Abstract
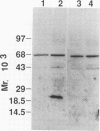
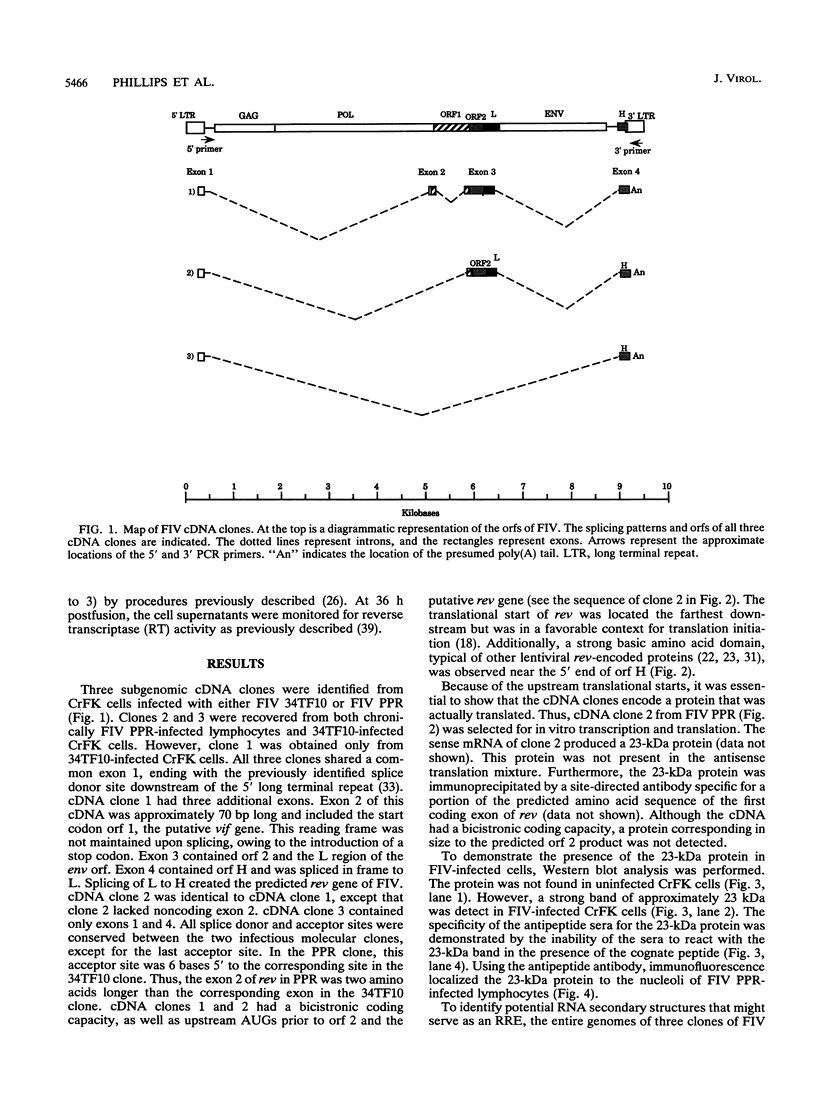
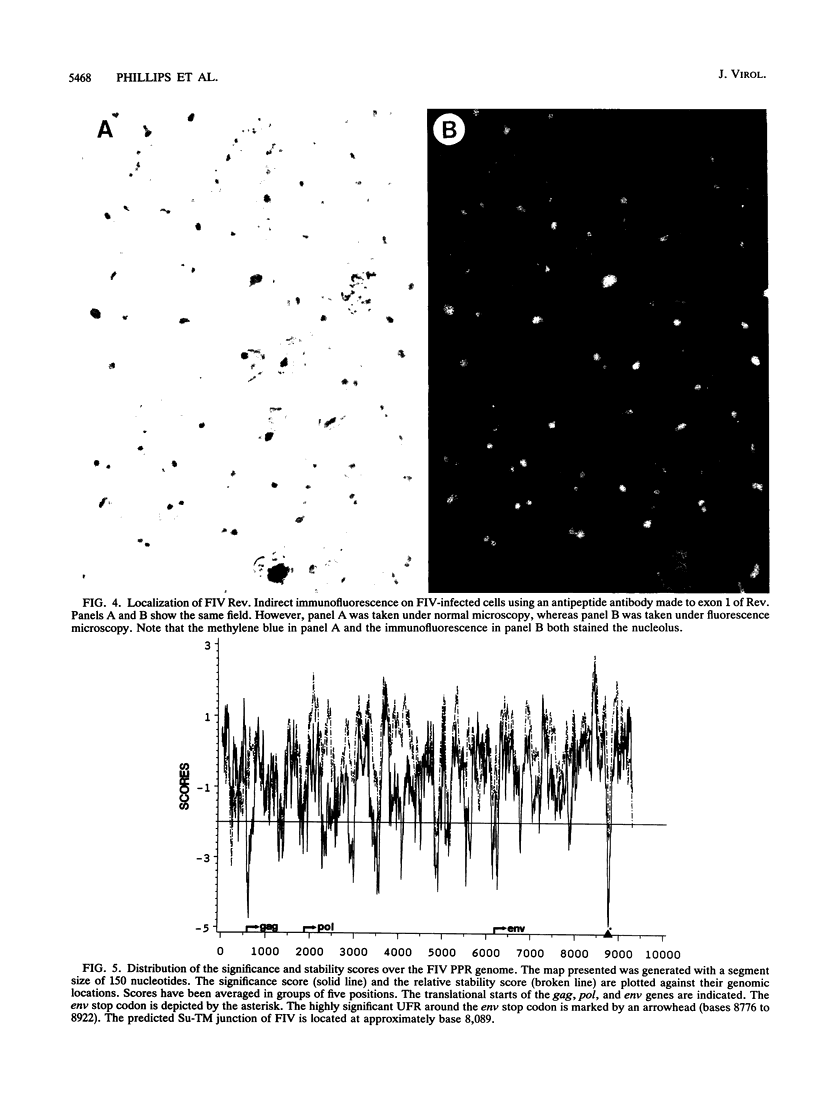
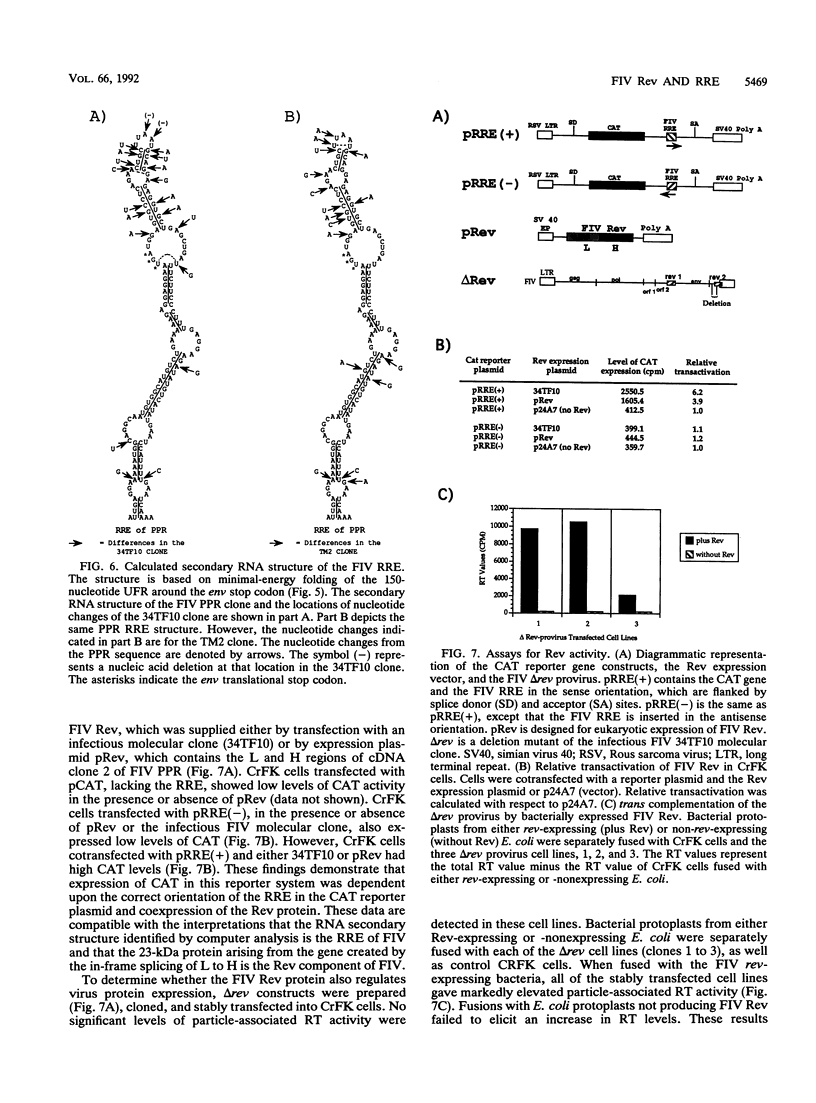
Spliced messages encoded by two distinct strains of feline immunodeficiency virus (FIV) were identified. Two of the cDNA clones represented mRNAs with bicistronic capacity. The first coding exon contained a short open reading frame (orf) of unknown function, designated orf 2. After a translational stop, this exon contained the L region of the env orf. The L region resides 5' to the predicted leader sequence of env. The second coding exon contained the H orf, which began 3' to env and extended into the U3 region of the long terminal repeat. The in-frame splicing of the L and H orfs created the FIV rev gene. Site-directed antibodies to the L orf recognized a 23-kDa protein in infected cells. Immunofluorescence studies localized Rev to the nucleoli of infected cells. The Rev-responsive element (RRE) of FIV was initially identified by computer analysis. Three independent isolates of FIV were searched in their entirety for regions with unusual RNA-folding properties. An unusual RNA-folding region was not found at the Su-TM junction but instead was located at the end of env. Minimal-energy foldings of this region revealed a structure that was highly conserved among the three isolates. Transient expression assays demonstrated that both the Rev and RRE components of FIV were necessary for efficient reporter gene expression. Cells stably transfected with rev-deleted proviruses produced virion-associated reverse transcriptase activity only when FIV Rev was supplied in trans. Thus, FIV is dependent on a fully functional Rev protein and an RRE for productive infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aepinus C., Voll R., Bröker M., Fleckenstein B. A rev/beta-galactosidase fusion protein binds in vitro transcripts spanning the rev-responsive element of human immunodeficiency virus type 1 (HIV-1). FEBS Lett. 1990 Apr 24;263(2):217–221. doi: 10.1016/0014-5793(90)81377-z. [DOI] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Peterlin B. M., Luciw P. A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Sharp P. A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989 Dec 1;59(5):789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Blume M., Lee S. G., Hung P. P., Hirsch V. M., Johnson P. R. The simian immunodeficiency virus (SIV) rev gene regulates env expression. J Med Primatol. 1990;19(3-4):167–176. [PubMed] [Google Scholar]
- Cullen B. R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991 Mar;65(3):1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Clements J. E. Characterization of a cDNA clone encoding the visna virus transactivating protein. Proc Natl Acad Sci U S A. 1989 Jan;86(2):414–418. doi: 10.1073/pnas.86.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Molineaux S., Clements J. E. Visna virus exhibits a complex transcriptional pattern: one aspect of gene expression shared with the acquired immunodeficiency syndrome retrovirus. J Virol. 1987 May;61(5):1325–1331. doi: 10.1128/jvi.61.5.1325-1331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Vazeux R., Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989 Jun 30;57(7):1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Hadzopoulou-Cladaras M., Cladaras C., Copeland T., Pavlakis G. N. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Heimer J., Hammarskjöld B., Sangwan I., Albert L., Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989 May;63(5):1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy S., Finch J. T., Gait M. J., Karn J., Singh M. Human immunodeficiency virus type 1 regulator of virion expression, rev, forms nucleoprotein filaments after binding to a purine-rich "bubble" located within the rev-responsive region of viral mRNAs. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7366–7370. doi: 10.1073/pnas.88.16.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. J., Hope T. J., Bond B. L., McDonald D., Grahl K., Parslow T. G. Minimal Rev-response element for type 1 human immunodeficiency virus. J Virol. 1991 Apr;65(4):2131–2134. doi: 10.1128/jvi.65.4.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomasu T., Miyazawa T., Furuya T., Shibata R., Sakai H., Sakuragi J., Fukasawa M., Maki N., Hasegawa A., Mikami T. Identification of feline immunodeficiency virus rev gene activity. J Virol. 1991 Aug;65(8):4539–4542. doi: 10.1128/jvi.65.8.4539-4542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Malim M. H., Cullen B. R., Maizel J. V. A highly conserved RNA folding region coincident with the Rev response element of primate immunodeficiency viruses. Nucleic Acids Res. 1990 Mar 25;18(6):1613–1623. doi: 10.1093/nar/18.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Fenrick R., Le S. Y., Maizel J. V., Cullen B. R. Functional comparison of the Rev trans-activators encoded by different primate immunodeficiency virus species. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8222–8226. doi: 10.1073/pnas.86.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Hauber J., Cullen B. R. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Cullen B. R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991 Apr 19;65(2):241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Mermer B., Felber B. K., Campbell M., Pavlakis G. N. Identification of trans-dominant HIV-1 rev protein mutants by direct transfer of bacterially produced proteins into human cells. Nucleic Acids Res. 1990 Apr 25;18(8):2037–2044. doi: 10.1093/nar/18.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N. L., Morrow P., Mosca J., Vahey M., Burke D. S., Redfield R. R. Induction of human immunodeficiency virus type 1 expression in chronically infected cells is associated primarily with a shift in RNA splicing patterns. J Virol. 1991 Mar;65(3):1291–1303. doi: 10.1128/jvi.65.3.1291-1303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Nicolaisen-Strouss K., Kumar H. P., Fitting T., Grant C. K., Elder J. H. Natural feline leukemia virus variant escapes neutralization by a monoclonal antibody via an amino acid change outside the antibody-binding epitope. J Virol. 1987 Nov;61(11):3410–3415. doi: 10.1128/jvi.61.11.3410-3415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Olsen H. S., Cochrane A. W., Dillon P. J., Nalin C. M., Rosen C. A. Interaction of the human immunodeficiency virus type 1 Rev protein with a structured region in env mRNA is dependent on multimer formation mediated through a basic stretch of amino acids. Genes Dev. 1990 Aug;4(8):1357–1364. doi: 10.1101/gad.4.8.1357. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips T. R., Talbott R. L., Lamont C., Muir S., Lovelace K., Elder J. H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990 Oct;64(10):4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Pavlakis G. N. Tat and Rev: positive regulators of HIV gene expression. AIDS. 1990 Jun;4(6):499–509. [PubMed] [Google Scholar]
- Saltarelli M., Querat G., Konings D. A., Vigne R., Clements J. E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990 Nov;179(1):347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. RNA amplification with transcript sequencing (RAWTS). Nucleic Acids Res. 1988 Jun 10;16(11):5197–5197. doi: 10.1093/nar/16.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley L. S., Brown P. H., Le S. Y., Maizel J. V., Clements J. E., Cullen B. R. Visna virus encodes a post-transcriptional regulator of viral structural gene expression. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7497–7501. doi: 10.1073/pnas.87.19.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley L. S., Malim M. H., Cullen B. R. Conserved functional organization of the human immunodeficiency virus type 1 and visna virus Rev proteins. J Virol. 1991 Jul;65(7):3877–3881. doi: 10.1128/jvi.65.7.3877-3881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Barban V., Quérat G., Mazarin V., Gourdou I., Sauze N. Transcription of visna virus during its lytic cycle: evidence for a sequential early and late gene expression. Virology. 1987 Nov;161(1):218–227. doi: 10.1016/0042-6822(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]