Abstract
Hyaluronan (HA) is expressed by cells in bone marrow where it contributes to the regulation of hematopoietic homeostasis. In this study, we have demonstrated that exogenous low molecular weight HA (LMW HA) polymers mobilize leukocytes, but not hematopoietic progenitor cells, to peripheral blood within a 3 hour time period following HA administration. Mobilization of leukocytes correlated with increased extracellular MMP-9 concentrations induced by LMW HA, but not high molecular weight (HMW) HA. In contrast, HMW HA up-regulated TIMP-1 expression in bone marrow cells. In vitro, HMW HA did not influence SDF-1 – mediated chemotaxis of hematopoietic progenitors, whereas LMW HA polymers demonstrated inhibitory activity. These findings suggest that the effects of HA on cell motility depend on the size of the HA polymers and on the type of target cells.
Introduction
Mobilization of hematopoietic stem/progenitor cells (HSPCs) is currently a routine clinical procedure that enables the harvesting of HSPCs from peripheral blood and their further use for transplantation and tissue regeneration. Despite of the recent progress in HSPC mobilization strategies, it is generally agreed that further improvement of mobilization protocols is needed to achieve a better quantity and quality of harvested HSPC in a higher percentage of donors (Pelus 2008).
The protocols for HSPC mobilization are based on understanding the molecular mechanisms that regulate homing of these cells into the bone marrow and their retention within the niche. In particular, several chemokines, cytokines, growth factors, their receptors, adhesion molecules as well as hormones and proteases have been identified as contributors to the complex network that regulates migration of HSPCs (Lapidot and Petit 2002; Cottler-Fox, Lapidot et al. 2003; Levesque and Winkler 2008; Pelus 2008; Schulz, von Andrian et al. 2009). Apparently, agents used for HSPC mobilization interrupt these pathways and enable HSPC to detach from the niche, migrate through the matrix, egress from the bone marrow and enter the circulation.
In addition to the currently established pathways, CD44 has been implicated in the regulation of HSPC homing (Khaldoyanidi, Denzel et al. 1996; Vermeulen, Le Pesteur et al. 1998). However, the mechanisms by which CD44 contributes to the network regulating HSPC migration remain illusive because of the enormous complexity of the pathways mediated by the CD44 family of glycoproteins, which differ in the structure of their extracellular domain due to alternative splicing and different patterns of glycosylation. The complexity of the structure of the CD44 molecules correlates with a differential affinity for multiple ligands (Lesley, Hyman et al. 1997; Knudson 1998). A major ligand, hyaluronan (HA), a member of the glycosaminoglycan family, constitutes a substantial part of the bone marrow microenvironment where it participates in local extracellular matrix (ECM) assembly (Fraser, Laurent et al. 1997). In addition to its interactions with CD44, HA is implicated in specific receptor-ligand interactions with other molecules including RHAMM, HARE and TLR4 (Pilarski, Masellis-Smith et al. 1994; Zhou, Weigel et al. 2000) that consequently influence cell behavior including cell migration (Pilarski, Miszta et al. 1993; Masellis-Smith, Belch et al. 1996; Andreutti, Geinoz et al. 1999; Nilsson, Haylock et al. 2003). In addition to directly influencing cell motility, HA can contribute to the regulation of cell migration by affecting the expression and activation of MMPs. Depending on the cell type and the size of HA, the expression of different MMPs can be either up-regulated or down-regulated (Sakuma, Miyachi et al. 2000; Habara, Nakatsuka et al. 2002; Spessotto, Rossi et al. 2002; Isnard, Robert et al. 2003; Alaniz, Garcia et al. 2004; Fieber, Baumann et al. 2004).
Since RHAMM and CD44 are differentially expressed by bone marrow-derived steady-state HSPC versus G-CSF-mobilized HSPC, a potential role for HA in the mobilization of HSPCs was suggested, where CD44 serves as an anchoring molecule while RHAMM mediates cell mobilization (Pilarski, Pruski et al. 1999). We found that within a 3 hour time window LMW HA polymers mobilize leukocytes, but not hematopoietic progenitors, in peripheral blood. Leukocyte mobilization correlated with increased levels of extracellular MMP-9, which can be released by activated neutrophils. While HMW HA did not influence MMP-9 release, the expression of its natural inhibitor TIPM-1 was up-regulated in bone marrow cells. This study demonstrates that the effect of HA on cell migration depends on the size of the HA polymers.
Materials and Methods
Mice
BALB/c mice obtained from Jackson Laboratory (Bar Harbor, Maine, USA) were maintained under standard laboratory conditions. All experimental procedure were performed according to the NIH Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC). Eight week old females were used in all experiments. Where indicated mice were injected with 100µl PBS or 100µg HA (Sigma, CA and Lifecord, Chaska, MN) diluted in 100µl PBS. Both HA preparations were proven to be endotoxin-free. Mice were euthanized by a CO2 overdose. Blood was collected by aspiration from the heart. To harvest bone marrow cells, femurs and tibias were dissected, epiphyses were cut off with scissors at each end of the bone. The contents of the bones were flushed out with PBS supplemented with 5% FCS using a needle (21G) attached to a 1-ml syringe. To ensure the preparation of a single cell suspension, the cell suspension was aspirated several times through a smaller needle (25G). The cells were kept on ice until use.
Colony Forming Unit (CFU) Assay
For the in vitro assay, bone marrow cells (2 × 104 cells/ml) or peripheral blood cells depleted of erythrocytes (5 × 105 cells/ml) were mixed with semisolid methylcellulose medium supplemented with 10% FCS, 1% BSA, L-glutamine, 5 × 10−4 M 2-mercaptoethanol, 10ng/ml IL-3, 10ng/ml IL-6, 50ng/ml SCF, and 3 U/ml erythropoietin (StemCell Technologies, Canada). The cultures were incubated in a humidified incubator with 5% CO2 in air at 37°C for 7–14 days. Colonies were scored microscopically. For the in vivo spleen colony forming unit (CFUs) assay, peripheral blood cells were transplanted into lethally irradiated recipients as previously described (Till and Mc 1961). After 8 days, mice were sacrificed, spleens were collected, fixed in Tellesnicky’s solution and colonies (CFUs-8) were counted after several hours of fixation.
Transmigration Assay
The assay was performed in triplicate using 5-µm pore Transwell filters coated with Matrigel (Transwell, 24-well cell clusters; Costar, Boston, MA). Bone marrow cells (1 × 106) were suspended in 100µl migration medium (Ultraculture with 0.5% BSA) and loaded onto each Transwell filter (upper well). The inserts were then transferred to the lower well, containing 600µl of migration medium. Where indicated, the media was supplemented with 50ng/ml stroma derived factor-1 (SDF-1, Upstate Biotechnology, NY). HA was added into the culture media at a concentration of 100µg/ml. The plates were incubated at 37°C in 5% CO2. After 4 hours of incubation, the upper wells were removed, and the cells in the bottom compartment were collected, counted and examined for the presence of HSPC by CFU assays as described above.
Flow Cytometry
CD44, TLR2 and TLR4 antibodies were from (Sigma, CA and Strategic Biosolutions, DE). The RHAMM specific monoclonal antibody (clone 3T3.5) was provided by Dr. Linda Pilarski (University of Alberta, Canada). Cell surface associated HA was detected by biotin-conjugated HA-binding protein followed by incubation with FITC-conjugated avidin (both from Sigma). For flow cytometry, 5 × 105 cells were stained according to standard procedures. Briefly, the cells were incubated with a primary antibody (10µg/ml) for 30 min at 4°C. Control cells were incubated with isotype-matched IgG (Strategic Biosolutions, DE). After washing with FACS buffer (2%FCS, 0.1% BSA, 0.01%NaN3 in PBS), the cells were incubated with a FITC-conjugated secondary antibody (Biosource International, CA). Fluorescence intensity was analyzed on a FACScan (Becton Dickinson, San Jose, CA) according to standard procedures.
Confocal Microscopy
Bone marrow cells were cultured on collagen-coated glass cover slips until 50% confluent. The cells were fixed with 4% paraformaldehyde in phosphate buffered saline for 30 min. After washing and blocking with 2% FCS for 2 hours at room temperature, the cells were treated with biotin-conjugated HA-binding protein (Sigma) for 2 h at 4°C. After washing, the cells were incubated with FITC-conjugated avidin in PBS containing 2% FCS for 1 h at room temperature. Negative controls were without HA-binding protein. After washing and staining the nuclei with DAPI (4'-6-diamidino-2-phenylindole) (Sigma Chemical Co.) for 10 min, the cells were washed and covered with a drop of AntiFade (Molecular Probes). Images were taken on an Olympus Fluoview FV1000 confocal microscope.
MMP-9 Detection in Neutrophils
To detect the expression of MMP-9, neutrophils were purified as previously described (DiScipio, Daffern et al. 1998). The isolated cells were plated on poly-D-lysine coated cover-slips and exposed to 100µg/ml LMW HA or HMW HA or 50nM C5a as a positive control for 30 min at 37°C. The cellular supernatants were used to detect MMP-9 by ELISA as previously described (DiScipio, Schraufstatter et al. 2006). Intracellular MMP-9 in the adherent neutrophils was detected by confocal microscopy using an affinity-purified rabbit polyclonal anti-MMP-9 antibody raised against the catalytic domain of MMP-9 in New Zealand White rabbits (DiScipio, Schraufstatter et al. 2006) followed by anti-rabbit Alexa-488-IgG (Invitrogen, CA) as the detection antibody.
Zymogram Analysis
The assay was performed according to the manufacturer’s recommendations (Invitrogen Corporation). Serum free samples of conditioned medium from control and HA-treated cells were mixed with Tris-Glycine SDS Sample Buffer (2x) and applied on zymogram gels. Following electrophoresis, gels were washed with renaturing and developing buffers. MMP activity was visualized by detection of degradation of gelatin-staining in Coomassie Blue stained zymogram gels.
Western Blotting
Bone marrow cells or peripheral blood leukocytes were lysed with modified RIPA buffer (50 mM Tris- HCl, pH 7.4, 10% glycerol, 1% NP-40, 150 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 0.2 mM PMSF, 2 µg/ml leupeptin, 2 µg/ml aprotinin, 2 mM sodium pyrophosphate, 2 mM sodium vanadate and 10 mM NaF) and clarified by centrifugation. The cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 4% dry milk in TBS-Tween, and exposed to HAS2-specific primary antibody provided by Dr. Paul DeAngelis (The University of Oklahoma). Antibody binding was detected using secondary horseradish peroxidase (HRP)-conjugated antibodies and enhanced chemiluminescence (ECL Plus, GE Lifesciences).
Statistical Analysis
Statistical analysis was carried out by Student’s t-test.
Results
HA Synthases, HA and HA Receptors Are Detected in Bone Marrow Cells
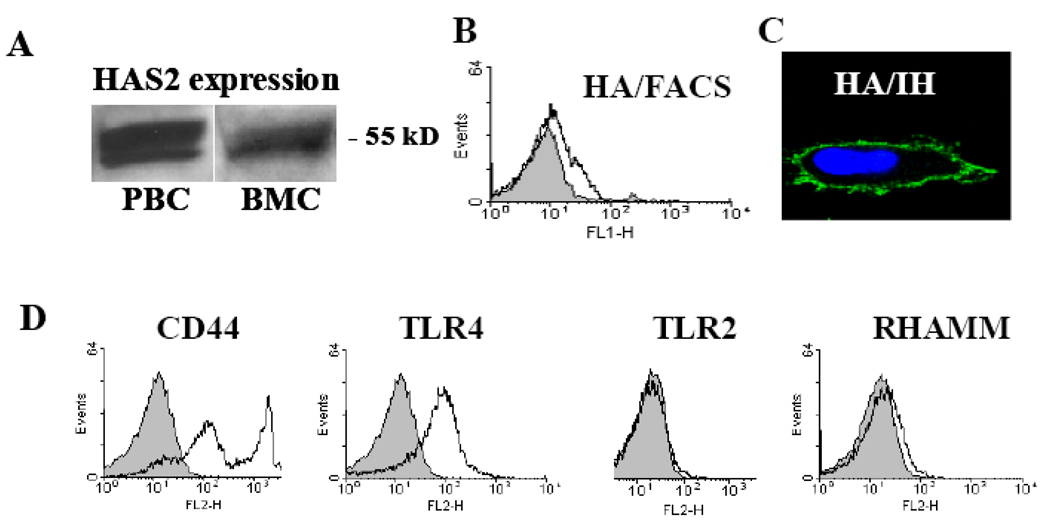
The involvement of HA in many cell functions suggests a requirement for continuous HA synthesis by specific enzymes, HA synthases (HAS), which are integrated in the cell membrane (DeAngelis, Papaconstantinou et al. 1993). Since the presence of the HA polymers regulates hematopoietic activity (Khaldoyanidi, Moll et al. 1999), it was important to investigate the expression of HAS in hematopoietic cells. Using HAS2-specific antibodies we demonstrated that HAS2 protein is expressed in both bone marrow cells and in peripheral blood cells (Figure 1A). HAS2 expression correlated with detectable levels of cell surface associated HA by bone marrow cells (Figure 1B,C). We further confirmed that HA receptors including CD44s, TLR4 and RHAMM, but not TLR2, are expressed in bone marrow cells (Figure 1D).
Figure 1.
Expression of HAS2, HA and HA receptors in hematopoietic cells. A: Expression of HAS2 was detected by Western blot analysis. The presence of HA on the cell surface was detected by FACS (B) or immune-fluorescence microscopy (C). D: The expression of the HA receptors CD44, RHAMM, TLR2 and TLR4 was detected by FACS.
HA Induces Cell Mobilization from Tissue Sites to the Peripheral Blood in Healthy Mice
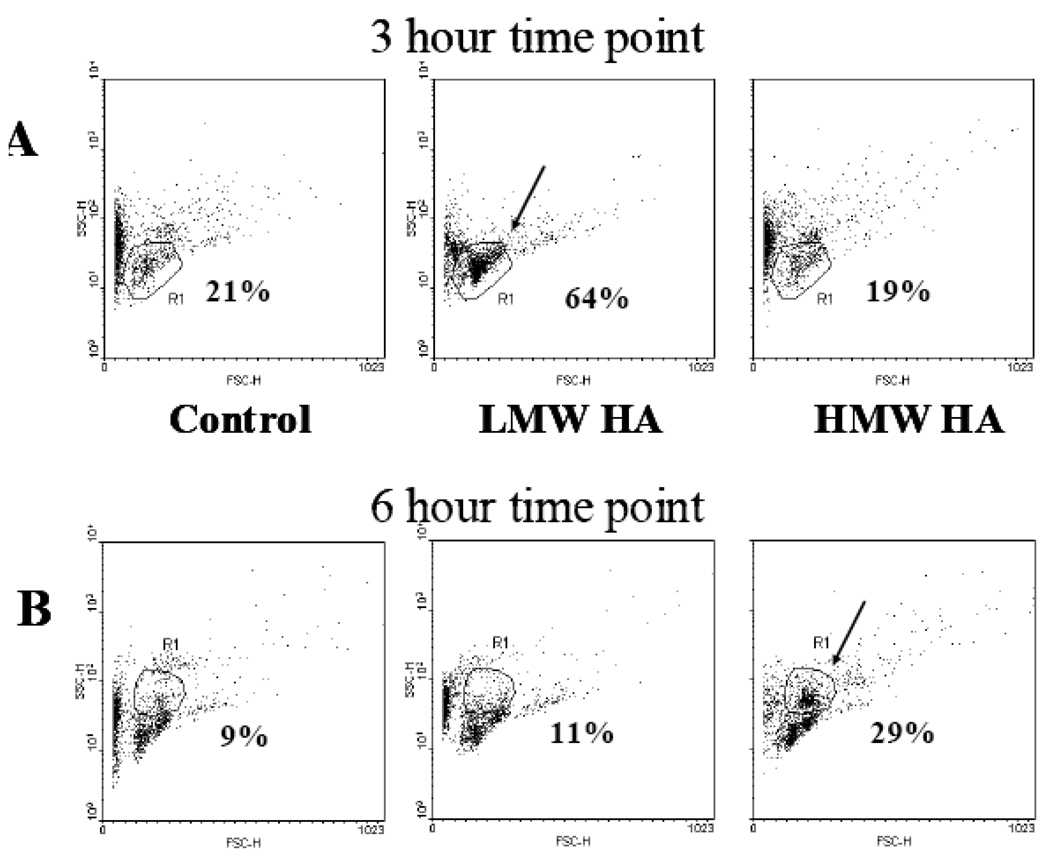
Excess of HA, induced in particular by injection of exogenous polymers, may disrupt homeostasis in bone marrow and result in increased cell motility as previously suggested (Pilarski, Pruski et al. 1999). Since the biological effects of HA depend on the size of the polymers (Hodge-Dufour, Noble et al. 1997), we tested the effect of high molecular weight HA (HMW HA) and low molecular weight HA (LMW HA) polymers on hematopoietic cell mobilization. We infused 100 µg/ml HA polymers of different sizes i.v. into the healthy mice. The control group of mice received infusions of PBS (vehicle). Peripheral blood cells were harvested 1 hour, 3 hours, 6 hours, 12 hours and 24 hours following the HA infusions. The collected samples were analyzed by FACS to assess the distribution of cell subpopulations of blood. Cell size was determined by forward scatter (FSC) and cell granularity was monitored by side scatter (SSC). Four cell populations were gated based on their size and granularity. Each control sample was analyzed using FSC and SSC parameters, which were used to gate on different cell populations, and compared to those from HA-treated mice. LMW HA induced a transient 3-fold increase in leukocyte numbers in the peripheral blood 3 hours after LMW HA infusion (Figure 2A), which returned to normal levels at the 6 hour examination point. HMW HA also induced cell mobilization but at a later time point, 6 hours following the HMW HA infusion (Figure 2B).
Figure 2.
Effect of HA on hematopoietic cell mobilization in healthy mice. Mice (3 mice/group) were intravenously injected with 200µl HMW HA (100µg/mouse) or 200µl LMW HA (100µg/mouse). The composition of cellular sub-populations in blood was examined by FACS analysis after 3 hours (A) and 6 hours (B) following HA administration.
LMW HA Polymers Increase MMP-9 Expression
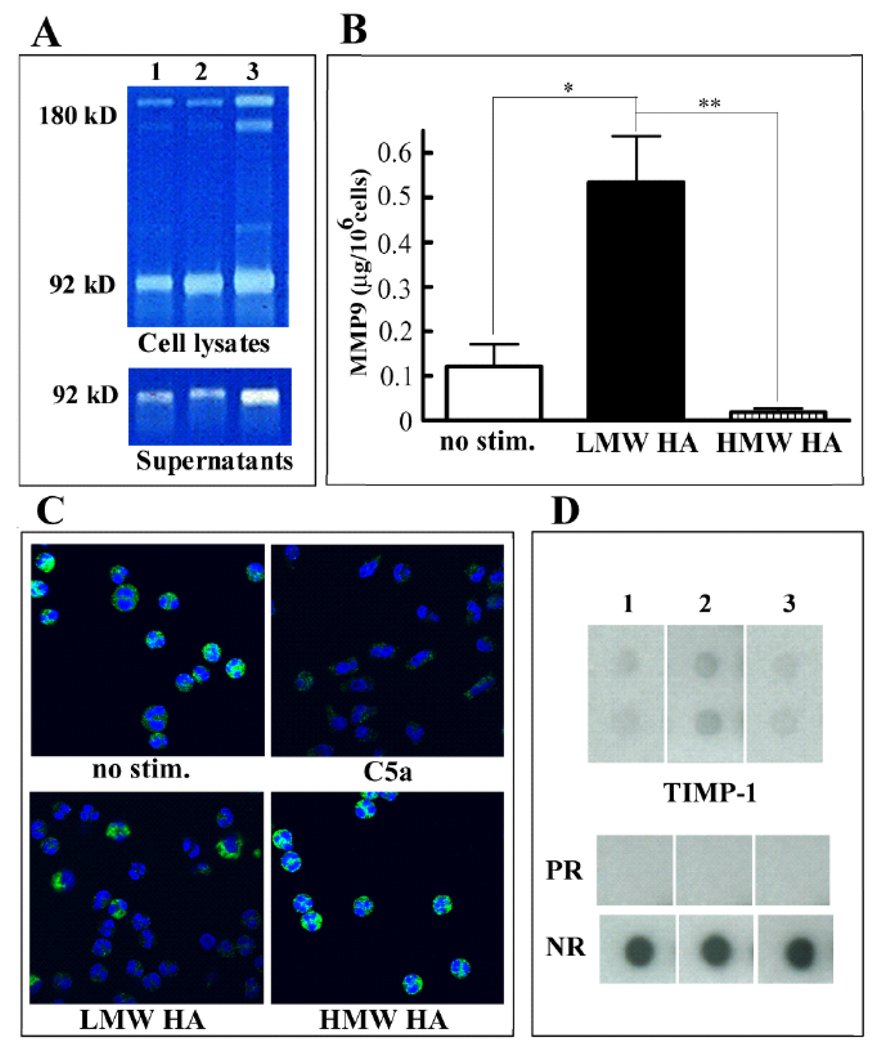
Migration of cells is associated with MMP-9 release and activation. Since HA influences MMP-9 expression and activation in various cell types (Sakuma, Miyachi et al. 2000; Isnard, Robert et al. 2003; Alaniz, Garcia et al. 2004), we investigated the effect of HMW HA and LMW HA on MMP-9 expression in bone marrow cells. While the expression of MMP-9 in bone marrow cells treated with HMW HA was not changed, the expression of both the soluble and the cell-associated MMP-9 was increased in cultures treated with LMW HA (Figure 3A).
Figure 3.
Effect of HA on MMP-9 and TIMP-1 expression. A: Bone marrow cells were untreated (lane 1) or treated with 100 µg/ml HMW HA (lane 2) or LMW HA polymers (lane 3). Supernatants and lysates of the adherent layer were harvested and analyzed using zymogram gels. B: Neutrophils purified from the peripheral blood were unstimulated or exposed to 100µg/ml LMW HA or 100µg/ml HMW HA for 30 min. MMP-9 was detected in the cellular supernatants by ELISA (mean ±S.D, n=4–6). Differences between unstimulated cells and LMW HA-treated cells, and between HMW HA and LMW HA-treated cells were statistically significant (p=0.014 and p=0.001 respectively). C: Neutrophils were unstimulated or stimulated with 50nM C5a, 100µg/ml LMW HA or 100µg/ml HMW HA for 30 min as indicated and the intracellular expression of MMP-9 was determined by immunofluorescence. The nuclei were counterstained with DAPI. D: The expression of TIMP-1 in murine bone marrow cell supernatants was examined by using the RayBio Mouse Cytokine Antibody Array III&3.1. Where indicated, bone marrow cells were not treated (lane 1) or treated with HMW HA (lane 2) or LMW HA (lane 3). Dots indicated as PR show the positive reference and blank shows the negative reference (NR). The experiment was performed twice with similar results.
Since high levels of soluble MMP-9 can be caused by the release of MMP-9 from intracellular storage, for instance by neutrophils, we investigated the levels of MMP-9 expression in neutrophils. It was demonstrated that LMW HA, but not HMW HA caused significant release of MMP-9 into the supernatant of neutrophils (Figure 3B), although the release was not as high as with the traditional neutrophil activator C5a, which caused 5 times as much release as LMW HA. Higher extracellular MMP-9 concentrations correlated with loss of intracellular MMP-9 containing granules from 75–80% of neutrophils incubated in the presence of LMW HA (Figure 3C), but not in neutrophils exposed to HMW HA (Figure 3C). Since activation of MMP-9 is regulated by TIMP-1, we examined the effect of HA on the expression of TIMP-1. Using protein array technology, we found that TIMP-1 is increased in HMW HA supernatant but was unchanged in LMW HA supernatant (Figure 3D) as compared to control cultures.
HA Does Not Influence the Number of Hematopoietic Progenitors in the Peripheral Blood of Healthy Mice
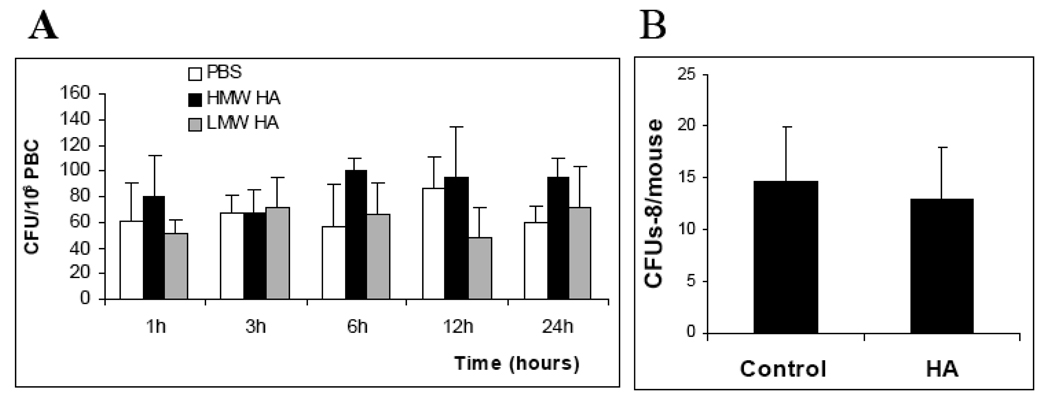
We next investigated whether hematopoietic stem/progenitor cells (HSPC) can be found among the mobilized cells in the peripheral blood of HA-treated mice. To examine the number of HSPC, the cells recovered from the peripheral blood samples were tested by the in vitro clonogenic assay. No significant changes in the number of progenitor cells were found in HA-treated mice as compared to controls (Figure 4A).
Figure 4.
Effect of HA on hematopoietic progenitors in the peripheral blood of healthy mice. The mice were treated as described in Figure 2. Control mice received 200µl PBS. At 1, 3, 6, 12 and 24 hours following the injections, the mice were sacrificed; peripheral blood was collected and analyzed by methylcellulose assays. The number of committed progenitors (colony forming units (CFU)/106 cells) is shown (A). The number of multipotent progenitors determined by the spleen colony forming unit assay (CFUs-8) in the peripheral blood after 24 hours following the HA injection is shown in B. No significant difference between the samples (p>0.5) was observed. The results are from one representative experiment out of two similar experiments.
In addition, peripheral blood from control and HA-treated mice was analyzed for the presence of multipotent hematopoietic progenitors using the in vivo CFUs assay. We found that the infusion of HA did not change the number of CFUs-8 in the peripheral blood (Figure 4B). These findings suggest that HA does not induce mobilization of HSPC in mice, whereas transient mobilization of mature hematopoietic cells was observed (Figure 2).
HA Does Not Induce Cell Mobilization in 5FU-Treated Mice
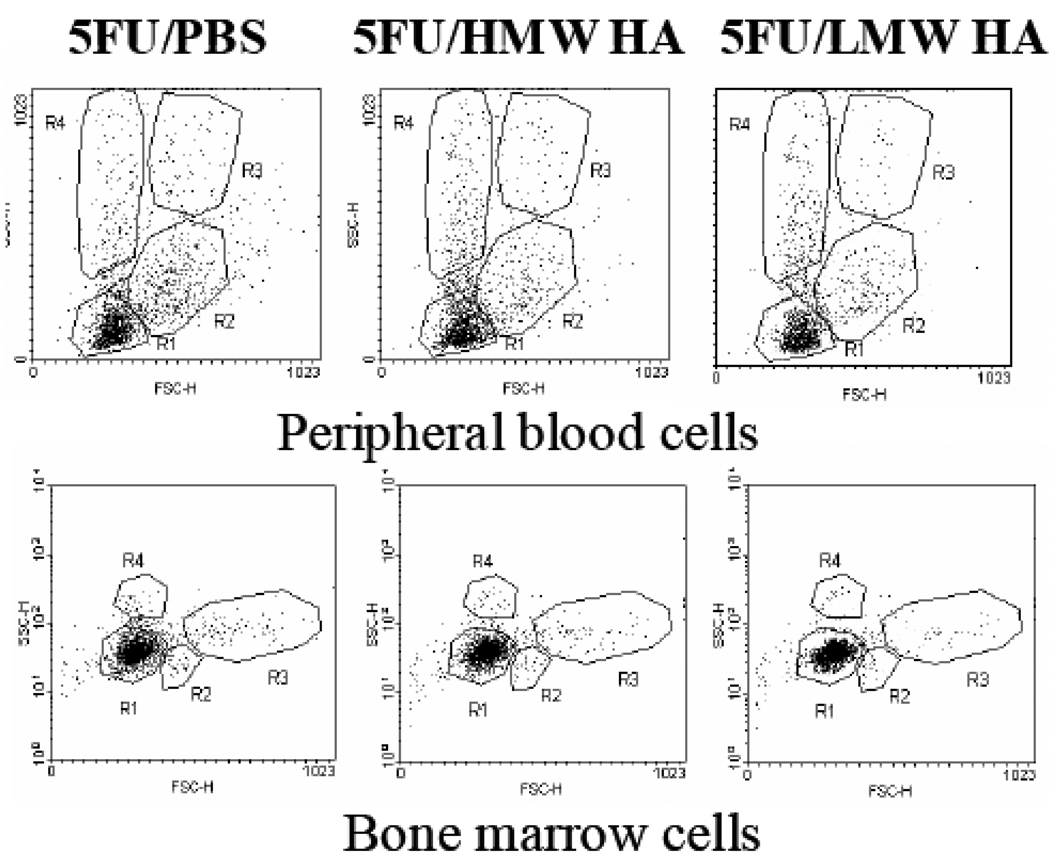
We further tested whether the increased numbers of leukocytes observed in the peripheral blood of 5FU-treated mice following HA infusions (Matrosova, Orlovskaya et al. 2004) could be explained by cell mobilization. Mice were administered 5FU followed by HA infusion on day 4. One hour, 3 hours, 6 hours, 12 hours and 24 hours after the HA infusion, mice were sacrificed, peripheral blood and bone marrow cells were harvested and examined by FACS. Neither the size of the cells nor the number of cells in each subpopulation in peripheral blood was changed after HA administration at any of the tested time points (representative data for the 6 hour time point are shown, Figure 5).
Figure 5.
Effect of HA on cell mobilization in 5FU-treated mice. A: Peripheral blood cell composition in 5FU treated mice 24 hours after HMW HA administration. Mice were administered 150mg/kg 5FU followed by HA or PBS infusion (3 mice per group). Peripheral blood cells were harvested and analyzed by flow cytometry. Four cell populations are gated based on their size and granularity. Dot plots were created using the CellQuestPro software program. B: Bone marrow cell composition in 5FU treated mice 24 hours after HMW HA administration. Mice were administered 150mg/kg 5FU followed by HA or PBS infusion (3 mice per group). Bone marrow cells were harvested and analyzed by flow cytometry. Four cell populations are gated based on their size and granularity. Dot plots were created using the CellQuestPro software program.
A similar analysis was performed with bone marrow cell samples harvested from the same control (5FU/PBS) and HA-treated (5FU/HA) mice. Similar to the results for peripheral blood, we found no difference in the cell composition in bone marrow between these two groups (Figure 5). Thus, we conclude that HA does not induce mobilization of cells from the bone marrow to the peripheral blood in 5FU treated mice.
HMW HA Does Not Influence Chemotaxis of Hematopoietic Progenitors
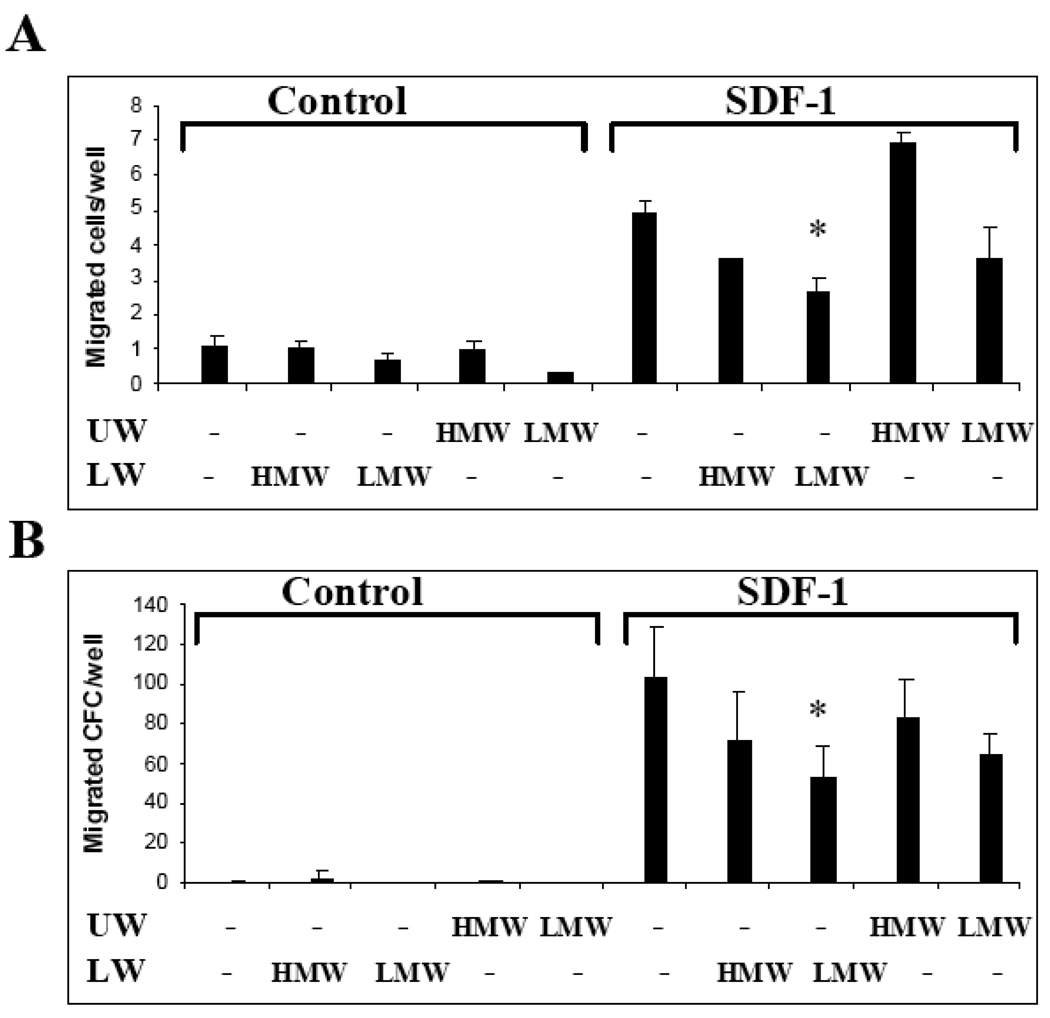
It has been reported recently that soluble HA inhibits transmigration of human HSPC (Avigdor, Goichberg et al. 2004). Since the size of the tested HA used in this study has not been reported, we tested the effect of HMW HA versus LMW HA on SDF-1 mediated chemotaxis of HSPCs. We found that the total number of hematopoietic cells that migrated through Matrigel toward SDF-1 was not significantly changed (Figure 6A). The transmigrated cells were further collected from the lower well and assayed for the number of HSPC using the clonogenic assay. It was found that LMW HA, but not HMW HA, decreased the number of HSPC that migrated toward SDF-1 (p=0.05) (Figure 6B).
Figure 6.
Effect of HA on chemotaxis of HSPC. A–B: Migration of bone marrow cells was tested by using matrigel-coated Transwells. 100 µg/ml HMW HA or LMW HA was added in the insert/upper well (UW) of the Transwells. Where indicated, 50ng/ml SDF-1 was added into the lower well (LW). The number of transmigrated colony forming cells (CFC) was determined by methylcellulose assay (B). Data shown are the means±SD of triplicates from one representative out of two similar experiments.
Discussion
In this study we have demonstrated that HA can mobilize leukocytes, but not hematopoietic progenitor cells, from tissue sites to the peripheral blood. We found that the effect of LMW HA is detected earlier as compared to HMW HA and is associated with increased extracellular MMP-9 concentrations
We demonstrated that bone marrow and peripheral blood cells express HAS2, which correlated with the expression of HA on the cell surface of the hematopoietic cells. Since all bone marrow cells express high levels of the HA receptors (CD44 and TLR4), any cell type can be a target for HA. Since the level of RHAMM expression is low in non-stimulated marrow, this pathway may be involved at later stages following cell activation. Under normal steady state conditions, there is a balance of HA synthesis by HASs and degradation by the specific HA degrading enzyme hyaluronidase (Frost, Csoka et al. 1997). The injection of exogenous HA polymers to healthy mice disturbs this HA homeostasis as is reflected by a short-term increase in the number of cells in the peripheral blood.
Interestingly, an additional sub-population of cells in peripheral blood appeared sooner (3 hours) when LMW HA polymers are injected as compared to HMW HA, which induced cell mobilization after a 6 hour period of time following the injection. One explanation for this phenomenon is that LMW HA, but not HMW HA induces MMP-9 expression or release, which is associated with increased cell motility (Lapidot, Dar et al. 2005). Since HMW HA undergoes degradation by hyaluronidase resulting in LMW HA polymers over time, a delayed effect of HMW HA on cell mobilization is observed.
It has been reported that the effect of HA on MMP expression and activation is mediated by HA receptors (Alaniz, Garcia et al. 2004). However, we were not able to detect any differences in receptor binding ability between HMW and LMW HA polymers (not shown). Therefore, we examined the effect of HA on the expression of TIMP-1, an inhibitor of MMP activation (Kugler 1999). Interestingly, we found that HMW HA, but not LMW HA, increased the expression of TIMP-1 in bone marrow cells. Therefore, we suggest that when cells are exposed to HMW HA, the effect of HA on MMP expression and activation is neutralized by TIMP-1 induced by the HMW HA. In addition, neutrophil granules are the likely source for much of the observed extracellular MMP-9. As tertiary neutrophilic granules are released by numerous stimuli that activate various MAPK cascades (Chakrabarti and Patel 2005), activation of such pathways is the likely means by which LMW HA causes MMP-9 release. Neutrophilic pro-MMP-9 – in contrast to pro-MMP-9 from all other cellular sources, is not bound to TIMP (Ardi, Kupriyanova et al. 2007) and can therefore readily support the release of neutrophils from the bone marrow, which is observed in LMW HA-treated mice.
Since an effect of HA on human HSPC mobilization has been previously suggested (Pilarski, Pruski et al. 1999), we next asked whether the number of hematopoietic progenitors in the peripheral blood of HA-treated mice was also increased following HA administration. Given that there was no statistically significant difference between the number of progenitors in blood of control and HA treated mice during different time points, we concluded that in mice HA can mobilize mature hematopoietic cells, but not their progenitors. Whether the effect of HA on HSPC mobilization is species-specific and does occur in humans requires further investigation.
We have previously reported that administration of HA to 5FU–treated mice results in a gradual increase in the number of both peripheral blood and bone marrow cells (Matrosova, Orlovskaya et al. 2004). This effect was mediated by an increased hematopoietic activity in the bone marrow. To investigate whether cell mobilization contributes to the increased cell numbers in peripheral blood in 5FU-treated mice, we tested the effect of HA on cell mobilization following 5FU injection. Since we did not detect any changes in cell sub-populations in peripheral blood or in bone marrow, we conclude that neither HMW nor LMW HA induce mobilization of hematopoietic cells in 5FU-treated mice. One explanation for the lack of an effect of HA on cell mobilization in 5FU-treated mice could be that the chemotherapy induces pancytopenia and hypoplasia of bone marrow and, therefore, eliminates the cellular supply for detectable cell mobilization. Furthermore, we have previously reported that 5FU decreased the expression of HA receptors, in particular CD44 (Matrosova, Orlovskaya et al. 2004). This, in turn, may lead to insufficient signaling through the HA receptors in bone marrow cells and subsequently lack of HA-induced MMP activation/release in 5FU-treated mice.
To test whether HA can directly influence the motility of hematopoietic progenitors, we investigated the effect of HA on spontaneous and chemokine-mediated migration of progenitors in vitro. Spontaneous migration of either bone marrow cells or progenitors was not affected by the addition of HA into the cultures. However, the addition of LMW HA decreased the number of progenitors migrating towards SDF-1. The process of migration of stem and progenitor cells towards a gradient of chemoattractant is important for cell homing, a process that is the opposite of cell mobilization. Thus, our findings suggest that an excess of LMW HA polymers in blood could interfere with effective homing of transplanted HSPCs. However, additional in vivo studies are required to test this possibility.
Acknowledgment
We thank Valentina Goncharova for excellent technical support. We thank Dr. Linda Pilarski (University of Alberta, Canada) for providing us with RHAMM-specific antibody and Dr. Paul DeAngelis (The University of Oklahoma) for HAS2-specific antibody. This project was funded by NIH/NCI (R41CA126004), the University of California Tobacco Related Disease Research Program (TRDRP-16RT-0134) and the SSPPIR Federal Program (grant 02.740.11.5087) to SKK.
References
- Alaniz L, Garcia M, et al. Modulation of matrix metalloproteinase-9 activity by hyaluronan is dependent on NF-kappaB activity in lymphoma cell lines with dissimilar invasive behavior. Biochem. Biophys. Res. Commun. 2004;324(2):736–743. doi: 10.1016/j.bbrc.2004.09.120. [DOI] [PubMed] [Google Scholar]
- Andreutti D, Geinoz A, et al. Effect of hyaluronic acid on migration, proliferation and alpha-smooth muscle actin expression by cultured rat and human fibroblasts. J. Submicrosc. Cytol. Pathol. 1999;31(2):173–177. [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, et al. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2007;104(51):20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J. Leukoc. Biol. 2005;78(1):279–288. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- Cottler-Fox MH, Lapidot T, et al. Stem cell mobilization. Hematology Am. Soc. Hematol. Educ. Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL, Papaconstantinou J, et al. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J. Biol. Chem. 1993;268(26):19181–19184. [PubMed] [Google Scholar]
- DiScipio RG, Daffern PJ, et al. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves alphaMbeta2 (CD11b/CD18) J. Immunol. 1998;160(8):4057–4066. [PubMed] [Google Scholar]
- DiScipio RG, Schraufstatter IU, et al. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int. Immunopharmacol. 2006;6(7):1109–1118. doi: 10.1016/j.intimp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Fieber C, Baumann P, et al. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J. Cell Sci. 2004;117(Pt 2):359–367. doi: 10.1242/jcs.00831. [DOI] [PubMed] [Google Scholar]
- Fraser JR, Laurent TC, et al. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- Frost GI, Csoka AB, et al. Purification, cloning, and expression of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 1997;236(1):10–15. doi: 10.1006/bbrc.1997.6773. [DOI] [PubMed] [Google Scholar]
- Habara T, Nakatsuka M, et al. The biological effects of antiadhesion agents on activated RAW264.7 macrophages. J. Biomed. Mater. Res. 2002;61(4):628–633. doi: 10.1002/jbm.10247. [DOI] [PubMed] [Google Scholar]
- Hodge-Dufour J, Noble PW, et al. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 1997;159(5):2492–2500. [PubMed] [Google Scholar]
- Isnard N, Robert L, et al. Effect of sulfated GAGs on the expression and activation of MMP-2 and MMP-9 in corneal and dermal explant cultures. Cell Biol. Int. 2003;27(9):779–784. doi: 10.1016/s1065-6995(03)00167-7. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S, Denzel A, et al. Requirement for CD44 in proliferation and homing of hematopoietic precursor cells. J. Leukoc. Biol. 1996;60(5):579–592. doi: 10.1002/jlb.60.5.579. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S, Moll J, et al. Hyaluronate-enhanced hematopoiesis: two different receptors trigger the release of interleukin-1beta and interleukin-6 from bone marrow macrophages. Blood. 1999;94(3):940–949. [PubMed] [Google Scholar]
- Knudson W. The role of CD44 as a cell surface hyaluronan receptor during tumor invasion of connective tissue. Front Biosci. 1998;3:d604–d615. doi: 10.2741/a305. [DOI] [PubMed] [Google Scholar]
- Kugler A. Matrix metalloproteinases and their inhibitors. Anticancer Res. 1999;19(2C):1589–1592. [PubMed] [Google Scholar]
- Lapidot T, Dar A, et al. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002;30(9):973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hyman R, et al. CD44 in inflammation and metastasis. Glycoconj J. 1997;14(5):611–622. doi: 10.1023/a:1018540610858. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr. Opin. Organ Transplant. 2008;13(1):53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- Masellis-Smith A, Belch AR, et al. Hyaluronan-dependent motility of B cells and leukemic plasma cells in blood, but not of bone marrow plasma cells, in multiple myeloma: alternate use of receptor for hyaluronan-mediated motility (RHAMM) and CD44. Blood. 1996;87(5):1891–1899. [PubMed] [Google Scholar]
- Matrosova VY, Orlovskaya IA, et al. Hyaluronic acid facilitates the recovery of hematopoiesis following 5-fluorouracil administration. Stem Cells. 2004;22(4):544–555. doi: 10.1634/stemcells.22-4-544. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Haylock DN, et al. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101(3):856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr. Opin. Hematol. 2008;15(4):285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski LM, Masellis-Smith A, et al. RHAMM, a receptor for hyaluronan-mediated motility, on normal human lymphocytes, thymocytes and malignant B cells: a mediator in B cell malignancy? Leuk Lymphoma. 1994;14(5–6):363–374. doi: 10.3109/10428199409049691. [DOI] [PubMed] [Google Scholar]
- Pilarski LM, Miszta H, et al. Regulated expression of a receptor for hyaluronan-mediated motility on human thymocytes and T cells. J. Immunol. 1993;150(10):4292–4302. [PubMed] [Google Scholar]
- Pilarski LM, Pruski E, et al. Potential role for hyaluronan and the hyaluronan receptor RHAMM in mobilization and trafficking of hematopoietic progenitor cells. Blood. 1999;93(9):2918–2927. [PubMed] [Google Scholar]
- Sakuma M, Miyachi S, et al. The effect of sodium hyaluronate on the expression of gelatinases in rabbit corneal epithelial wound healing. Jpn J. Ophthalmol. 2000;44(5):475–481. doi: 10.1016/s0021-5155(00)00208-2. [DOI] [PubMed] [Google Scholar]
- Schulz C, von Andrian UH, et al. Hematopoietic stem and progenitor cells: their mobilization and homing to bone marrow and peripheral tissue. Immunol. Res. 2009;44(1–3):160–168. doi: 10.1007/s12026-009-8109-6. [DOI] [PubMed] [Google Scholar]
- Spessotto P, Rossi FM, et al. Hyaluronan-CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. J. Cell Biol. 2002;158(6):1133–1144. doi: 10.1083/jcb.200202120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Vermeulen M, Le Pesteur F, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92(3):894–900. [PubMed] [Google Scholar]
- Zhou B, Weigel JA, et al. Identification of the hyaluronan receptor for endocytosis (HARE) J. Biol. Chem. 2000;275(48):37733–37741. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]