Abstract
Substance use disorder comorbidity in schizophrenia may reflect dysfunctional cortical-striatal-limbic circuitry commonly involved in the addiction process and the pathogenesis of schizophrenia. Rats with neonatal ventral hippocampal lesions (NVHL) demonstrate post-adolescent onset of schizophrenia-like symptoms and increased addiction vulnerability in paradigms using cocaine in adulthood. Here, we investigated response profiles of young adult NVHL vs. SHAM rats to ethanol, an addictive drug with many psychopharmacological effects divergent from those of cocaine, in a locomotor sensitization paradigm. Over 15 days of daily injections of saline, low (0.15 g/kg) or high (1.0 g/kg) doses of ethanol, NVHL rats showed stimulatory effects at the low dose compared to saline and high-dose conditions, while SHAM rats showed expected patterns of dose-dependent suppression of locomotor activity. In a challenge session 2 weeks later in which a moderate dose (0.25 g/kg) of ethanol was given to all subjects, NVHL rats with history of prior ethanol exposure showed greater locomotor activity consistent with installment of alcohol-induced sensitization not present in SHAMs. These findings provide further evidence of enhanced short- and long-term responsivity to abused drugs in a neurodevelopmental model of schizophrenia, and may reflect potentiation of common mechanisms of addiction shared between pharmacologically diverse addictive drugs.
Keywords: Schizophrenia, Alcohol, Sensitization, Addiction, Neonatal ventral hippocampal lesions, Dopamine, Dual diagnosis
1. Introduction
Even when excluding smoking, up to 50% of patients with schizophrenia meet criteria for substance use disorders (SUDs), putting them at 4.6 times the risk of the general population (Regier et al., 1990). Schizophrenia and comorbid SUDs are associated with lower quality of life ratings, decreased treatment compliance, increased violence, suicide, and homelessness, more frequent hospitalization and greater costs of care (Addington and Addington, 1996; Dixon, 1999). Ethanol use, like other types of addictive drugs including cocaine, amphetamine, and nicotine, are associated with SUDs in schizophrenia at rates greater than the general population, with up to 33.7% meeting criteria for alcohol abuse or dependence (Regier et al., 1990). As with SUD comorbidity in general, alcohol abuse/dependence in schizophrenia is also associated with greater psychiatric and medical complications (Bowie et al., 2005; Drake et al., 1989; Gerding et al., 1999).
Prefrontal cortical and limbic abnormalities underlying schizophrenia may potentiate motivational responses to addictive drugs mediated by mesolimbic dopamine (DA) systems and related circuitry, including the Nucleus Accumbers (NAc) (Chambers et al., 2001). Explorations of this hypothesis have employed the neonatal ventral hippocampal lesion (NVHL) rat model of schizophrenia in conjunction with preclinical addiction paradigms. The ventral hippocampus sends glutamatergic afferents directly into the NAc, and there influences cortical–striatal information processing that is also modulated by mesolimbic DA (Kelley and Domesick, 1982; O’Donnell and Grace, 1995). Adult NVHL rats show abnormal firing responses in the NAc upon stimulation of midbrain sources of mesolimbic DA (Goto and O’Donnell, 2002), suggestive that intrinsic or extrinsic (e.g. drug induced) DA efflux in this region produces abnormal physiological consequences.
Phenotypically, NVHLs produce a spectrum of schizophrenia-like symptoms, encompassing positive, negative, and cognitive symptom domains, many of which are ameliorated by neuroleptic treatment (Becker and Grecksch, 2003; Le Pen and Moreau, 2002; Lipska and Weinberger, 1994). Positive-like symptoms include post-adolescent-onset of hyperresponsivity to dopamine-mediated stimuli, including novelty, stress, and psychostimulants (Lipska et al., 1993). Social deficits (Becker et al., 1999; Sams-Dodd et al., 1997) and decreases in reward-seeking behavior (Le Pen et al., 2002) mimic schizophrenia’s negative symptoms. Cognitive symptoms include working memory, spatial learning, avoidance learning deficits (Chambers et al., 1996; Le Pen et al., 2000), and impairments of prepulse inhibition (Lipska et al., 1995) and latent inhibiton (Grecksch et al., 1999). In studies modeling dual diagnosis, NVHL rats acquire cocaine self-administration faster, exhibit binge-like behavior, and show greater drug-seeking after withdrawal and upon drug-induced relapse (Chambers and Self, 2002). Additionally, they show elevated baseline measures of natural reward-related impulsivity as a possible endophenotypic trait marker of addiction vulnerability that appears further accentuated by history of cocaine exposure (Chambers et al., 2005).
As a means to model dual diagnosis schizophrenia involving a non-psychostimulant addictive drug, we here examined the capacity of ethanol to induce locomotor sensitization in NVHL rats. Locomotor sensitization has long been utilized as an indirect model-paradigm of addiction, in which repeated doses of addictive drugs incrementally and semi-permanently up-regulate behavioral responsivity to the drug, akin to what is thought to occur with induction of pathological motivation for drug use (Robinson and Berridge, 1993). As a possible correlate of increased addiction vulnerability, NVHL rats show enhanced response patterns over both short and long-term courses of repeated cocaine injections (Chambers and Taylor, 2004). Compared to the psychostimulant cocaine, which is known to have pharmacologic effects that mostly target monoamine transporter systems, ethanol is primarily a central nervous system depressant with complex effects involving a wider range of transmitter systems, including GABA, glutamate and the mono-amines (Faingold et al., 1998). Although both ethanol and cocaine have been shown to increase mesolimbic DA transmission (Di Chiara and Imperato, 1988; Gessa et al., 1985; Imperato and Di Chiara, 1986), ethanol-induced locomotor activity and locomotor sensitization to ethanol has proven difficult to demonstrate in rats, likely owing to its predominant sedative effects. However, the biphasic actions of ethanol, in which low doses activate and high doses sedate, are well documented, particularly in mice (Dudek et al., 1991; Phillips et al., 1995). Hoshaw and Lewis (2001) managed to show ethanol sensitization in the Sprague-Dawley rat strain using a subgroup that were high responders to novelty, and by using a challenge dose of ethanol (0.25 g/kg) lower than the relatively more sedating induction dose (1 g/kg). Based on these findings, as a means for optimizing detection of ethanol sensitization in NVHL vs. SHAM rats, we used a similar approach in which saline, low (0.15 g/kg) or high doses (1.0 g/kg) of ethanol were given i.p. in 15 daily injections, followed by a moderate challenge dose (0.25 g/kg) to all subjects 2 weeks later. The low (0.15 g/kg) dose was included, as this dose given i.v. has previously been shown to be near the lower dose limit for stimulating the ventral tegmental area source of mesolimibic DA (Gessa et al., 1985). Based on Bloom et al. (1982) these doses given i.p. to naïve male rats produce estimated blood alcohol concentrations ranging <0.02% (0.15 g/kg), 0.02–0.04% (0.25 g/kg), and 0.08–0.12% (1 g/kg), where mild to moderate levels of intoxication occur with the latter two doses (Chuck et al., 2006).
2. Materials and methods
2.1. Subjects and NVHL surgeries
Pregnant Sprague-Dawley rats (Harlan, Indianapolis, Indiana) arrived in the laboratory at 14–15 days gestation and were individually housed on a 12-h light/dark cycle with ad libitum access to food and water. Seven days after birth (PD 7), male pups weighing 15–18 g were removed for 1–2 h for surgery according to the protocol developed by Lipska et al. (1993). Pups were randomly assigned sham or lesion status and anesthetized by hypothermia on ice for 15–20 min. An anterior–posterior incision was made on the dorsal surface of the head, and pups were secured to a stereotaxic platform with tape. A 26S gauge Hamilton syringe needle was lowered bilaterally into the ventral hippopcampal formation (AP −3.0 mm, ML ±3.5 mm, VD −5.0 mm relative to bregma). Infusions were delivered over 135 s, after which the needle was left in place for an additional 3 min to prevent backflow. The lesion group received bilateral infusions of 0.3 μL of 10 μg/μL ibotenic acid (Sigma, St. Louis, Missouri) dissolved in artificial cerebrospinal fluid (CSF), while the sham group received only 0.3 μL of artificial CSF. The surgical wound was closed with Vetbond (3 M), and pups were warmed on a heating pad before return to their mothers in litters of 4–6 pups, balanced by lesion status. Pups and mothers were left undisturbed until weaning at PD 21; from PD 35 until the end of the experiment, animals were housed in pairs. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Indiana University Institutional Animal Care and Use Committee.
2.2. Locomotor activity
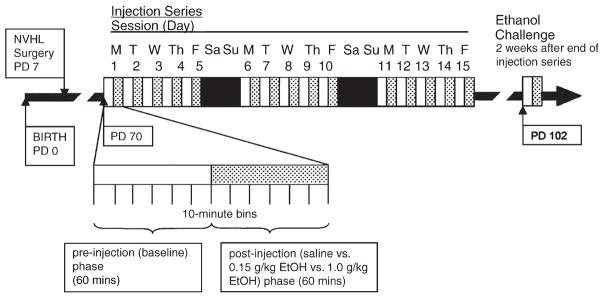
Starting at PD 70, locomotor behavior was assessed over fifteen 2-h sessions (1 session/day), each of which consisted of a 60-min baseline phase and a 60-min postinjection phase, all divided into 10-min bins (Fig. 1). After the baseline period ended, animals received intraperitoneal injections of either 0.9% saline (2.11 mL/kg), 0.15 g/kg ethanol (1.0 mL/kg of 15% v/v ethanol in water), or 1.0 g/kg ethanol (8.45 mL/kg of 15% v/v ethanol in water), according to randomly assigned drug treatment group. Two weeks later, in another 2-h session, all animals received a challenge dose of 0.25 g/kg ethanol (2.11 mL/kg of 15% v/v ethanol in water) after the baseline period.
Fig. 1.
Experimental timeline with respect to rat age. All rats received 0.25 g/kg ethanol in the challenge session.
Locomotor activity sessions took place in 43.2 cm × 43.2 cm×30.5 cm high recording chambers, which were made of clear plastic and equipped with three sets of infrared beam transmitters and detectors (Med Associates, Inc., St. Albans, VT). Translational motion (x, y dimension) transmitter and detector arrays were located 5 cm from the chamber floor, and rearing (z dimension) arrays were located 17.5 cm from the chamber floor. Each array consisted of 16 beams located 2.5 cm apart. Data were collected using Activity Monitor 5.0 software (Med Associates, Inc., St. Albans, VT), which was configured to separate small, quick movements (stereotypic beam break counts) from gross translational locomotion (ambulatory beam break counts, distance traveled) based on pilot work with cocaine-induced stereotypy.
2.3. Lesion verification
Following the challenge session, animals were anesthetized with isoflurane and decapitated. Brains were immediately frozen in isopentane, and 20 μm coronal sections were taken on a cryostat every 300 μm from −3.3 to −5.8 mm relative to bregma. Sections were mounted, fixed in chloroform, and stained with 0.5% thionin. Only lesioned animals with histologically confirmed bilateral damage to the ventral hippocampus as assessed microscopically were included in the analysis; animals with unilateral damage or damage extending beyond the hippocampus were excluded.
2.4. Data analysis
Locomotor distance traveled in the novel open field, and pre-and post-injection locomotion over the 15 sensitization days and in the challenge session were analyzed using separate two- or three-way repeated measures analysis of variance (ANOVA). Drug dose and/or lesion status served as between subjects factors, with bins or days serving as the within subjects repeated measure. Where appropriate to specify subgroup contributions to significant main effects, post-hoc testing using the least significant difference (LSD) method was employed. Statistical significance was assumed at p<0.05. Data in all figures are presented as mean±SEM.
3. Results
3.1. Lesion analysis
After histological analysis, seventeen rats with appropriate bilateral ibotenic acid lesions to the ventral hippocampus and 23 SHAM rats with no evidence of excitotoxic damage were included in data analysis. The included animals had been divided into the following groups during the experiment: NVHL-saline (n =6), NVHL-0.15 g/kg ethanol (n=6), NVHL-1.0 g/kg ethanol (n =5), SHAM-saline (n=7), SHAM-0.15 g/kg ethanol (n=7), and SHAM-1.0 g/kg ethanol (n=9).
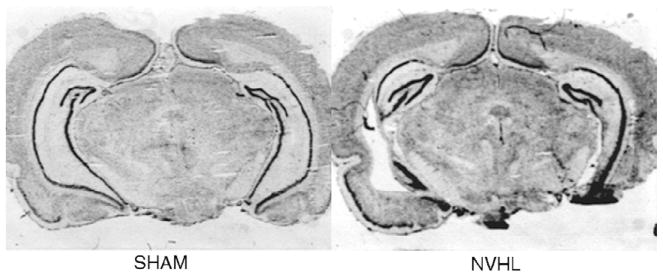
Fig. 2 shows typical coronal sections from SHAM and NVHL rats. Included NVHL rats all had hippocampal neuronal loss in at least two consecutive sections taken 300 μm apart. Smaller lesions were confined to the meeting of the dorsal and ventral regions of CA3 and extended approximately 600 μm posterior. Larger lesions encompassed more anterior dorsal sections of CA3, and extended ventral-caudally to ventral CA3, ventral CA1 and subiculum, and ventral-caudal DG. Most NVHL rats showed enlargement of lateral ventricles.
Fig. 2.
Photomicrographs of coronal sections through the hippocampus of SHAM and NVHL rats.
3.2. Novel field response and daily pre-injection activity
In response to the novel field (pre-injection phase on the first day of testing; Fig. 3), NVHL and SHAM rats showed similar declines in activity to baseline levels across the six pre-injection bins (bins: F(5,190)=367.6, p<0.001). The groups did not differ significantly in terms of main effect of lesion status or lesion status×bins interaction.
Fig. 3.
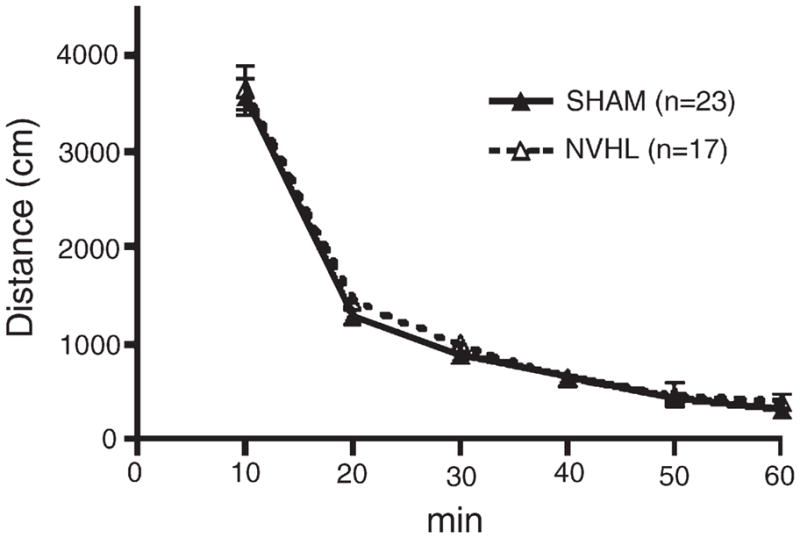
Pre-injection distance traveled during the initial locomotor sensitization session for SHAM and NVHL rats. No significant differences were found between NVHL and SHAM rats. Data are expressed as group means of distance traveled (cm)±SEM.
To assess baseline activity levels during the pre-injection period across the series of fifteen injections, data from the bin immediately preceding injections (bin 6) were analyzed. Bin 6 activity declined significantly across all groups over the 15 days (days: F(14,476)=5.6, p<0.001). Lesion status produced no significant main effect or interactions. The main effect of dose, though, was significant (dose: F(2,34)=7.8, p<0.01). Post hoc testing revealed that while none of 4 groups receiving saline or low doses of ethanol (0.15 g/kg) were mutually different, 1.0 g/kg ethanol significantly suppressed the overall pre-injection activity of both SHAM and NVHL rats compared to the SHAM-SAL group (p<0.05), and these two high dose groups were not mutually different.
3.3. Daily post-injection activity
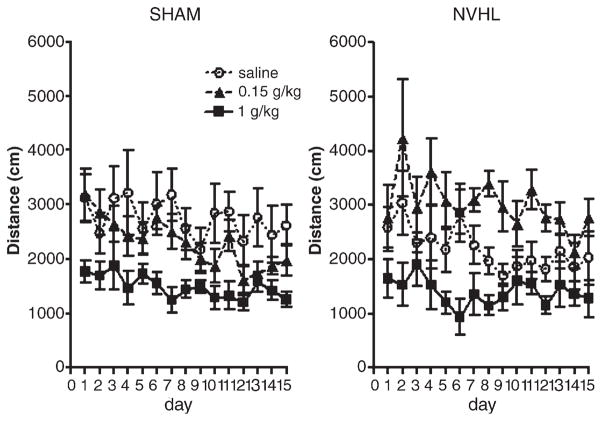
Fig. 4 shows group means of post-injection distance traveled across the fifteen sensitization sessions. Activity was significantly variable across days (days: F(14,476)=4.127, p<0.001) and dose of ethanol produced a significant main effect on activity (dose: F(2,34)=13.7, p<0.001). Ethanol dose differentially modulated activity levels of SHAM vs. NVHL rats, as indicated by the significant lesion status×dose interaction but lack of a lesion main effect (lesion×dose: F(2,34)=3.3, p<0.05). Post-hoc analysis revealed that NVHL rats receiving 0.15 g/kg ethanol showed stimulatory effects of the drug compared to NVHL rats receiving saline (p<0.05) or high doses (1.0 g/kg) of ethanol (p<0.001), while high doses depressed locomotor activity compared to saline (p<0.05). NVHL rats receiving 0.15 g/kg ethanol were marginally more active than SHAM rats receiving the same dose (p=0.053). For SHAM rats, low dose ethanol non-significantly tended to suppress locomotor activity compared to saline, while the high dose significantly suppressed locomotor activity compared to the 0.15 g/kg ethanol (p<0.05) or saline (p<0.001) groups.
Fig. 4.
Post-injection distance traveled during the 15-session injection series. NVHL and SHAM rats are depicted separately for clarity but all data was examined in one analysis. An overall repeated measures ANOVA revealed a main effect of dose [F(2,34)=13.7, p<0.001] and a significant interaction of lesion status and dose [F(2,34)=3.3, p<0.05]. In post-hoc testing, the 0.15 g/kg dose stimulated activity compared to saline in NVHL rats (p<0.05) but not in SHAM rats. The 1.0 g/kg dose was sedating compared to saline for both SHAM and NVHL groups (p<0.05). Data are expressed as group means of distance traveled (cm)±SEM.
In order to discern whether group differences in gross locomotor activity could relate to differential proportions of stereotypic activity, we examined the percentage of post-injection beam breaks that were classified as stereotypic rather than ambulatory according to the following formula: [stereotypic counts/(stereotypic counts+ ambulatory counts)]×100% (Table 1). A main effect of dose emerged (dose: F(2, 34)=18.708, p<0.001). Post hoc analysis revealed that NVHL and SHAM rats treated with 1.0 g/kg ethanol showed similar percentages of stereotypic activity across sessions while both these high dose groups demonstrated higher percentages (p<0.05) than all rat groups treated with either 0.15 g/kg ethanol or saline. Meanwhile, none of the groups receiving saline or low doses of ethanol were mutually different. A secondary analysis of the absolute number of stereotypic counts (rather than taken as a percentage of total movement) revealed no lesion or dose effects.
Table 1.
Percents (group mean±SEM) of total post-injection beam breaks classified as stereotypic during the 15-session injection series and challenge session
| Day | SHAM saline |
NVHL saline |
SHAM 0.15 g/kg |
NVHL 0.15 g/kg |
SHAM 1.0 g/kg |
NVHL 1.0 g/kg |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| 1 | 59.8 | 1.6 | 57.4 | 2.7 | 58.3 | 2.0 | 65.5 | 2.3 | 66.1 | 3.2 | 68.5 | 3.2 |
| 2 | 63.4 | 2.8 | 58.7 | 3.5 | 60.4 | 2.0 | 58.4 | 4.6 | 66.8 | 2.0 | 67.8 | 2.1 |
| 3 | 60.0 | 2.4 | 61.6 | 2.2 | 61.7 | 1.2 | 62.9 | 2.5 | 70.4 | 4.3 | 67.1 | 5.0 |
| 4 | 60.6 | 5.9 | 57.8 | 2.2 | 63.1 | 2.4 | 59.0 | 2.6 | 72.4 | 2.9 | 70.5 | 3.2 |
| 5 | 60.3 | 2.9 | 59.4 | 3.3 | 65.8 | 1.9 | 59.5 | 3.3 | 68.3 | 2.0 | 73.0 | 3.8 |
| 6 | 57.0 | 2.0 | 56.6 | 4.2 | 61.2 | 2.8 | 61.0 | 3.1 | 66.5 | 2.0 | 75.5 | 2.9 |
| 7 | 55.7 | 2.1 | 60.9 | 3.9 | 61.5 | 2.0 | 56.8 | 2.4 | 74.0 | 3.8 | 67.9 | 6.6 |
| 8 | 57.8 | 1.9 | 62.4 | 3.3 | 62.7 | 2.2 | 59.1 | 1.7 | 68.0 | 3.0 | 68.9 | 3.0 |
| 9 | 62.7 | 2.0 | 63.0 | 3.9 | 63.3 | 2.2 | 57.9 | 2.9 | 66.7 | 2.4 | 70.3 | 3.4 |
| 10 | 59.6 | 2.7 | 60.3 | 3.9 | 60.6 | 1.7 | 61.1 | 3.3 | 70.6 | 3.5 | 67.2 | 3.2 |
| 11 | 58.8 | 2.4 | 61.2 | 4.0 | 60.8 | 1.4 | 58.8 | 2.2 | 71.6 | 2.8 | 66.5 | 1.1 |
| 12 | 62.8 | 1.5 | 60.7 | 4.6 | 66.8 | 2.1 | 56.8 | 2.3 | 73.0 | 2.3 | 69.1 | 2.1 |
| 13 | 60.9 | 2.5 | 58.5 | 3.0 | 66.6 | 1.7 | 58.2 | 1.6 | 71.0 | 2.8 | 64.9 | 3.2 |
| 14 | 60.0 | 2.7 | 63.4 | 3.6 | 65.7 | 1.3 | 65.7 | 2.3 | 66.3 | 3.0 | 66.1 | 2.1 |
| 15 | 60.0 | 1.9 | 60.6 | 3.1 | 67.3 | 2.3 | 61.0 | 2.5 | 70.5 | 2.4 | 70.5 | 5.0 |
| Challenge | 59.4 | 1.9 | 59.1 | 3.9 | 62.9 | 1.6 | 57.0 | 2.9 | 59.7 | 2.1 | 57.0 | 4.4 |
3.4. Ethanol challenge session
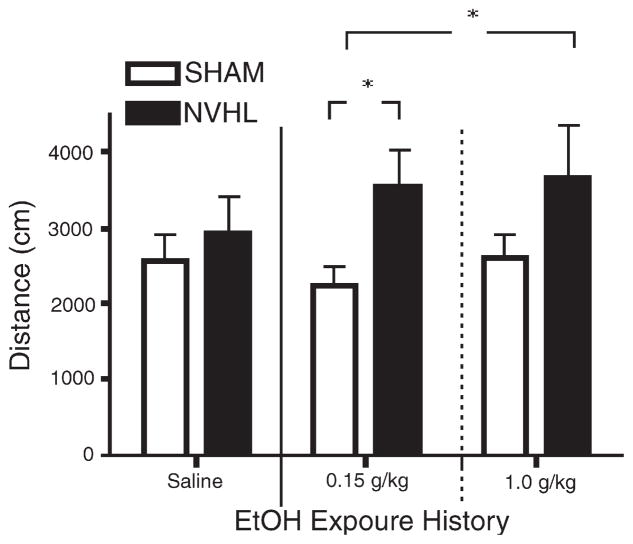
All rats, regardless of previous treatment, received a challenge dose of 0.25 g/kg ethanol during a challenge session 2 weeks after the last day of the initial injection series (Fig. 5). In analyzing post-injection distance traveled, a main effect of lesion status was observed (lesion status: F(1,34)=7.258, p<0.02). Post hoc tests further specified group differences where NVHL rats previously treated with either 0.15 g/kg or 1.0 g/kg ethanol showed greater activity than SHAM rats who received the 0.15 g/kg dose (p<0.05). NVHL rats previously treated with saline, and SHAM groups previously treated with saline, low or high doses of ethanol, were not mutually different.
Fig. 5.
Postinjection distance traveled during the challenge session, in which all groups received 0.25 g/kg ethanol. Two-way ANOVA revealed a significant effect of Lesion Status [F(1,34)=7.3, p<0.05]. Post-hoc analysis showed that NVHL rats with a history of receiving repeated doses of either 0.15 g/kg or 1.0 g/kg ethanol had significantly greater locomotor activation than SHAM rats with a history of 0.15 g/kg doses (*p<0.05), whereas NVHL and SHAM rats with a history of saline did not differ. Data are expressed as group means of distance traveled (cm)±SEM.
In order to confirm that equal baseline activity levels were achieved by all groups during the pre-injection phase of the challenge session, the distance traveled during the bin just prior to injection (bin 6) was examined. This analysis revealed no main effects or interaction of lesion status or dose of ethanol. Percentages of post-injection beam breaks during the challenge session categorized as stereotypic rather than ambulatory were also analyzed (Table 1). In contrast to the prior series of 15 injections, no dose or other group differences were observed in the challenge session.
4. Discussion
This study demonstrates that NVHL rats show altered short-and long-term responses to repeated ethanol injections. In contrast to SHAM rats, a low dose of ethanol (0.15 g/kg) given in daily injections is stimulatory in NVHL rats, while both NVHL and SHAM groups show similar depression of locomotion with high doses (1.0 g/kg). Two weeks after the initial series of injections, a moderate dose (0.25 g/kg) of ethanol produced an augmented psychomotor response in NVHL rats with histories of either low or high doses of ethanol.
To the extent that behavioral sensitization reflects the installment of an augmented behavioral response due to neuroadaptative processes resulting from prior drug history, these results indicate a greater sensitivity to ethanol sensitization in NVHL compared to SHAM rats. The effects of the initial low doses of ethanol in NVHL rats, and the results of the challenge session are analogous to the effects of cocaine (15 mg/kg), in which both SHAM and NVHL rats show sensitization, but NVHL rats show elevated short and long-term locomotor activity patterns compared to SHAM rats, consistent with enhanced installment of the sensitized phenotype (Chambers and Taylor, 2004). Taken together, these studies indicate that NVHL rats show elevated activity patterns to repeated injections of two addictive drugs, despite differential classifications as either a psychostimulant (cocaine) or central nervous system depressant (alcohol). Previous studies suggest that in animal models of dual diagnosis or alcohol addiction, elevated activity patterns to repeated doses of an addictive drug may represent a general trait marker of enhanced addiction vulnerability. Rats with either NVHLs or adult-age olfactory bulbecomy (model of affective-spectrum disorders) show elevated patterns of locomotor sensitization to cocaine (Chambers et al., 2004; Chambers and Taylor, 2004) as well as increased acquisition of psychostimulant self-administration (Chambers and Self, 2002; Holmes et al., 2002). Similarly, rats selectively bred for high preference for ethanol self-administration also show psychostimulant-like effects of ethanol (Agabio et al., 2001; Parvarinta and Korpi, 1993; Quintanilla, 1999; Rodd et al., 2004; Waller et al., 1986).
Although greater novelty-induced activation has also been identified as predictor of enhanced drug-self-administration (Piazza et al., 1989), novelty-induced activity prior to injection during the first locomotor sensitization session was indistinguishable between NVHL and SHAM rats in the current study (Fig. 3). NVHL hyperresponsivity to novelty has been observed in some circumstances but not others, whereas pharmacological stimulation of DA systems appears to be more reliable in provoking hyperactive responses in NVHL rats (Chambers and Taylor, 2004; Lipska et al., 1993). Nevertheless, based on the lack of group differences here, it is apparent that lesion-induced changes in the novelty response taken as baseline difference in locomotion could not have accounted for group differences in subsequent injection-induced behavior.
The stimulatory effect of low doses of ethanol specifically identified in NVHL rats was arguably the most interesting aspect of the initial 15 injection days. Analysis of activity in the 10-min bins prior to injection across the 15 sessions did not reveal significant differences in baseline activity based on lesion group at any dose, but did indicate that high dose regimens suppressed subsequent pre-injection activity for SHAM and NVHL rats alike. On the one hand, this result reinforces the interpretation that the hyperlocomotive effects of low-dose ethanol in NVHL rats was drug specific rather than reflecting differential baselines of activity. On the other hand it suggests that high doses of ethanol produce suppression of locomotor activity that lasts at least 24 h to a similar extent in both groups. Likewise, analysis of the proportion of post-injection stereotypic movements across the 15 sessions demonstrated no lesion based differences, while increased proportions of stereotypic movement were observed to a similar extent in both NVHL and SHAM rats receiving the high dose. Thus the stimulatory effects of low dose ethanol in NVHL rats also did not involve lesion-specific effects of the drug on different classes of movement. The mechanism of the suppression of daily pre-injection activity occurring at the high dose is unclear. Possibly, this represented a manifestation of acute daily alcohol withdrawal or ‘hangover’ as could be expected under certain conditions with high daily doses of ethanol (Sinclair and Gustafsson, 1987), and given that 1 g/kg doses of alcohol reduce to nearly 50% of maximal BACs as soon as 2 h after injection (Bloom et al., 1982). Alternatively, this effect could reflect an expectation response to the potentially aversive effects of higher doses of ethanol. Regardless, there were no group differences in pre-injection activity or post-injection proportions of stereotypic behavior occurring in the challenge session 2 weeks after the initial series of doses, after which time acute hangover effects or expectations of aversive stimuli may have disappeared.
The hyperresponsivity of NVHL rats to the low dose of ethanol over the initial series of 15 injections is reminiscent of studies using psychostimulants, and is suggestive of a differential DA-related effect that may be most easily demonstrable with low does of ethanol. Hyperlocomotion to initial injections of amphetamine and cocaine, which ranks among the broad family of addictive drugs as the most directly stimulatory of DA-systems, is a clear and highly replicable feature of NVHLs (Chambers and Taylor, 2004; Lipska et al., 1993; Wood et al., 1997). Higher doses of ethanol, where lesion-based differences were not seen, would be expected to introduce overriding sedative effects that would obliterate behavioral stimulatory effects carried by DA stimulation (Hoshaw and Lewis, 2001). DA release in the accumbens as measured by microdialysis has been shown to increase as dose of ethanol increases from 0.25 to 2.5 g/kg (Imperato and Di Chiara, 1986), despite increasing depression of locomotor activity (Gingras and Cools, 1996). The 0.15 g/kg dose used in this study, despite being comparatively low, has also been shown to increase firing rates in VTA neurons (Gessa et al., 1985). There is also evidence that human subjects show stimulatory responses to initial low doses of alcohol (Davidson et al., 2002), which could be a feature more easily modeled by NVHL as opposed to SHAMS in the rat species owing to lesion-induced alterations in DA-related circuit sensitivity. Notably, however, while low doses of ethanol produced psychostimulant-like effects in NVHL rats, the plot of their daily post-injection activity across the initial 15 days does not resemble the fairly regular incremental increases in locomotion characteristic of repeated daily psychostimulant dosing in healthy rats. Possibly, this is a complex effect of the non-transmitter-specific (‘dirty’) effects of ethanol involving GABA, glutamatergic as well as monoaminergic transmittier systems (Faingold et al., 1998), each of which may follow differential dynamics of tolerance and sensitization in daily dosing regimens.
Evidence for long-term sensitization in NVHL but not SHAM rats was observed in the challenge session two weeks after the final day of the 15-injection series, when all animals were given a moderate 0.25 g/kg ethanol dose (Fig. 5). Statistically, this effect was carried more strongly in NVHL vs. SHAM comparisons at the low as opposed to the high dose possibly suggesting that history of stimulatory doses more robustly carries long-term sensitizing effects than history of higher depressant doses. However, this possibility remains speculative owing to the relatively low number (n=5) of the NVHL-high dose group, which would weaken statistical effects at this dose. In any case, NVHL rats showed evidence for sensitizing effects with history of both doses of ethanol, even though the higher dose was acutely sedating to a similar extent in both NVHL and SHAM rats alike over the prior 15 sessions. Evidently, the choice of using a moderate dose of ethanol for the challenge session, as informed by Hoshaw and Lewis (2001), was sufficiently non-sedating for uncovering lesion-based sensitizing effects, while also not stimulatory enough to produce significant locomotor differences between NVHL and SHAM rats with histories of saline injections.
Several features of our results cast doubt on the possibility that the sensitization-like effects observed in NVHL rats in the challenge session merely represented tolerance to the sedative effects of ethanol. First, over 15 initial sessions, where the sedating effects of ethanol were most clearly evident at the high dose, NVHL and SHAM rats performed similarly, and their response curves were largely flat if not slightly decreasing over sessions. If tolerance to sedation were to manifest, it would be expected in the form of a pseudo-sensitization effect in which high dose rats show incremental increases in post-injection activity. Second, only the high-dose receiving rats showed significant suppression of pre-injection locomotion. To the extent that this could represent acute withdrawal effects, and tolerance and withdrawal are phenomenologically linked, this is not the dose where lesion-based differences in locomotor stimulation occurred. Third, the challenge session, in which long-term sensitization-like effects were observed, was carried out 2 weeks after the initial injection series, over which time significant tolerance effects would be expected to have dissipated. Here, and unlike in the initial injection series, neither lesion nor dose history produced differences in activity in the 10-min prior to injection.
Considering the neurobiological basis for enhanced long-term sensitization to both cocaine and ethanol in NVHL rats may have important implications for understanding dual diagnosis phenomena involving drug types of divergent psychoactive profiles in schizophrenia. Despite differential behavioral and neurochemical profiles among various addictive drugs including amphetamine, cocaine, opiates, nicotine, and ethanol, all these drugs share a common feature in their capacity to elicit mesolimbic DA-systems activation resulting in increased DA concentration in the NAc (Di Chiara and Imperato, 1988; Wise and Bozarth, 1987). DA is also heavily implicated in mediating neuroplastic changes in cortical–striatal circuits underlying incremental increases in either overt behavior or more occult motivational processes (Robinson and Berridge, 1993; Vanderschuren and Kalivas, 2000). Specifically, DA may play a key role in alcohol-induced behavioral sensitization routinely observed in mice (Broadbent et al., 2005), and may undergo neurochemical sensitization with alcohol in rats (Nestby et al., 1997). Together with evidence that NVHL rats respond robustly to agents that directly stimulate or antagonize DA systems (Lipska and Weinberger, 1994), this literature points to a general role of altered DA functionality in NVHL rats as a basis for increased markers of addiction vulnerability, and potentially, increased ethanol sensitization as observed in this study.
The specific nature of DA-systems dysfunction in NVHL rats, and the precise role of DA in behavioral and motivational sensitization in general remain uncertain. Since DA modulates glutamatergic and/or GABAergic transmission and related plasticity occurring in cortical-striatal circuits that directly control psychomotor behavior, alterations in these target substrates of DA transmission may be critical (Goto and Grace, 2005). Consistent with this view, studies examining evidence for concrete changes in DA transmission in NVHL rats have only produced subtle or largely negative results thus far (Chrapusta et al., 2003; Lillirank et al., 1999; Schroeder et al., 1999; Wan et al., 1996), while mounting evidence implicate neuroadaptations involving glutamate and GABA transmission in behavioral sensitization (Everitt and Wolf, 2002; Goto and Grace, 2005; Vanderschuren and Kalivas, 2000).
Numerous studies have identified alterations in both glutamate and GABAergic transmitter systems within cortical-striatal circuits in both schizophrenia (Benes and Berretta, 2001; Laruelle et al., 2003; Olney and Farber, 1995; Woo et al., 2004) and NVHL rats (Lipska et al., 2003; Schroeder et al., 1999; Stine et al., 2001). Moreover, alterations in glutamate and/or GABA systems may be present in patients with increased genetic susceptibility to alcoholism (Lappalainen et al., 2005; Petrakis et al., 2004). These conditions may represent a setting in which endogenous or drug-induced DA-efflux could be operating on an abnormal substrate (e.g. frontal cortical–ventral striatal circuits) to alter psychomotor sensitization phenomena and/or increase addiction vulnerability. Given the activity of ethanol as both a strong potentiator of the GABAA receptor and an NMDA antagonist (Faingold et al., 1998), we also cannot discount a primary, DA-independent, role for these transmitter systems in producing ethanol sensitization in NVHL rats.
In summary, this report finds that in repeated dose regimens of ethanol designed to minimize its predominant sedative effects, NVHL as opposed to SHAM rats show enhanced short-term activity and long-term sensitization responses, analogous to previous findings using the psychostimulant cocaine. Together, these results demonstrate that neurodevelopmental lesions leading to a schizophrenia-like syndrome accentuate the capacity of two addictive drugs, despite their differential classification as stimulants vs. depressants, to introduce long-term behavioral changes mediated by circuits closely linked with the process of addiction. Although these data are consistent with the idea that dual diagnosis schizophrenia involving several drug classes may represent common neurocircuit involvement underlying both primary psychiatric symptoms and increased addiction vulnerability (Chambers et al., 2001), further work is needed to more precisely elucidate the interactive roles of DA, glutamate and GABA in these processes. Since many features of the NVHL syndrome involving dopamine emerge after adolescence, further studies should examine how sensitizing regimens of addictive drugs given at different developmental stages (e.g. post-natal/peri-adolescence) might interact with the NVHL phenotype in adulthood. Characterization of the behavioral and neurobiological correlates of a wider variety of addictive drugs in this and other neurodevelopmental models of mental illness will be instructive toward finding more definitive and parsimonious treatments for dual diagnosis conditions emerging in peri-adolescent phases of development.
Acknowledgments
This work was funded by the National Institutes of Drug Abuse NIDA: K08 DA019850-01 and Indiana University (R.A.C.).
Footnotes
Publisher's Disclaimer: This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author’s benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution’s administrator.
All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier’s permissions site at:
References
- Addington J, Addington D. Substance abuse and cognitive functioning in schizophrenia. J Psychiatry Neurosci. 1996;22:99–104. [PMC free article] [PubMed] [Google Scholar]
- Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, et al. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–6. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G. Haloperidol and clozapine affect social behavior in rats postnatally lesioned in the ventral hippocampus. Pharmacol Biochem Behav. 2003;76:1–8. doi: 10.1016/s0091-3057(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Bernstein H-G, Hollt V, Bogerts B. Social behavior in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology (Berl) 1999;144:333–8. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bloom F, Lad P, Pittman Q, Rogers J. Blood alcohol levels in rats: non-uniform yields from intraperitoneal doses based on body weight. Br J Pharmacol. 1982;75:251–4. doi: 10.1111/j.1476-5381.1982.tb08780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Serper MR, Riggio S, Harvey PD. Neurocognition, symptomatology, and functional skills in older alcohol-abusing schizophrenia patients. Schizophr Bull. 2005;31:175–82. doi: 10.1093/jschbul/sbi001. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. Role of dopamine in behavioral sensitization to ethanol in DBA/2J mice. Alcohol. 2005;35:137–48. doi: 10.1016/j.alcohol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–16. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–94. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sheehan T, Taylor JR. Locomotor sensitization to cocaine in rats with olfactory bulbectomy. Synapse. 2004;52:167–75. doi: 10.1002/syn.20017. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward-related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology (Berl) 2005;179:470–8. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK. Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague-Dawley rats. Synapse. 2003;47:270–7. doi: 10.1002/syn.10179. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–61. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Davidson D, Hutchison K, Dagon C, Swift R. Assessing the stimulant effect of alcohol in humans. Pharmacol Biochem Behav. 2002;72:151–6. doi: 10.1016/s0091-3057(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Drake RE, Osher FC, Wallach MA. Alcohol use and abuse in schizophrenia: a prospective community study. J Nerv Ment Dis. 1989;177:408–14. doi: 10.1097/00005053-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–9. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, N’Gouemo P, Riaz A. Ethanol and neurotransmitter interactions-from molecular to integrative effects. Prog Neurobiol. 1998;55:509–35. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Gerding LB, Labbate LA, Measom MO, Santos AB, Arana GW. Alcohol dependence and hospitalization in schizophrenia. Schizophr Res. 1999;38:71–5. doi: 10.1016/s0920-9964(98)00177-7. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. Analysis of the biphasic locomotor response to ethanol in high and low responders to novelty: a study in Nijmegen Wistar rats. Psychopharmacology (Berl) 1996;125:258–64. doi: 10.1007/BF02247337. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–66. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–7. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Bernstein H-G, Becker A, Hollt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20:525–32. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Masini CV, Primeaux SD, Garrett JL, Zellner A, Stogner KS, et al. Intravenous self-administration of amphetamine in a rat model of depression. Synapse. 2002;46:4–10. doi: 10.1002/syn.10105. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Lewis MJ. Behavioral sensitization to ethanol in rats: evidence from the Sprague-Dawley strain. Pharmacol Biochem Behav. 2001;68:685–90. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–28. [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rats: an anterograde and retrograde-horseradishperoxidase study. Neuroscience. 1982;7:2321–35. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–8. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–58. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau J-L. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine, and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Martin JR, Jenck F, Moreau J-L. Spatial and associative learning deficits induced by neonatal excitotoxic hippocampal damage in rats: further evaluation of an animal model of schizophrenia. Behav Pharmacol. 2000;11:257–68. doi: 10.1097/00008877-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gaudet L, Mortas P, Mory R, Moreau J-L. Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2002;161:434–41. doi: 10.1007/s00213-002-1092-4. [DOI] [PubMed] [Google Scholar]
- Lillirank SM, Lipska BK, Kolachana BS, Weinberger DR. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999;106:183–96. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal exitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:65–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibiotn of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur J Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJMJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, et al. Ethanol, like psychostimulants and morphine, causes long-lasting hyper-reactivity of dopamine and acetylcholine neurons of the rat nucleus accumbens: possible role in behavioral sensitization. Psychopharmacology. 1997;133:69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to the nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Parvarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;44:127–33. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–82. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–78. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME. Effect of low doses of ethanol on spontaneous locomotor activity in UChB and UChA rats. Addict Biol. 1999;4:443–8. doi: 10.1080/13556219971434. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area Study. JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-salience theory of addiction. Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, et al. Low-dose stimulatory effect of ethanol during adolescence in rat lines selectively bred for high alcohol preference. Alcohol Clin Exp Res. 2004;28:535–43. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–10. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Grecksch G, Becker A, Bogerts B, Hoellt V. Alterations of the dopaminergic and gluatmatergic neurotransmission in adult rats with postnatal ibotenic acid hippocampal lesion. Psychopharmacology. 1999;145:61–6. doi: 10.1007/s002130051032. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Gustafsson K. Behavioral changes in rats on the day after acute ethanol intoxication. Alcohol. 1987;4:503–7. doi: 10.1016/0741-8329(87)90093-0. [DOI] [PubMed] [Google Scholar]
- Stine CD, Lu W, Wolf ME. Expression of AMPA receptor flip and flop mRNAs in the nucleus accumbens and prefrontal cortex after neonatal ventral hippocampal lesions. Neuropsychopharmacology. 2001;24:253–66. doi: 10.1016/S0893-133X(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Kalivas P. Alterations in dopaminergic and glutamatergic transmissioin in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24:617–23. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Wan R-Q, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behav Brain Res. 1996;78:211–23. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Woo T-UW, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Wood GK, Lipska BK, Weinberger DR. Behavioral changes in rats with early ventral hippocampal damage vary with age at damage. Brain Res Dev Brain Res. 1997;101:17–25. doi: 10.1016/s0165-3806(97)00050-3. [DOI] [PubMed] [Google Scholar]