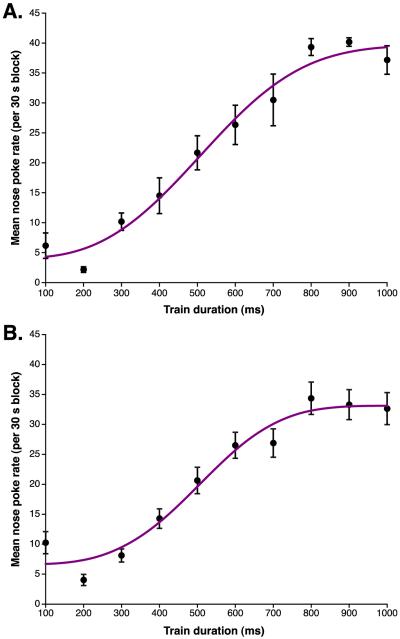
Fig. 1.
Psychometric functions of nose poke rates over 10 different train durations (100–1000 ms in 100 ms increments). Each train duration was given for 30 s in randomized order. A Weibull function was fitted to the nose poke responses. (A) Example of a psychometric function from a single subject. (B) Average psychometric function from the last session of all experimental subjects prior to training.
In the third and final phase of the experiment, subjects underwent tone training once a day. Each tone training session consisted of 51 trials with a 45 s average intertrial interval (ITI, end of one tone to start of next tone). During ITIs, NPs were reinforced on a 1:1 fixed ratio schedule with the low reward train duration. For each trial, a pure tone (2.0 kHz, 20 s, 70 dB SPL, cosine-shaped 10 ms rise/fall times [10–90% of maximum]) was presented as the conditioned stimulus (CS) using Tucker–Davis Technologies (TDT, Alachua, FL) System 3 hardware controlled by Coulbourn hardware and software. NPs made during the first 10 s of the tone continued to deliver stimulation at the low duration of rewarding stimulation. However, NPs made during the last 10 s of the tone received the high duration of rewarding stimulation (see Fig. 2). Training continued until either asymptotic tone-controlled NP behavior was achieved with three consecutive sessions of stable performance, or nose poking during the tone remained substantially irregular after a minimum of 11 sessions. Subjects then underwent a terminal neurophysiological session to map the frequency organization of A1.
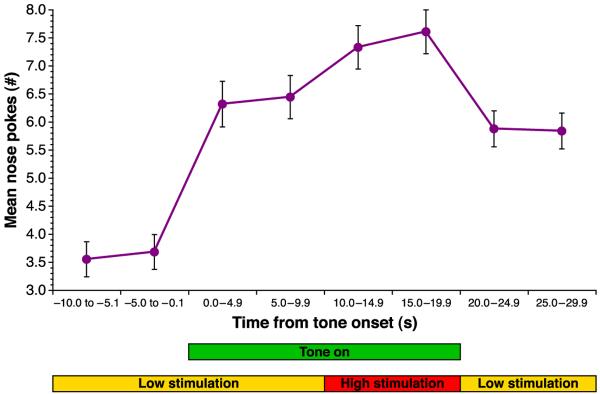
Fig. 2.
Example of response from a tone-controlled subject. The subject received a low reward for each nose poke during intertrial intervals and the first 10 s of a 20 s tone. During the last 10 s of the tone, the subject received high reward. Although the magnitude of brain stimulation reward did not change during the first 10 s of the tone, the mean number of nose pokes increased, demonstrating conditioned control of NPs by the tone, i.e., anticipation of the larger reward.
Tone control was defined as a statistically-significant increase in the rate of NPs during the first 10 s of the CS tone presentation (preceding the increase in reward duration) compared to the 10 s immediately preceding tone onset. Data were analyzed using customized Matlab (MathWorks, Natick, MA), SPSS Statistics (Chicago, IL) and StatView (SAS Institute, Cary, NC) software. Trials that did not have NPs during both the pre-tone and tone periods were eliminated. Wilcoxon signed ranks test were used to analyze the behavior of each subject.
2.4. Electrophysiological recording
Neurophysiological recordings were obtained with animals under sodium pentobarbital anesthesia (50 mg/kg, i.p., Nembutal), in a double-walled acoustic chamber (IAC). Supplements (approximately 15 mg/kg, i.p., per hour) were administered throughout the terminal recording to maintain a state of areflexia as measured by suppression of the forepaw withdrawal, pinna and eye blink reflexes. Bronchial secretions were reduced by treatment with atropine sulfate (0.25 mg/kg, i.m.) prior to surgery. Core body temperature was maintained at 37 °C. The animal was placed in a stereotaxic frame, the area behind the pedestal was excised and the cisterna magna was opened to draw off cerebral spinal fluid in order to prevent cerebral edema. To permit removal of the ear bars while maintaining the subject's head in a fixed position, additional stainless steel screws were inserted into the calvarium and the pedestal plus screws were bonded to two 13 mm threaded aluminum hexagonal standoffs that were bolted to the stereotaxic frame. For naïve subjects, six anchoring screws were inserted into the calvarium and the pedestal was constructed around them at this time. Bone and dura were removed over the right auditory cortex and a speaker (Aiwa, San Diego, CA) was placed abutting the ear canal contralateral to the auditory cortex.
Extracellular multi-unit discharges were obtained with a four-electrode array of tungsten microelectrodes spaced 305–505 μm apart (0.2–2.0 MΩ, FHC, Bowdoin, ME) at a depth of 400–700 μm throughout the primary auditory cortex. Penetrations were accomplished with an Inchworm motor controller (model 8200, EXFO Burleigh Instruments, Victor, NY). Images of the surface of the cortex and the location of the points of penetration were captured with a digital camera (Coolpix 4500, Nikon, Melville, NY) attached to a zoom stereomicroscope (Nikon model SMZ660) so that the precise positions could be used to reconstruct the tonotopic maps using Illustrator (Adobe, San Jose, CA). Acoustic stimuli of pseudo-randomly presented pure tones of 0.5–53.8 kHz (0.25 octave spacing, 10 repetitions) and 0–80 dB SPL (10 dB spacing) were generated with TDT System 3 hardware and software. The 50 ms tone bursts (cosine-shaped 8 ms rise/fall times [10–90% of maximum]) were presented every 700 ms.
Frequency response areas (FRA) were produced offline for each penetration site using customized Matlab software. The threshold, characteristic frequency (CF, defined as an onset response to the stimulus frequency at the lowest threshold) and bandwidths at 10, 20, 30 and 40 dB above threshold were determined at each site if possible. Primary auditory cortex (A1) was defined conventionally as the auditory-responsive cortical area with lowest thresholds for pure tones and containing a general progression of low to high CFs along the posterior to anterior axis (i.e., tonotopic gradient). Anterior sites that constituted a reversal in the CF progression were considered to be a part of the anterior auditory field (AAF) and were excluded from A1 analysis (e.g., Berlau & Weinberger, 2008; Rutkowski, Miasnikov, & Weinberger, 2003; Sally & Kelly, 1988). The remaining borders of A1 were outlined by sites that had a lower threshold for noise than to tones, and thus were considered to lie outside of A1. Voronoi tessellation maps were created using the CFs and coordinates of the penetrations. The proportional areas of half-octave frequency bands were calculated for each subject.
2.5. Histology
After recording, all animals were sacrificed with a sodium pentobarbital euthanasia solution (Euthasol, Virbac Animal Health, Fort Worth, TX). Experimental subjects were perfused through the heart with saline followed by 10% buffered formalin (Fisher Scientific, Hampton, NH) and the brain removed. Following several days of post-fixation in a 0.8 M sucrose/formalin solution for subsequent tissue cryoprotection, the brain was blocked and sectioned at 50 lm intervals with a freezing microtome. The sections were mounted onto gelatin-coated slides, dried and stained with 0.25% thionin for Nissl substance to determine the AP, ML and DV coordinates of the tip of the stimulating electrode using Paxinos and Watson (2007).
3. Results
3.1. Psychometric testing
Before training, psychometric plots of NP rate vs. train duration were obtained from subjects for a minimum of 2 days. Weibull functions were then fitted to these plots. Fig. 1A gives an example of such a function for a single session and Fig. 1B provides the group-averaged plot using the data obtained on the final day of testing for the 11 experimental subjects, prior to the beginning of training. Asymptote was reached at 800–900 ms. Across the group, the mode of high reward duration was 900 ms and the mode of low reward duration was 400 ms. The group mean NP rate was significantly different between 400 and 900 ms (Wilcoxon signed ranks test, z = 2.93, p = .005). Additionally, the NP rate was significantly different between the highest low reward duration of 500 ms, and the lowest high reward duration of 800 ms (z = 2.93, p = .005).
3.2. Tone control of behavior
As noted in Methods, the 2.0 kHz tone was presented while the subjects were nose poking to receive the low duration of reward. The tone was given for 20 s with the high duration of reward present during the last 10 s. Naturally, the NP rate increased when the reward duration was increased. However, as noted in Methods, conditioned tone control would be evident if the subjects increased their NP rate during the first 10 s of the tone, before the reward value was increased. Fig. 2 presents an example of the performance of a representative subject during a single session. Note that its mean NP increased during the first 10 s after tone onset although the reward duration had not yet increased, demonstrating conditioned control by the CS tone.
Seven of the eleven subjects exhibited this pattern of tone control, attaining a criterion of 3 consecutive days of significant increase in NP rate during the first 10 s after tone onset (Wilcoxon signed ranks test, all p < .05). This criterion was attained for these subjects in a mean of 9.3 ± 1.6 daily sessions. Four subjects did not meet this criterion (all p > .23). This led to the categorization of two groups: subjects that successfully acquired tone-controlled behavior (TC, n = 7) and subjects that failed to acquire tone-controlled behavior (NTC, n = 4).
NP rate was analyzed during consecutive 5 s time periods starting 10 s immediately preceding tone onset and continuing until 10 s after tone offset (40 s total). A difference score was calculated by subtracting the baseline rate (mean of the two 5 s pre-tone onset periods) from the NP rate after the tone onset. A detailed analysis of behavior for both groups for the mean of the last five sessions prior to neurophysiological mapping is presented in Fig. 3.
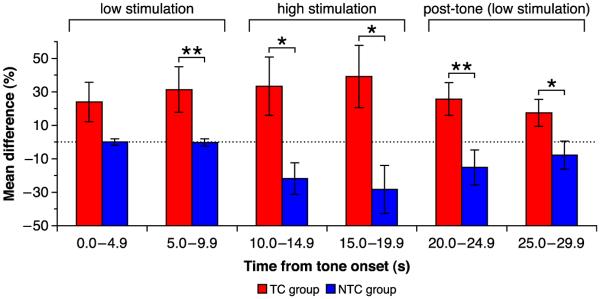
Fig. 3.
Average group performance of TC and NTC groups (percent difference from pre-tone baseline). Nose poke (NP) rate was calculated in consecutive 5 s periods from 10 s before tone onset to 10 s after tone offset. The 10 s before the tone constituted the pre-tone baseline. During the first 10 s after tone onset, the subjects received low train duration stimulation in response to NPs. For the last 10 s of the tone, the subjects received high train duration stimulation. After the tone, the stimulation returned to the low train duration. The TC group showed a significant increase in NP rate relative to the NTC group during the second 5 s after tone onset, and thereafter. *p ≤ .05, **p ≤ .01.
Note the qualitatively different patterns of behavior for the two groups. The TC group exhibited an increase in NPs during the first 10 s of the CS tone, maintained a high level of responding during high VTAstm, and showed the beginning of a decline toward baseline (pre-tone level) after the offset of tone and return to the low duration of reward. In contrast, the NTC group exhibited no change in NP rate during the first 10 s of tone presentation, then showed a decline in response when NPs produced the high reward duration, finally increasing toward baseline after tone offset. Their decrease in NP rate during high VTAstm might reflect distraction because they did not regard the tone as a signal (it had no control during the first 10 s), so that the high reward duration would have occurred suddenly and without warning for NTC animals.
A direct comparison of NPs for the two groups revealed that, while the TC group exhibited a greater percent change from baseline than the NTC group during the first 5 s of tone presentation, this difference did not quite reach statistical significance (Wilcoxon–Mann–Whitney test [WMW]; 0.0–4.9 s: z = 1.89, p = .07). However, the TC group did exhibit significantly greater increase in NP rate during the next 5 s, before the VTA reward duration was increased (5.0–9.9 s: z = 2.46, p = .01). This indicates that the tone exerted differential control on NPs in the two groups. The TC group also exhibited a greater change in NPs when the reward duration was increased during the next two 5 s periods (10.0–14.9 s: z = 2.27, p = .02; 15.0–19.9 s: z = 2.27, p = .02) and also after tone offset and a return to the low duration of reward (20.0–24.9 s: z = 2.65, p = .006; 25.0–29.9 s: z = 2.08, p = .04).
Insofar as the first 10 s of tone presentation provides for the critical test of tone control of NPs, we conducted an analysis of this period to determine if the groups differed in the pattern of their NPs. Within-group comparisons of the first and second 5 s periods revealed that the TC group exhibited a significant increase in NP rate (WMW, z = 2.20, p = .028) whereas the NTC group did not (z = 0.00, p > .999).
The reasons for the failure of the NTC group to develop tone control are not apparent. During the phase of psychophysical testing, their behaviors were indistinguishable. There was no significant difference in the mean durations of either the high VTAstm reward (TC = 885.71 ± 14.29 ms, NTC = 900 ± 0.0 ms; WMW, z = 0.76, p = .45) or low reward (TC = 400 ± 21.82 ms, NTC = 450 ± 28.87 ms; WMW, z = 1.33, p = .18). Furthermore, the groups did not differ in the pulses per second of stimulation received (TC = 26.8 ± 3.98, NTC = 28.0 ± 4.90; WMW, z = 0.19, p = .85). Not only were these VTAstm parameters maintained during training, but their NPs did not differ within the training phase during the intertrial (intertone) intervals (TC = 1.70 ± 0.30, NTC = 1.37 ± 0.38 [nose pokes/5 s period]; WMW, z = 0.57, p = .57, last five sessions). Finally, the groups did not differ in the number of days of training (TC = 15.71 ± 1.80, NTC = 14.50 ± 1.76, mean ± s.e.; WMW, z = 0.28, p = .78).
3.3. Histology
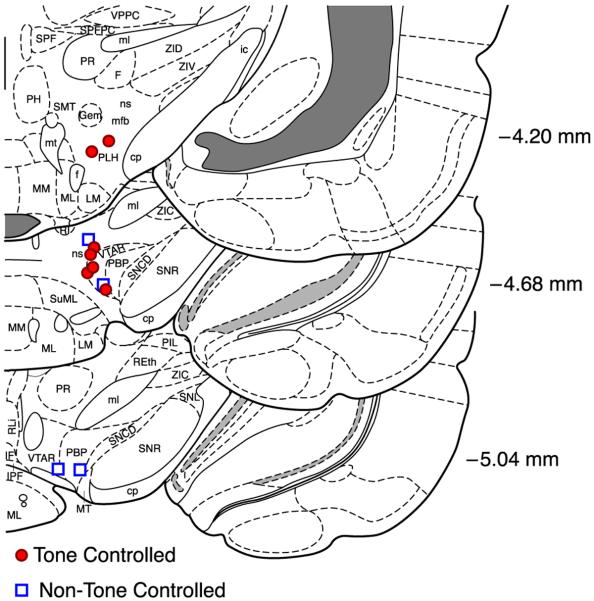
There were no significant differences in electrode placements across the AP, ML, or DV dimensions between the TC and the NTC groups (unpaired t-tests, all p > .05) (Fig. 4).
Fig. 4.
Location of stimulating electrodes. Reconstructed from histology, the sites of the tips of the electrodes are represented by closed circles for TC subjects and open squares for NTC subjects. The stimulating sites of the TC and NTC groups were not statistically different in AP, ML or DV planes. Some abbreviations: mfb, medial forebrain bundle; ns, nigrostriatal bundle; PBP, parabrachial pigmented nucleus of the VTA; PLH, peduncular part of lateral hypothalamus; VTAR, ventral tegmental area, rostral part (from Paxinos & Watson, 2007).
3.4. Electrophysiological mapping
The primary auditory cortices of the TC (n = 7), NTC (n = 4) and Naïve (n = 9) groups were mapped and the area of characteristic frequency distribution was determined for half-octave bands. Table 1 summarizes the half-octave bands and the number of penetrations (recording sites) for each of the three groups.
Table 1.
Number of penetrations recorded from the TC, NTC and karthi Naïve groups in half-octave frequency bands.
| Frequency band (kHz) | Frequency range (kHz) | TC group (n = 7) | NTC group (n = 4) | Naïve group (n = 9) |
|---|---|---|---|---|
| 0.7 | 0.595–0.840 | 1 | 0 | 2 |
| 1.0 | 0.841–1.189 | 13 | 2 | 2 |
| 1.4 | 1.190–1.681 | 9 | 5 | 12 |
| 2.0 | 1.682–2.378 | 33 | 9 | 17 |
| 2.8 | 2.379–3.363 | 8 | 7 | 12 |
| 4.0 | 3.364–4.756 | 5 | 5 | 19 |
| 5.7 | 4.757–6.727 | 18 | 5 | 37 |
| 8.0 | 6.728–9.513 | 13 | 3 | 17 |
| 11.3 | 9.514–13.454 | 27 | 15 | 20 |
| 16.0 | 13.455–19.027 | 30 | 17 | 58 |
| 22.6 | 19.028–26.908 | 13 | 0 | 30 |
| 32.0 | 26.909–38.054 | 42 | 31 | 38 |
| 45.3 | 38.055–53.817 | 25 | 27 | 44 |
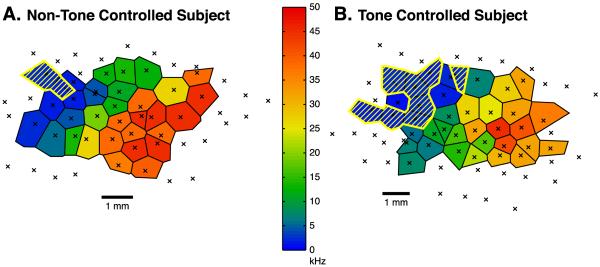
The TC group had a greater mean percentage of area devoted to the half-octave band centered on the CS frequency of 2.0 kHz than either the NTC or the Naïve groups. Fig. 5 presents examples of frequency maps for individual subjects in the TC and NTC groups. The polygons representing this CS-centered frequency band are outlined and hatched. Note that the NTC subject's area is clearly smaller than that of the TC subject.
Fig. 5.
Tonotopic maps (Voronoi tessellations) in the primary auditory cortex showing the organization of characteristic frequencies (CFs) for representative subjects within the NTC and TC groups. Recording sites are marked by crosses. Polygons outline the areas representing the CFs, with the color representing the frequency as denoted by the color bar. Regions indicated by yellow hatch lines denote the representational areas for CFs within the 2.0 kHz CS tone ± 0.25 octave band (half-octave = 1.682–2.378 kHz). Crosses not surrounded by colored polygons represent sites classified as outside of A1. Scale bar = 1 mm. (A) Example of a NTC subject; the representational area of CFs within the 2.0 kHz half-octave band was 5.8% of the primary auditory cortex. (B) Example of a TC subject; note the larger representational area for the CS half-octave band, which was 22.6% of the total area of A1.
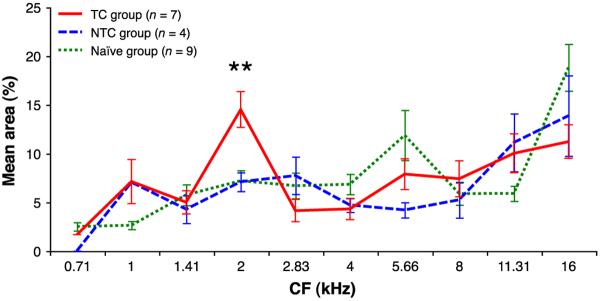
A statistical analysis of the mapping of representational area showed a significant difference among the three groups in the 2.0 kHz band (Kruskal–Wallis, χ2 = 10.82, p = .0045). Post-hoc analyses (Wilcoxon–Mann–Whitney tests) showed that the TC group was significantly different from both the NTC and Naïve groups (TC vs. NTC: z = 2.457, p = .0140; TC vs. Naïve: z = 3.017, p = .0026) but there was no significant difference between the NTC and Naïve groups (z = 0.000, p > .9999). The only significant difference among groups was at the CS frequency band (all other frequency bands, Kruskal–Wallis, p > .05) (Fig. 6 and Table 2).
Fig. 6.
Mean percent area of representation of half-octave frequency bands for TC, NTC and Naïve groups. For clarity, only data for the lowest 10 bands are shown. The TC group showed a significant increase in percent area represented at the 2.0 kHz half-octave band compared to the NTC and Naïve groups. There were no other significant differences. **TC vs. NTC, p = .0140; TC vs. Naïve, p = .0026.
Table 2.
Mean percent area frequency representation for TC, NTC and Naïve groups in half-octave bands.
| Frequency band (kHz) | Frequency range (kHz) | TC group (n = 7) | NTC group (n = 4) | Naïve group (n = 9) |
|---|---|---|---|---|
| 0.7 | 0.595–0.840 | 1.64 | - | 2.48 ± 0.42 |
| 1.0 | 0.841–1.189 | 7.09 ± 2.26 | 7.02 | 2.62 ± 0.39 |
| 1.4 | 1.190–1.681 | 4.96 ± 1.19 | 4.26 ± 1.43 | 5.74 ± 1.07 |
| →2.0 | 1.682–2.378 | 14.47 ± 1.83* | 7.07 ± 0.97 | 7.18 ± 1.07 |
| 2.8 | 2.379–3.363 | 4.12 ± 1.14 | 7.72 ± 1.92 | 6.68 ± 1.32 |
| 4.0 | 3.364–4.756 | 4.28 ± 1.09 | 4.68 ± 0.71 | 6.83 ± 1.03 |
| 5.7 | 4.757–6.727 | 7.85 ± 1.58 | 4.19 ± 0.79 | 11.85 ± 2.58 |
| 8.0 | 6.728–9.513 | 7.38 ± 1.84 | 5.22 ± 1.83 | 5.85 ± 1.14 |
| 11.3 | 9.514–13.454 | 9.98 ± 1.99 | 11.10 ± 2.97 | 5.87 ± 0.78 |
| 16.0 | 13.455–19.027 | 11.19 ± 1.72 | 13.85 ± 4.13 | 18.79 ± 2.40 |
| 22.6 | 19.028–26.908 | 6.35 ± 1.65 | - | 10.84 ± 1.28 |
| 32.0 | 26.909–38.054 | 17.64 ± 2.55 | 27.01 ± 7.27 | 11.95 ± 2.50 |
| 45.3 | 38.055–53.817 | 15.20 ± 3.16 | 19.92 ± 7.27 | 13.65 ± 1.97 |
Wilcoxon–Mann–Whitney; TC vs. NTC, p = .0140; TC vs. Naïve, p = .0026.
Parameters other than area of frequency representation were analyzed across the three groups. The absolute threshold and the bandwidths at 10, 20, 30 and 40 dB above threshold were examined at the half-octave band resolution. This was comprised of 65 statistical tests, i.e., five neural measures across 13 half-octave bands. Kruskal–Wallis one-way non-parametric analyses showed evidence for only two significant effects: the 1.0 kHz band for threshold (p = .03) and the 45.3 kHz band for bandwidth at 40 dB (p = .05). As this number of significant outcomes was expected by chance, and given the lack of any systematic relationship between them, these particular data are considered not to be functionally significant.
4. Discussion
4.1. Validity of the findings
There are two major findings. First is the demonstration that a tone can gain control over an instrumental behavior that anticipated delivery of a substantial increase in the value of a powerful reward, electrical stimulation of the VTA. Second, and of greater importance, subjects for whom the 2.0 kHz tone gained control of behavior also developed a highly specific increase in the area of representation of this same frequency within the primary auditory cortex.
We interpret the behavioral data as demonstrating tone control of nose poke behavior on the basis of two factors. First, the reward value was equated across subjects using psychophysical procedures to identify, separately for each rat, the train duration supporting half-maximal responding and the train duration supporting maximal responding. The half-maximal train duration was then used to maintain ongoing NPs, while the longer duration was used to provide the most powerful reward we could deliver under the current circumstances. These data provided validation of the reward value of VTA stimulation independent of the tone training situation. Second, 7 of 11 subjects significantly increased their NPs during the first 10 s of tone presentation. This was before the reward value was increased during the second 10 s of the tone. Therefore, we infer that the increase in NPs was caused by presentation of the tone because it signaled a forthcoming increase in reward value. In short, the first 10 s of the tone served as an effective Pavlovian conditioned stimulus, and Pavlovian conditioning modulated the instrumental behavior of nose poking. Such modulation has been reported in other tasks which used normal reinforcers rather than brain reward (reviewed in Rescorla, 1988; Rescorla & Solomon, 1967; see also Holland, 2004). Thus, the current behavioral findings fall within the domain of normative conditioning phenomena.
The key finding is that the specific expansion of the frequency band centered on the CS frequency of 2.0 kHz. The results provide strong support for the conclusion that this highly specific cortical plasticity is a consequence of prior tone control of behavior because the four subjects that failed to develop tone control (NTC group) also failed to develop cortical plasticity. In fact, the NTC group's cortical frequency organization was not different from that of naïve subjects. The dependence of specific representational cortical gain on the acquired signal importance of the CS tone is underscored by the lack of other differences between the TC and NTC groups. Their VTAstm parameters were statistically the same. Also, the reward efficacy of low VTAstm was identical during the intertrial periods lacking tone. Moreover, they received the same amount of training. Therefore, either or both exposure to the tone and behaviorally-dependent receipt of VTA stimulation are not sufficient to induce the CS-specific gain in 2.0 kHz representation.
An alternative account might be that animals that had an expanded representation of 2.0 kHz prior to training were able to develop 2.0 kHz tone control of behavior, whereas those that did not have such an expansion were unable to do so. However, the tonotopic organization of the NTC group was not different from that of the naïve animals. If seven out of the 11 experimental rats happened to have an expanded 2.0 kHz representation prior to training, then one would expect a similar proportion of the nine naïve rats to also have a similar expanded representation. However, the Naïve group did not exhibit such representation and in fact, its cortical organization was not different from that of the NTC group. Furthermore, the probability that seven rats had a pre-existing increased 2.0 kHz representation and that we happened to select 2.0 kHz among all other possible stimuli as a training frequency is extremely low. Therefore, the findings are most parsimoniously interpreted as indicating that successful tone control was responsible for the increased 2.0 kHz representation.
It is interesting to note that the NTC group did not have to acquire tone control to receive the high reward duration during the last 10 s of tone, so their behavior was in some sense more adaptive than the TC group, whose “extra” NPs preceding the increase in reward duration did not themselves yield a high reward duration. That the NTC group failed to obtain as much high reward as the TC group is probably attributable to distraction produced by the sudden increase in reward duration, for it was “un-signaled from their point-of-view”. Nonetheless, conditioned control in the TC group did yield more reward than the NTC group anyway, because it produced higher rates of response to low reward durations than otherwise. This behavioral pattern is consistent with the effects of powerful rewards.
The present findings extend support for the hypothesis that the primary auditory cortex is a site of the storage of the behavioral importance of acoustic frequencies, and that the amount of gain in representational area may serve as a “memory code” for acquired stimulus significance (e.g., Rutkowski & Weinberger, 2005). Although the current results are strongly suggestive, they were not intended to be decisive. Naturally, while correlational studies are essential in establishing relationships, interventionistic experiments of a more causal nature would provide a stronger test of these hypotheses. The direct induction of specific auditory associative memory by nucleus basalis stimulation, with concomitant induction of specific plasticity of frequency tuning in primary auditory cortex, is one such step in that direction (Miasnikov, Chen, & Weinberger, 2006).
4.2. Specificity of cortical plasticity
The degree of specificity of cortical plasticity is remarkable. Representational gain was limited to the CS frequency plus or minus 0.25 octaves, i.e., a 0.5 octave “window” in the frequency spectrum. Such a high degree of specificity would ordinarily require two-tone discrimination training with a gradually decreasing distance between rewarded and non-rewarded frequencies (e.g., see Mackintosh, 1974). That single-tone training could produce such a striking effect suggests that the use of brain reward was responsible. Moreover, it may have been the use of maximum durations of reward that exerted such a powerful effect.
This factor may shed light on the mechanisms by which tuning shifts are formed in the brain. It is already established that direct application of acetylcholine or anticholinesterases to the auditory cortex can produce specific tuning changes (Ashe, McKenna, & Weinberger, 1989; Metherate & Weinberger, 1990). Further, pairing a tone with stimulation of the cholinergic nucleus basalis, which itself is motivationally neutral (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008), induces both specific plasticity in A1 (Bakin & Weinberger, 1996; Bjordahl, Dimyan, & Weinberger, 1998; Kilgard & Merzenich, 1998) and specific associative behavioral auditory memory (McLin, Miasnikov, & Weinberger, 2002; Miasnikov et al., 2006). Thus, the VTA dopaminergic system might engage or “drive” the nucleus basalis to release an extraordinary “pulse” of acetylcholine into the auditory cortex to produce the highly specific cortical gains observed here. In addition, or alternatively, dopamine itself could have a powerful effect on the auditory cortex, as it appears to be released there during auditory learning (Stark & Scheich, 1997) and can also interact with other modulators in A1 (Atzori, Kanold, Pineda, Flores-Hernandez, & Paz, 2005).
4.3. Relationship to previous studies
The present study demonstrates that instrumental conditioning produces specific gains in cortical representation for rewarding stimulation of the VTA, and that this plasticity is tightly linked to tone control of behavior. Thus, it extends two previous reports of cortical plasticity formed by classical conditioning using VTA stimulation as the unconditioned stimulus.
Bao, Chan, and Merzenich (2001) reported a gain in the representational area of a tone that was followed by VTA stimulation. No plasticity was found with tone or VTAstm presented alone. However, the appropriate control for associative processes would have been to present both tone and VTA stimulation unpaired or randomly within the same animal. They also did not include any behavioral measures to assess actual learning. Hence, whether the results reflect associative processes in classical conditioning was unknown.
Kisley and Gerstein (2001) studied classical conditioning with rats under light ketamine anesthesia. The authors did find that tone paired with VTAstm produced associative CS-specific plasticity, including tuning shifts toward or to the frequency of the conditioned stimulus. Their tuning shifts, if extended across the tonotopic map, would be expected to produce an increase in representational area, as was found in the Bao et al. (2001) and present studies.
Kisley and Gerstein also reported that shifts lasted only a few days, based upon reduced successive correlations of all frequencies in their tuning curves. If specific plasticity in A1 was of such short duration, then it would be a poor candidate for involvement of stimulus control in addictive and other behaviors. However, Kisley and Gerstein did not actually measure tuning by tracking the best frequency over days, so the reduced correlations might have reflected fluctuations in weak responses to frequencies distant from the best frequency (BF). In contrast, a direct study of tuning stability, including analysis of the BFs, thresholds and bandwidths, found little or no changes in tuning or other features of neuronal response for periods of 2–4 weeks (Galván, Chen, & Weinberger, 2001). Moreover, CS-specific tuning shifts become even more, rather than less, specific over days (Galván & Weinberger, 2002) and are retained for weeks or months (Weinberger, Javid, & Lepan, 1993). The present study could not track the long-term retention of specific representational gains because detailed mapping of the auditory cortex is necessarily a terminal procedure. However, insofar as associative tuning shifts exhibit long-term retention, and as such shifts underlie gains in cortical area, it seems likely that the specific plasticity induced in this study is not transient.
4.4. Future directions
The present findings extend the domain of associatively-induced frequency-specific plasticity in the primary auditory cortex to gains in representational area using brain stimulation reward. However, they do not directly address the issue of the neural bases of stimulus control of behaviors involving powerful reinforcers. Future studies will need to link the expanded representation to additional behavior. One approach would be to induce different amounts of expansion by training subjects with different amounts of high brain reward. (In this initial study, we used the maximum reward based on the psychometric functions in order to avoid a false negative conclusion.) If the amount of expansion is a substrate of behavioral control, then the amount of behavioral control should be an increasing function of the area of representation. Extinction, both in the present task, and in a novel task using the same CS frequency, might then be retarded as a direct function of the amount of cortical gain. Regardless of the particular strategies used, the current findings do provide a way to address issues concerning the properties and neural substrates of stimulus control in addictive and other behaviors. Finally, the findings are consistent with the possibility that the amount of cortical territory that comes to best represent a stimulus may be a substrate for the ability of that stimulus to gain control over behavior in situations of particularly powerful rewards.
Acknowledgements
We thank Katarzyna M. Bieszczad for raw naïve subject data. This research was supported by a research grant from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIDCD) DC-02938 to N.M.W. and by the American Psychological Association/Diversity Program in Neuroscience predoctoral fellowship 5-T32-MH18882 to C.M.C.
References
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4(1):44–54. doi: 10.1002/syn.890040106. [DOI] [PubMed] [Google Scholar]
- Atzori M, Kanold PO, Pineda JC, Flores-Hernandez J, Paz RD. Dopamine prevents muscarinic-induced decrease of glutamate release in the auditory cortex. Neuroscience. 2005;134(4):1153–1165. doi: 10.1016/j.neuroscience.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research. 1990;536(1–2):271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412(6842):79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Berlau KM, Weinberger NM. Learning strategy determines auditory cortical plasticity. Neurobiology of Learning and Memory. 2008;89(2):153–166. doi: 10.1016/j.nlm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Campbell AW. Histological studies on the localisation of cerebral function. Cambridge University Press; Cambridge, UK: 1905. [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13(2):148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT. A history of the study of the cortex: Changes in the concept of the sensory pathway. In: Kimble GA, Schlesinger K, editors. Topics in the history of psychology. Vol. 1. Lawrence Erlbaum Associates; Hillsdale, NJ: 1985. pp. 305–387. Chap. 8. [Google Scholar]
- Edeline J-M. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: A critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Progress in Neurobiology. 1999;57(2):165–224. doi: 10.1016/s0301-0082(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Galván VV, Chen J, Weinberger NM. Long-term frequency tuning of local field potentials in the auditory cortex of the waking guinea pig. Journal of the Association for Research in Otolaryngology. 2001;2(3):199–215. doi: 10.1007/s101620010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiology of Learning and Memory. 2002;77(1):78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: Role of the corticofugal system. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology. 2004;30(2):104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. European Journal of Neuroscience. 2001;13(10):1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. Academic Press; New York: 1974. [Google Scholar]
- Mark TA, Gallistel CR. Subjective reward magnitude of medial forebrain stimulation as a function of train duration and pulse frequency. Behavioral Neuroscience. 1993;107(2):389–401. doi: 10.1037//0735-7044.107.2.389. [DOI] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6(2):133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proceedings of the Royal Society of London Series B: Biological Sciences. 1998;265(1397):649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. Journal of Neuroscience. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It's not what you think it is. American Psychologist. 1988;43(3):151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychological Review. 1967;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Miasnikov AA, Weinberger NM. Characterisation of multiple physiological fields within the anatomical core of rat auditory cortex. Hearing Research. 2003;181(1–2):116–130. doi: 10.1016/s0378-5955(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: Sound frequency. Journal of Neurophysiology. 1988;59(5):1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. British Journal of Pharmacology. 2008;154(2):369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Scheich H. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: A long-term microdialysis study of metabolites. Journal of Neurochemistry. 1997;68(2):691–697. doi: 10.1046/j.1471-4159.1997.68020691.x. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Diamond DM. Physiological plasticity in auditory cortex: Rapid induction by learning. Progress in Neurobiology. 1987;29(1):1–55. doi: 10.1016/0301-0082(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RM. Mind, brain and adaptation in the nineteenth century: Cerebral localization and its biological context from Gall to Ferrier. Clarendon Press; Oxford, UK: 1970. [Google Scholar]