Abstract
Introduction
No previous study has concurrently assessed the association between meat intake, meat cooking methods and doneness levels, meat mutagens (heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons), heme iron, and nitrite from meat and colorectal adenoma in asymptomatic women undergoing colonoscopy.
Methods
Of 807 eligible women in a cross-sectional multi-center colonoscopy screening study, 158 prevalent colorectal adenoma cases and 649 controls satisfactorily completed validated food frequency and meat questionnaires. Using an established meat mutagen database and new iron and nitrite databases, we comprehensively investigated components of meat that may be involved in carcinogenesis. Using logistic regression we estimated odds ratios (OR) and 95% confidence intervals (CI) within quartiles of meat-related variables.
Results
Red meat was positively associated with colorectal adenoma (OR fourth versus first quartile = 2.02; 95% CI = 1.06–3.83; P trend = 0.38). Intake of pan fried meat (OR = 1.72; 95% CI = 0.96–3.07; P trend = 0.01) and the HCA: 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) (OR = 1.90; 95% CI = 1.05–3.42; P trend = 0.07) were also associated with increased risk of colorectal adenoma. The new databases yielded lower estimates of heme iron and nitrite than previous assessment methods, although the two methods were highly correlated for both exposures. Although not statistically significant, there were positive associations between iron and heme iron from meat and colorectal adenoma.
Conclusions
In asymptomatic women undergoing colonoscopy, colorectal adenomas were associated with high intake of red meat, pan fried meat, and the HCA MeIQx. Other meat-related exposures require further investigation.
Introduction
Although much of the current epidemiologic research has found positive associations between both red meat and processed meat and colorectal cancer (1), the mechanisms to explain these associations remain largely unknown. In addition, the evidence for the role of meat in relation to colorectal adenomas, known precursors of colorectal cancer (2–4), has been mixed. There are a limited number of studies with full colonoscopy to verify adenoma status (5–7), as well as a lack of detail on meat intake and on the effect of different components of meat, including meat mutagens associated with cooking practices, heme iron and nitrite on colorectal adenoma development. Of these various components of meat, each has an independent and viable mechanism to impact carcinogenesis.
Meat mutagens, such as heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs), are formed in meats cooked well done at high temperatures (8–10). HCAs and PAHs produce intestinal tumors in rodents (11–13) and have been associated with an increased risk of colorectal adenoma in several epidemiologic studies (5, 14–17).
Both non-heme iron and heme iron can induce oxidative DNA damage, by catalyzing the formation of reactive oxygen species (18, 19). However, heme iron, which is found predominantly in red meat, may be more important in the carcinogenic process due to its greater bioavailability. Heme iron is associated with increased cytotoxicity of fecal water (20, 21) and the promotion of chemically-induced colorectal cancer in rats (22). Human studies have also found a positive association between heme iron intake and endogenous formation of N-nitroso compounds (NOCs) (23, 24), some of the most powerful chemical carcinogens (25).
Epidemiologic studies of dietary iron or serum iron indices in relation to colorectal adenoma have produced contradictory results (26–29), perhaps due to the inability to assess heme iron intake separately from total iron from meat. All previous analyses of heme iron have relied on two methods to estimate intake based on standard proportions of total iron from meat (30, 31). Using these approximations for heme iron intake, one study of colorectal adenoma found no evidence of an association (29) and studies of colorectal cancer observed weak positive associations (31–33) or no association (34). However, research indicates that heme iron in meat can be partially converted to non-heme iron depending on the type and duration of the cooking method employed (35–37). A new heme iron database being developed at the National Cancer Institute (NCI), to be used in conjunction with a detailed meat-cooking module, allows for this consideration.
More recently, research has begun to focus on exposure to nitrite and NOCs related to the intake of processed meat. The addition of nitrite to processed meat can lead to the formation of NOCs, which cause tumors in both the colon and rectum of numerous animal species (38). Limited epidemiological research indicates N-nitrosodimethylamine intake may be associated with colorectal cancer (39) and combined nitrate and nitrite intake from meat may be associated with colorectal adenoma (40).
We examined the relationship between dietary meat intake and colorectal adenoma in asymptomatic women enrolled in a colonoscopy screening study by exploring several meat-related exposures that may impact carcinogenesis. This is the first study to assess the association between meat intake, method of meat cooking and doneness levels, meat mutagens, heme iron, and nitrite from meat and colorectal adenoma in asymptomatic women undergoing screening colonoscopy. Furthermore, this is the first study to utilize a heme iron database that estimates heme iron intake based on laboratory measures of various meats prepared according to different cooking methods and doneness levels (41), which may allow for a better quantification of heme iron intake. This is only the second study to examine nitrite intake in relation to colorectal adenoma with a database based on measured values of this compound in processed meats. We hypothesized that women with high intakes of red meat, well done meat, pan fried or grilled meat, meat mutagens, heme iron, and nitrite would have an increased risk of colorectal adenoma compared to those with low intakes.
Materials and methods
The CONCeRN Study
The CONCeRN (COlorectal Neoplasia screening with Colonoscopy in asymptomatic women at Regional Navy/army medical centers) study, previously described in detail (42), is a multi-center screening study designed to determine the relative benefits of colonoscopy versus sigmoidoscopy for colorectal cancer screening in asymptomatic, average-risk women. From January 1, 2000 to December 31, 2002, women were invited to participate in an etiologic component of the CONCeRN study involving additional biospecimen and questionnaire data collection. Prior to colonoscopy, participants returned two self-administered questionnaires they had received in the mail: a risk factor questionnaire (RFQ) (e.g. family history, height, weight, etc.) and the NCI’s diet history questionnaire (DHQ) (43).
Case Ascertainment
Any polyp found during colonoscopy was reviewed by an expert gastrointestinal pathologist, and participants were classified as cases of colorectal adenoma if they had a pathologically verified adenomatous (i.e. tubular, villous, or serrated) polyp of any size in the proximal or distal colon or rectum. The protocol was approved by the institutional review boards at the NCI and each participating medical center. All participants provided written informed consent.
Assessment of meat, meat mutagens, iron and heme iron from meat, and nitrite from meat
Estimates of meat intake (g/day) were based on responses to the DHQ that assessed usual intake (frequency and portion size) of 124 food items over the past year. The DHQ has been validated against four 24-hour dietary recalls and found to be comparable to other food frequency questionnaires (44). Nutrient intake was estimated using the Diet*Calc Analysis Program (version 1.4.3, 2005), based on nutrient content information from the U.S. Department of Agriculture (USDA) Survey Nutrient Database and the Nutrition Data Systems for Research from the University of Minnesota. Intake of red meat included bacon, beef, cheeseburgers, cold cuts, ham, hamburgers, hot dogs, liver, pork, sausage, veal, venison, and red meat from mixed dishes. White meat included chicken, fish, and turkey. Processed meat included bacon, cold cuts, ham, hot dogs, and sausage.
Usual meat cooking methods (e.g. baked, broiled, grilled, microwaved, pan fried) and doneness levels (e.g. rare, medium, well done, and very well done) were assessed in a detailed meat-cooking module administered as part of the RFQ. Using this information, along with the Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease (CHARRED) (http://www.charred.cancer.gov) software application, we generated estimates of three HCAs (ng/day): 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine (PhIP), as well as benzo[a]pyrene (B[a]P), a marker of total PAH exposure. CHARRED computes estimates based on data from more than 120 categories of meat cooked by different methods and to varying doneness levels. CHARRED also estimates mutagenic activity (revertant colonies/day), a measure of total mutagenic potential incorporating all meat-related mutagens.
Cooking method, doneness level, and meat intake data were further utilized to estimate heme iron intake from meat using preliminary data from a NCI heme iron database based on measured values from meat samples cooked by different methods and to varying doneness levels (41). Currently, the heme iron database includes data on bacon, chicken (not in mixed dishes), cold cuts, hamburgers, hot dogs (regular), pork chops, roast beef, sausage (regular), and steak cooked by a variety of cooking methods to a range of doneness levels. In addition, iron (heme and non-heme) from meat was estimated from nutrient content information from the USDA Survey Nutrient Database. Finally, we estimated exposure to nitrite from processed meat using a NCI database containing measured values of nitrite from 10 types of processed meat, representing 90% of the processed meat assessed in FFQs (40, 41). The database does not take into account other dietary sources of nitrite.
Statistical analysis
The demographic characteristics and dietary covariates of those with and without colorectal adenoma were compared using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. The dietary variables were nutrient density adjusted; using residual energy adjustment (45) did not alter our findings. Quartile cut-points for dietary variables were based on intake in those without colorectal adenomas; setting the cut-points using all the data did not alter our results. To assess total iron intake from diet and supplements, we residually energy adjusted the dietary iron so that it could be combined with supplemental iron and remain on the same scale (g/day). Odds ratios (OR) and 95% confidence intervals (CI) were computed using unconditional logistic regression, with the first quartile as the referent group. Trend tests were calculated using the median intake values of each quartile. We also calculated ORs based on continuous measures of intake for each exposure. To further evaluate whether the associations between red meat intake and colorectal adenoma were linear, we created restricted cubic regression splines with varying knots (3, 4, and 5) located at set percentiles of intake (46). All reported P values are two sided and analyses were conducted using SAS software (SAS Institute, Cary, NC, Version 9).
We compared previous methods of assessing heme iron and nitrite from meat to our measured values. For this, we calculated heme iron intake using a current method in the literature (40% of iron from the meats included in the NCI heme iron database) (30) and compared these to the estimates from the NCI heme iron database. In addition, we calculated heme iron as 40% of iron for all meats. For nitrite, we compared the measured values to average values from the historical literature (40). We computed Pearson correlation coefficients, as well as weighted Kappa statistics (47, 48) and percent agreement for agreement across quartiles to characterize descriptively the relationship between the different methods.
Multivariate models were adjusted for characteristics associated with colorectal adenomas in our dataset or associated with colorectal adenomas or colorectal cancer in the literature. The covariates were: age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), highest level of physical activity (no moderate or vigorous activity, moderate activity, vigorous activity), body mass index (BMI) (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current hormone replacement therapy (HRT) use (yes, no), history of colorectal polyps or cancer in a primary relative (yes, no), regular non-steroidal anti-inflammatory drug (NSAID) use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, mg/1,000 kcal), calcium from supplements (continuous, mg/day), and total caloric intake (continuous, kcal/day).
Results
A total of 910 women (92% participation) completed the RFQ and DHQ questionnaires and underwent colonoscopy. Participants were ineligible for this cross-sectional analysis if they had an incomplete colonoscopy (n = 11); missed more than seven items on the DHQ (n = 77); or were extreme outliers for reported total caloric intake (top and bottom 1% for intake, n = 22). After exclusions (some individuals met multiple criteria), there were 807 participants (158 with prevalent colorectal adenoma and 649 without).
Among the 158 women with prevalent colorectal adenoma, 37 (23.4%) had advanced adenomas, 89 (56.3%) had adenomas in the proximal colon, and 55 (34.8%) had adenomas in the distal colon (Table 1). Compared to participants without colorectal adenomas, cases were more likely to be older and to have a higher BMI. Cases had lower intake of dietary calcium and were less likely to report current use of HRT and regular use of NSAIDs.
Table 1.
Characteristics of asymptomatic women in the CONCeRN study (N=807).
| Characteristic | With Adenoma N = 158 n (%), mean ± SD |
Without Adenoma N = 649 n (%), mean ± SD |
P-value* |
|---|---|---|---|
| Age (years) | 60.2 ± 9.0 | 57.2 ± 7.6 | <0.01 |
| Study Center | 0.10 | ||
| Bethesda | 95 (60.1) | 420 (64.7) | |
| Portsmouth | 20 (12.7) | 66 (10.2) | |
| San Diego | 25 (15.8) | 122 (18.8) | |
| Washington D.C. | 18 (11.4) | 41 (6.3) | |
| Race | 0.06 | ||
| White | 115 (72.8) | 525 (80.9) | |
| African-American | 21 (13.3) | 54 (8.3) | |
| Other | 22 (13.9) | 70 (10.8) | |
| Education, Graduate or professional degree | 34 (21.5) | 164 (25.3) | 0.06 |
| Body mass index (kg/m2) | 27.6 ± 5.8 | 26.7 ± 5.7 | 0.03 |
| Physical activity, vigorous | 53 (33.5) | 239 (36.8) | 0.46 |
| Smoking status | 0.16 | ||
| Never | 82 (51.9) | 387 (59.6) | |
| Former | 64 (40.5) | 229 (35.3) | |
| Current | 12 (7.6) | 33 (5.1) | |
| Alcohol (g/day) | 6.0 ± 9.6 | 7.1 ± 13.3 | 0.14 |
| Current use of hormone replacement therapy | 86 (54.4) | 422 (65.0) | 0.01 |
| Positive family history of colorectal cancer or polyps | 37 (23.4) | 148 (22.8) | 0.87 |
| Regular use of non-steroidal anti-inflammatory drugs | 58 (36.7) | 334 (51.5) | <0.01 |
| Total energy intake (kcal/day) | 1471 ± 554 | 1556 ± 601 | 0.17 |
| Dietary folate (mcg/day) | 349.1 ± 158.5 | 365.0 ± 166.8 | 0.23 |
| Supplemental folate (mcg/day) | 233.3 ± 185.8 | 233.1 ± 187.9 | 0.88 |
| Dietary calcium (mg/day) | 599.1 ± 279.3 | 679.3 ± 339.2 | 0.01 |
| Supplemental calcium (mg/day) | 404.5 ± 397.3 | 430.0 ± 396.7 | 0.23 |
| Fiber (g/day) | 16.1 ± 8.7 | 17.3 ± 8.6 | 0.09 |
| Fruit (g/day) | 274.6 ± 336.9 | 280.7 ± 232.1 | 0.44 |
| Vegetables (g/day) | 274.9 ± 184.2 | 295.9 ± 238.4 | 0.53 |
| Adenoma Characteristics | |||
| Advanceda | 37 (23.4) | - | |
| Proximalb | 89 (56.3) | - | |
| Distalb | 55 (34.8) | - |
χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables.
Advanced adenomas met one of the following histologic criteria: greater than 10mm in diameter, villous, tubulovillous, high-grade dysplasia, or cancer (invasive or in situ).
Proximal adenomas were those in the cecum, ascending colon, hepatic flexure, transverse colon, or splenic flexure. Distal adenomas were those in the descending colon, sigmoid colon, or rectum. 14 cases had both proximal and distal adenomas and are not classified here.
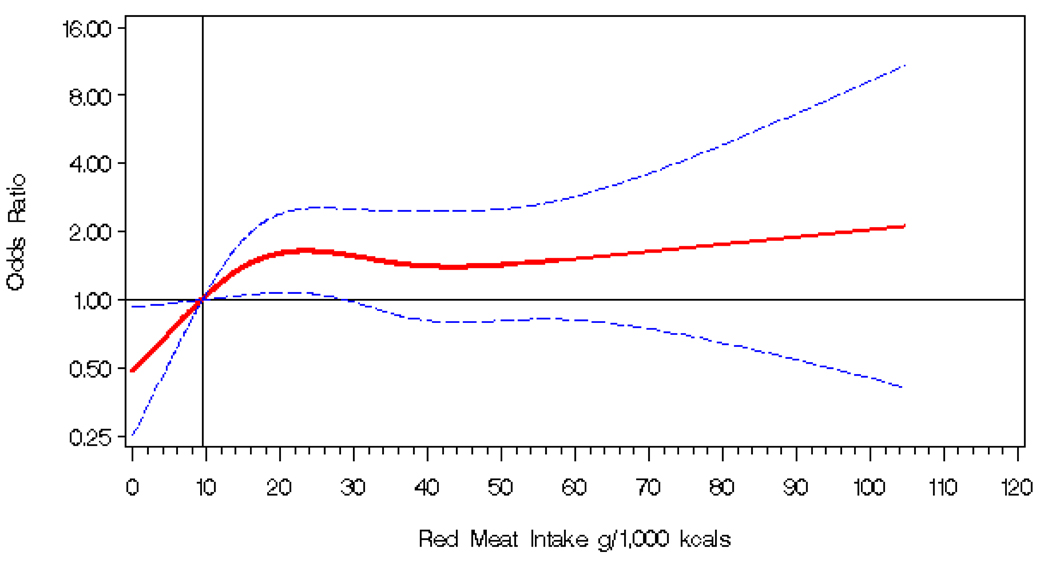
Although we observed no association for total meat intake, there was a positive association between red meat intake and colorectal adenoma (OR for the fourth compared to the first quartile = 2.02; 95% CI = 1.06–3.83), with a possible threshold effect in the second quartile (Table 2, Figure 1). There were no statistically significant associations for white meat, processed meat, chicken, or fish.
Table 2.
Multivariate ORs* for colorectal adenoma risk associated with intake of meat (g/1,000 kcal).
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Trenda | Continuous (per 10 g) |
|---|---|---|---|---|---|---|
| Total meat | ||||||
| Median (Cut-points) | 34.2 (≤44.4) | 53.6 (>44.4–64.2) | 77.9 (>64.2–90.8) | 111.1 (>90.8) | ||
| Cases/Controls | 36/161 | 37/158 | 51/159 | 31/158 | ||
| OR (95% CI) | 1.00 | 1.08 (0.62–1.89) | 1.79 (1.05–3.07) | 0.99 (0.55–1.81) | 0.75 | 1.00 (0.95–1.06) |
| Red meat | ||||||
| Median (Cut-points) | 9.4 (≤ 15.0) | 18.7 (>15.0–24.0) | 28.7 (>24.0–35.6) | 45.1 (>35.8) | ||
| Cases/Controls | 22/160 | 54/160 | 39/158 | 40/158 | ||
| OR (95% CI) | 1.00 | 2.97 (1.64–5.37) | 2.05 (1.10–3.84) | 2.02 (1.06–3.83) | 0.38 | 1.07 (0.95–1.21) |
| White meat | ||||||
| Median (Cut-points) | 13.3 (≤ 22.1) | 27.9 (>22.1–36.9) | 44.6 (>36.9–59.1) | 80.2 (>59.1) | ||
| Cases/Controls | 43/159 | 45/161 | 29/156 | 38/160 | ||
| OR (95% CI) | 1.00 | 1.06 (0.63–1.78) | 0.81 (0.46–1.43) | 0.98 (0.57–1.70) | 0.89 | 0.99 (0.93–1.05) |
| Processed meat b | ||||||
| Median (Cut-points) | 1.5 (≤ 2.6) | 3.9 (>2.6–5.1) | 6.7 (>5.1–9.7) | 15.7 (>9.7) | ||
| Cases/Controls | 36/160 | 37/161 | 41/160 | 41/155 | ||
| OR (95% CI) | 1.00 | 1.09 (0.62–1.92) | 0.99 (0.56–1.74) | 1.05 (0.59–1.85) | 0.84 | 0.98 (0.78–1.23) |
| Chicken | ||||||
| Median (Cut-points) | 5.4 (≤ 9.8) | 15.3 (>9.8–20.9) | 27.8 (>20.9–39.6) | 59.5 (>39.6) | ||
| Cases/Controls | 40/158 | 42/159 | 36/158 | 37/161 | ||
| OR (95% CI) | 1.00 | 1.20 (0.69–2.08) | 1.19 (0.67–2.10) | 1.11 (0.62–1.98) | 0.84 | 1.01 (0.93–1.08) |
| Fish | ||||||
| Median (Cut-points) | 3.0 (≤ 4.5) | 6.3 (>4.5–8.7) | 11.4 (>8.7–15.5) | 24.5 (>15.5) | ||
| Cases/Controls | 46/161 | 36/160 | 35/157 | 38/158 | ||
| OR (95% CI) | 1.00 | 0.74 (0.43–1.26) | 0.86 (0.50–1.49) | 1.15 (0.67–1.99) | 0.44 | 1.00 (0.86–1.16) |
OR adjusted for age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), physical activity (no moderate or vigorous activity, moderate, vigorous), BMI (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current HRT use (yes, no), family history of colorectal polyps or cancer (yes, no), regular NSAID use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, g/1,000 kcal), calcium from supplements (continuous, g/day), and total caloric intake (continuous, kcal/day).
P trend calculated using the median of each quartile.
Includes bacon, sausage, hot dogs, ham, and cold cuts.
Figure 1. Odd ratios* for colorectal adenoma risk in relation to red meat intake (g/1000 kcal) in the CONCeRN study (test for curvature p-value = 0.09).
*Odds ratios (1.00 indicated by solid horizontal black line) are modeled on a continuous basis using a four knot cubic regression spline. Odds ratios are indicated by the solid red line and 95% confidence intervals by dashed blue lines. The referent (solid vertical black line) is an intake of 9.4 g/1000kcal of red meat (median of the first quartile), with knots placed at the 5th, 25th, 75th, and 95th percentiles of the distribution of red meat intake. The odds ratios were adjusted for age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), physical activity (no moderate or vigorous activity, moderate, vigorous), BMI (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current HRT use (yes, no), family history of colorectal polyps or cancer (yes, no), regular NSAID use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, g/1,000 kcal), calcium from supplements (continuous, g/day), and total caloric intake (continuous, kcal/day).
Pan fried meat intake was positively associated with colorectal adenoma, with borderline statistical significance for the categorical data (OR for the fourth compared to the first quartile = 1.72; 95% CI = 0.96–3.07) and a statistically significant association for the continuous data (OR = 1.45 per additional 10g/1000kcal; 95% CI =1.01–2.08) (Table3). There was no evidence that grilled meat intake or meat doneness were associated with colorectal adenoma in this population.
Table 3.
Multivariate ORs* for colorectal adenoma risk associated with intake of meat cooking (g/1000 kcal), doneness levels (g/1000 kcal), and meat mutagens (ng/day).
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Trenda | Continuous (per 10 unit) |
|---|---|---|---|---|---|---|
| Grilled meat | ||||||
| Median (Cut-points) | 0.1 (≤ 0.8) | 1.8 (>0.8–3.1) | 5.1 (>3.1–8.1) | 15.0 (>8.1) | ||
| Cases/Controls | 33/160 | 50/157 | 35/161 | 37/158 | ||
| OR (95% CI) | 1.00 | 1.85 (1.07–3.21) | 1.21 (0.67–2.20) | 1.40 (0.75–2.60) | 0.88 | 0.89 (0.70–1.12) |
| Pan fried meat | ||||||
| Median (Cut-points) | 0.0 (≤ 0.3) | 0.7 (>0.3–1.2) | 2.1 (>1.2–3.4) | 5.3 (>3.4) | ||
| Cases/Controls | 31/162 | 27/157 | 34/160 | 63/157 | ||
| OR (95% CI) | 1.00 | 0.88 (0.47–1.63) | 1.00 (0.55–1.82) | 1.72 (0.96–3.07) | 0.01 | 1.45 (1.01–2.08) |
| Well/Very well meat | ||||||
| Median (Cut-points) | 0.0 (≤ 0.3) | 0.8 (>0.3–1.7) | 3.0 (>1.7–5.3) | 11.4 (>5.3) | ||
| Cases/Controls | 33/162 | 45/159 | 39/156 | 38/159 | ||
| OR (95% CI) | 1.00 | 1.39 (0.80–2.41) | 1.21 (0.68–2.15) | 1.00 (0.56–1.82) | 0.74 | 0.80 (0.60–1.05) |
| DiMeIQx | ||||||
| Median (Cut-points) | 0.0 (≤ 0.1) | 0.2 (>0.1–0.4) | 0.6 (>0.4–1.1) | 2.1(>1.1) | ||
| Cases/Controls | 35/162 | 34/159 | 46/158 | 40/156 | ||
| OR (95% CI) | 1.00 | 1.04 (0.59–1.82) | 1.41 (0.82–2.41) | 1.21 (0.69–2.13) | 0.60 | 2.00 (0.61–6.58) |
| MeIQx | ||||||
| Median (Cut-points) | 1.9 (≤ 3.7) | 5.8 (>3.7–8.5) | 12.2 (>8.5–18.2) | 27.9(> 18.2) | ||
| Cases/Controls | 30/162 | 37/160 | 40/159 | 48/155 | ||
| OR (95% CI) | 1.00 | 1.43 (0.81–2.54) | 1.81 (1.01–3.24) | 1.90 (1.05–3.42) | 0.07 | 1.11 (1.03–1.21) |
| PhIP | ||||||
| Median (Cut-points) | 4.5 (≤ 9.0) | 16.2 (>9.0–25.9) | 44.5 (>25.9–68.1) | 139.1 (>68.1) | ||
| Cases/Controls | 35/160 | 33/158 | 44/158 | 43/160 | ||
| OR (95% CI) | 1.00 | 1.16 (0.66–2.05) | 1.53 (0.89–2.62) | 1.49 (0.85–2.62) | 0.22 | 1.01 (0.98–1.03)b |
| B[a]P | ||||||
| Median (Cut-points) | 0.4 (≤ 1.6) | 4.2 (>1.6–6.8) | 13.4 (>6.8–22.2) | 47.1 (>22.2) | ||
| Cases/Controls | 41/161 | 34/157 | 41/159 | 39/159 | ||
| OR (95% CI) | 1.00 | 0.83 (0.48–1.42) | 1.15 (0.68–1.94) | 1.16 (0.67–2.00) | 0.40 | 1.01 (0.91–1.11)b |
|
Mutagenic activity (revertant colonies/day) |
||||||
| Median (Cut-points) | 260 (≤ 568) | 841 (>568–1308) | 2030 (>1308–3162) | 5654 (>3162) | ||
| Cases/Controls | 31/163 | 38/157 | 43/159 | 43/157 | ||
| OR (95% CI) | 1.00 | 1.67 (0.94–2.95) | 1.84 (1.04–3.26) | 1.69 (0.94–3.04) | 0.32 | 1.04 (0.99–1.08)c |
OR adjusted for age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), physical activity (no moderate or vigorous activity, moderate, vigorous), BMI (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current HRT use (yes, no), family history of colorectal polyps or cancer (yes, no), regular NSAID use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, g/1,000 kcal), calcium from supplements (continuous, g/day), and total caloric intake (continuous, kcal/day).
P trend calculated using the median of each quartile.
Per 20 ng/day.
Per 1000 revertant colonies/day.
Among the meat mutagens, there was a possible positive association between the HCA, MeIQx, and colorectal adenoma in the categorical data (OR for the fourth compared to the first quartile = 1.90; 95% CI = 1.05–3.42), as the P trend was borderline statistically significant (0.07) (Table 3). However, this relationship was statistically significant on the continuous scale (OR = 1.11 per additional 10ng/day, 95% CI = 1.03–1.21) (Table 3). There was no evidence that DiMeIQx, PhIP, B[a]P, or total mutagenic activity were associated with colorectal adenoma.
There were no statistically significant associations between total iron, dietary iron, or iron from supplements and colorectal adenoma (Table 4). However, there were positive associations between iron and heme iron from a subset of meats and colorectal adenoma, although these ORs did not reach statistical significance. There was no evidence of effect modification by alcohol status (< 5 g/day vs. ≥ 5 g/day) for the association between heme iron and colorectal adenoma (data not shown).
Table 4.
Multivariate ORs* for colorectal adenoma risk associated with intake of total iron (mg/day), dietary iron (mg/1000 kcal), iron from meat (mg/1000 kcal), heme iron from meat (mg/1000 kcal), and supplemental iron (mg/day).
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Trenda | Continuous (per 10 unit) |
|---|---|---|---|---|---|---|
| Total iron b | ||||||
| Median (Cut-points) | 10.8 (≤ 12.6) | 15.0 (>12.6–22.9) | 28.3 (>22.9–30.2) | 33.4 (>56.6) | ||
| Cases/Controls | 47/161 | 43/158 | 29/158 | 36/159 | ||
| OR (95% CI)c | 1.00 | 1.21 (0.66–1.92) | 0.73 (0.42–1.27) | 0.91 (0.52–1.59) | 0.34 | 0.96 (0.79–1.17) |
| Dietary iron | ||||||
| Median (Cut-points) | 6.3 (≤ 7.0) | 7.5 (>7.0–8.1) | 8.8 (>8.1–9.7) | 10.9 (>9.7) | ||
| Cases/Controls | 36/159 | 43/161 | 41/157 | 35/159 | ||
| OR (95% CI) | 1.00 | 1.30 (0.76–2.22) | 1.55 (0.87–2.75) | 1.11 (0.57–2.16) | 0.77 | 1.29 (0.50–3.34) |
| Iron from meat d | ||||||
| Median (Cut-points) | 0.16 (≤ 0.23) | 0.31 (>0.23–0.38) | 0.47 (>0.38–0.58) | 0.78 (>0.58) | ||
| Cases/Controls | 26/163 | 47/162 | 41/162 | 44/162 | ||
| OR (95% CI) | 1.00 | 1.67 (0.94–2.96) | 1.75 (0.97–3.15) | 1.71 (0.94–3.14) | 0.17 | 1.06 (0.87–1.28)e |
| Heme iron from meat | ||||||
| Median (Cut-points) | 0.05 (≤ 0.09) | 0.11 (>0.09–0.14) | 0.17 (>0.14–0.21) | 0.28 (>0.21) | ||
| Cases/Controls | 30/160 | 46/162 | 39/158 | 40/156 | ||
| OR (95% CI) | 1.00 | 1.53 (0.88–2.66) | 1.50 (0.84–2.66) | 1.50 (0.83–2.73) | 0.32 | 1.06 (0.89–1.27)f |
| Supplemental iron g | ||||||
| Cut-points | 0.0 | 0.3–12.9 | 18.0–39.4 | |||
| Cases/Controls | 76/247 | 22/130 | 57/259 | |||
| OR (95% CI) | 1.00 | 0.72 (0.41–1.26) | 0.82 (0.54–1.25) | 0.34 | 0.94 (0.76–1.15) |
OR adjusted for age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), physical activity (no moderate or vigorous activity, moderate, vigorous), BMI (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current HRT use (yes, no), family history of colorectal polyps or cancer (yes, no), regular NSAID use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, g/1,000 kcal), calcium from supplements (continuous, g/day), and total caloric intake (continuous, kcal/day).
P trend calculated using the median of each quartile.
Dietary iron (residual energy adjusted) plus iron from supplements.
Nutrients in this model were residual energy adjusted.
Limited to only those meats in the heme iron database.
Per 0.30 mg/1000 kcal.
Per 0.10 mg/1000 kcal.
Due to the small number of people taking supplements containing iron, iron from supplements was broken into three levels.
In our population, mean heme iron intake with the NCI heme iron database (mean = 0.16, standard deviation (SD) = 0.11 mg/1,000 kcal) was 88.9% of the amount estimated by calculating 40% of iron from the same meats for which we had measured values (mean = 0.18, SD = 0.12 mg/1,000 kcal). The Pearson correlation coefficient was 0.97 for comparing these two estimates of heme iron. When the heme iron estimates were categorized into quartiles there was high agreement (weighted Kappa = 0.85, 95% CI = 0.83–0.87; percent agreement = 81.5%, 95% CI = 78.8%–84.2%). Risk estimates (detailed data not shown) using 40% of iron from meat (limited to those in the heme iron database) to estimate heme iron intake were similar to those based on the NCI heme iron database (OR for the fourth compared to the first quartile = 1.71; 95% CI = 0.94–3.14, p-trend = 0.17). We also calculated heme iron as 40% of iron from all meats, not just those in the heme iron database, and saw no association with risk of colorectal adenoma (OR for the fourth compared to the first quartile = 1.00; 95% CI = 0.55–1.82, p-trend = 0.76).
Intake of nitrite from meat was not associated with colorectal adenoma (Table 5). Although highly correlated (Pearson correlation = 0.97), intake of nitrite estimated by the NCI database was 70.4% of the estimated intake from previously published values. When the measured and published nitrite values were categorized into quartiles, the weighted Kappa statistic was 0.88 (95% CI = 0.84–0.90) and percent agreement was 84.4% (81.9%–86.9%).
Table 5.
Multivariate ORs* for adenoma risk associated with intake of nitrite from meat (mg/day).
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Trenda | Continuous (per mg) |
|---|---|---|---|---|---|---|
| Nitrite | ||||||
| Median (Cut-points) | 0.02 (≤ 0.03) | 0.04 (>0.03–0.06) | 0.08 (>0.06–0.12) | 0.22 (>0.12) | ||
| Cases/Controls | 40/161 | 43/161 | 34/158 | 38/156 | ||
| OR (95% CI) | 1.00 | 1.14 (0.67–1.93) | 0.91 (0.52–1.59) | 1.05 (0.59–1.86) | 0.99 | 0.73 (0.15–3.58) |
OR adjusted for age (continuous), education (high school or less, vocational/technical/business, 1–3 years or college/junior college, college graduate, graduate/professional degree), race (White, African-American, other), smoking status (never, former, current), physical activity (no moderate or vigorous activity, moderate, vigorous), BMI (continuous, kg/m2), study center (Bethesda, Portsmouth, San Diego, Washington D.C.), current HRT use (yes, no), family history of colorectal polyps or cancer (yes, no), regular NSAID use (defined as once a week for at least one month at any time prior to three months before colonoscopy; yes, no, don’t know/missing), alcohol intake (<5 g/day, 5–14.9 g/day, ≥15 g/day), fiber (continuous, g/1,000 kcal), dietary calcium (continuous, g/1,000 kcal), calcium from supplements (continuous, g/day), and total caloric intake (continuous, kcal/day).
P trend calculated using the median of each quartile.
Discussion
This is the first study to concurrently assess the association of meat intake, meat cooking methods and doneness levels, meat mutagens, heme iron and nitrite with prevalent colorectal adenomas in asymptomatic women undergoing colonoscopy. It is the first study to utilize a new NCI heme iron database to quantify heme iron intake based on laboratory measured meat samples. In our study, we found an increased risk of colorectal adenoma for women who consumed the highest amounts of red meat, pan fried meat, and the HCA MeIQx. Although there was no association for total iron, dietary iron, iron from supplements, or nitrite, there were positive associations between iron and heme iron from meat and colorectal adenoma although these associations did not reach statistical significance.
Our findings for red meat and pan fried meat agree with several previous studies that have observed positive associations between red meat and meat cooked at high temperatures and colorectal adenoma (5, 14, 49–52). The motive for studying pan fried meat is based on the hypothesis that formation of HCAs during such high temperature cooking methods will increase the risk of neoplasia; therefore, it is particularly interesting that we also observed a positive association with MeIQx, which has been associated with increased risk of colorectal adenoma in previous studies (14, 15). Unlike some previous studies, we did not observe an elevated risk for grilled meat (17, 50) or for well/very well done meat (14, 50, 51).
Although some studies have linked high intake of processed meat intake to an increased risk of colorectal adenoma (40, 51), we did not observe such an association in our study population, which had a relatively low intake of processed meat and thus low levels of nitrite from meat. The one previous study that utilized the NCI nitrite database found a two-fold increased risk of colorectal adenoma for those in the highest quartile of nitrate and nitrite intake compared to the lowest quartile; however, the range of intake of processed meat was higher in the previous study compared to ours (40). Consequently, our study may have lacked statistical power to assess the association of nitrite with colorectal adenoma.
Our null findings for dietary iron are similar to those reported by three previous studies of colorectal adenoma (27–29). Several cohort studies have examined the association between dietary iron (34, 53) or iron stores (34, 53–55) and colorectal cancer, but results have been inconclusive. Nelson et al., evaluated 33 case-control studies and concluded that higher dietary iron and iron stores were associated with an increased risk of colorectal cancer (56). However, results from a recent nested case-control study among male smokers found inverse associations between several serum iron indices and colon cancer, but not rectal cancer (57). The inconsistency in the literature might be due to the different study populations, as well as the different sources and types of iron evaluated in these studies. Future research may also need to consider role of individual variation in genes related to iron absorption and metabolism in colorectal neoplasia. Finally, the role of iron in colorectal carcinogenesis is most likely very complex, as excess exposure to iron could be at the systemic level through excess iron absorption or directly through the lumen due to the passage of excess dietary iron through the gastrointestinal tract.
Our findings do not show a clear association between iron and heme iron from meat and colorectal adenoma in women, although there was a suggestion of an increased risk. This is the first study to assess heme iron intake using a database based on laboratory measured values of heme iron in various meats cooked by different methods and to varying degrees of doneness. Previous studies of colorectal cancer that have attempted to evaluate heme iron intake using a proportion of total iron (32, 33) or by employing a methodology that accounts for varying levels of heme iron by meat type (31, 34), have yielded inconsistent results. In this study, heme iron intake estimated as 40% of iron from those meats in the heme iron database was highly correlated with the heme iron database values and most individuals were classified in the same quartile of intake. However, had we only assessed heme iron as 40% of iron from all meats, as done in previous research, the risk estimate would have been completely null.
Our current findings on heme iron may be somewhat limited as this population of women had a higher consumption of white meat as compared to red meat and a narrow range of cooking methods and doneness levels. In addition, heme iron intake is most likely underestimated in this population as the heme database only accounted for 57.3% of the red meat intake in our population and we may have lacked the power to observe a statistically significant association for a low range of intake. In light of the inconsistent findings for heme iron, additional studies are necessary. The NCI heme iron database should also be investigated in other larger populations to further evaluate current estimation methods of exposure to heme iron.
The strengths of our study include colonoscopy confirmed colorectal adenoma status for all participants, detailed exposure assessment of meat intake, cooking methods and doneness levels, analysis of multiple potential mechanisms, and a high participation rate. This dataset provided a unique opportunity to comprehensively examine a wide range of meat-related exposures among asymptomatic women with little risk of dietary changes due to disease status. Participants also completed questionnaires prior to colonoscopy eliminating recall bias. Limitations of this study include the cross-sectional rather than longitudinal design, as well as the evaluation of usual dietary intake only during the year prior to colonoscopy, which may not be the time period most relevant to the development of colorectal adenoma. Although this study utilized comprehensive methods of assessment for meat, meat cooking methods, and doneness levels, the possibility of some degree of measurement error remains. As in any observational study, it is possible that the observed association is due to unmeasured or residual confounding, although this study did assess all known potential confounders. We also were not able to evaluate genetic variation that may be involved in colorectal carcinogenesis. Finally, the generalizability of these results is limited to predominantly Caucasian and highly educated women.
This is the first study to assess meat intake and the currently hypothesized potentially carcinogenic components of meat in relation to colorectal adenoma in a population of asymptomatic women undergoing screening colonoscopy. In this population, there was an increased risk of colorectal adenoma with high intake of red meat, pan fried meat, and, the HCA MeIQx. Future research on meat and colorectal neoplasia should focus on assessing all meat-related exposures to enhance our understanding of the mechanisms for the role of meat in cancer. Given our small sample size, evaluation of heme iron and nitrite from meat in large, prospective cohort studies is needed to clarify the role of these exposures in carcinogenesis.
Study Highlights
- What is current knowledge
- Red and processed meat positively associated with colorectal neoplasia
- Mechanisms are not well understood
- Possible meat-related exposures implicated include: meat mutagens, heme iron, nitrite
- No study has analyzed these exposures simultaneously
- Prior research has estimated heme iron intake by applying a standard factor to total iron from meat
- What is new here
- This is the first study of asymptomatic women with full colonoscopy and concurrent assessment of multiple components of meat intake, including meat cooking methods, doneness levels, meat mutagens, nitrite, and heme iron
- Estimated heme iron and nitrite using databases based on measured values
- Increased risk of adenoma with high intake of red meat, pan fried meat, and the heterocyclic amine MeIQx
- Possible association between iron and heme iron from meat and colorectal adenoma
Acknowledgment
Guarantor of the article: Leah M. Ferrucci, MPH
Financial Support: This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Cancer Institute and by grant TU2 CA105666 from the National Cancer Institute.
Abbreviations
- B[a]P
benzo[a]pyrene
- BMI
body mass index
- CHARRED
Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease
- CONCeRN
COlorectal Neoplasia screening with Colonoscopy in asymptomatic women at Regional Navy/army medical centers
- CI
confidence interval
- DHQ
diet history questionnaire
- DiMeIQx
2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline
- HCAs
heterocyclic amines
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- NCI
National Cancer Institute
- NOCs
N-nitroso compounds
- NSAID
non-steroidal anti-inflammatory drug
- OR
odds ratio
- PAHs
polycyclic aromatic hydrocarbons
- PhIP
2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine
- RFQ
risk factor questionnaire
- SD
standard deviation
- USDA
U.S. Department of Agriculture
Footnotes
Specific Author Contributions:
LMF performed and interpreted the statistical analyses and drafted the manuscript. AJC and RS participated in devising the hypothesis, interpreting the results, and edited the manuscript. BDC, AF, BIG, XM, STM, AS and PSS participated in interpreting the results and edited the manuscript. BDC, AF, AS, and PSS were also involved in the original design, conduct, and oversight of the CONCeRN study.
Potential Competing Interests: None
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF, Guyton KZ, Hiatt RA, et al. Colorectal cancer screening for persons at average risk. J Natl Cancer Inst. 2002;94:1126–1133. doi: 10.1093/jnci/94.15.1126. [DOI] [PubMed] [Google Scholar]
- 4.Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 5.Shin A, Shrubsole MJ, Ness RM, et al. Meat and meat-mutagen intake, doneness preference and the risk of colorectal polyps: the Tennessee Colorectal Polyp Study. Int J Cancer. 2007;121:136–142. doi: 10.1002/ijc.22664. [DOI] [PubMed] [Google Scholar]
- 6.Austin GL, Adair LS, Galanko JA, et al. A diet high in fruits and low in meats reduces the risk of colorectal adenomas. J Nutr. 2007;137:999–1004. doi: 10.1093/jn/137.4.999. [DOI] [PubMed] [Google Scholar]
- 7.Sandler RS, Lyles CM, Peipins LA, et al. Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst. 1993;85:884–891. doi: 10.1093/jnci/85.11.884. [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, Rothman N, Brown ED, et al. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo- [4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995;55:4516–4519. [PubMed] [Google Scholar]
- 9.Sinha R, Knize MG, Salmon CP, et al. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. 1998;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Rothman N, Salmon CP, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 11.Ito N, Hasegawa R, Sano M, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–1506. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 12.Ohgaki H, Takayama S, Sugimura T. Carcinogenicities of heterocyclic amines in cooked food. Mutat Res. 1991;259:399–410. doi: 10.1016/0165-1218(91)90130-e. [DOI] [PubMed] [Google Scholar]
- 13.Ochiai M, Imai H, Sugimura T, et al. Induction of intestinal tumors and lymphomas in C57BL/6N mice by a food-borne carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn J Cancer Res. 2002;93:478–483. doi: 10.1111/j.1349-7006.2002.tb01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha R, Peters U, Cross AJ, et al. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res. 2005;65:8034–8041. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Kulldorff M, Chow WH, et al. Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:559–562. [PubMed] [Google Scholar]
- 16.Wu K, Giovannucci E, Byrne C, et al. Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2006;15:1120–1125. doi: 10.1158/1055-9965.EPI-05-0782. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R, Kulldorff M, Gunter MJ, et al. Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2030–2034. doi: 10.1158/1055-9965.EPI-04-0854. [DOI] [PubMed] [Google Scholar]
- 18.Glei M, Latunde-Dada GO, Klinder A, et al. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–161. doi: 10.1016/s1383-5718(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 19.Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses. 2007;68:562–564. doi: 10.1016/j.mehy.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Sesink AL, Termont DS, Kleibeuker JH, et al. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704–5709. [PubMed] [Google Scholar]
- 21.Sesink AL, Termont DS, Kleibeuker JH, et al. Red meat and colon cancer: dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis. 2000;21:1909–1915. doi: 10.1093/carcin/21.10.1909. [DOI] [PubMed] [Google Scholar]
- 22.Pierre F, Freeman A, Tache S, et al. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. J Nutr. 2004;134:2711–2716. doi: 10.1093/jn/134.10.2711. [DOI] [PubMed] [Google Scholar]
- 23.Hughes R, Cross AJ, Pollock JR, et al. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22:199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- 24.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 25.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 26.Tseng M, Greenberg ER, Sandler RS, et al. Serum ferritin concentration and recurrence of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2000;9:625–630. [PubMed] [Google Scholar]
- 27.Tseng M, Murray SC, Kupper LL, et al. Micronutrients and the risk of colorectal adenomas. Am J Epidemiol. 1996;144:1005–1014. doi: 10.1093/oxfordjournals.aje.a008871. [DOI] [PubMed] [Google Scholar]
- 28.Tseng M, Sandler RS, Greenberg ER, et al. Dietary iron and recurrence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1997;6:1029–1032. [PubMed] [Google Scholar]
- 29.Chan AT, Ma J, Tranah GJ, et al. Hemochromatosis gene mutations, body iron stores, dietary iron, and risk of colorectal adenoma in women. J Natl Cancer Inst. 2005;97:917–926. doi: 10.1093/jnci/dji165. [DOI] [PubMed] [Google Scholar]
- 30.Monsen ER, Balintfy JL. Calculating dietary iron bioavailability: refinement and computerization. J Am Diet Assoc. 1982;80:307–311. [PubMed] [Google Scholar]
- 31.Balder HF, Vogel J, Jansen MC, et al. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:717–725. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- 32.Larsson SC, Adami HO, Giovannucci E, et al. Re: Heme iron, zinc, alcohol consumption, and risk of colon cancer. J Natl Cancer Inst. 2005;97:232–233. doi: 10.1093/jnci/dji032. author reply 233-4. [DOI] [PubMed] [Google Scholar]
- 33.Lee DH, Anderson KE, Harnack LJ, et al. Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women's Health Study. J Natl Cancer Inst. 2004;96:403–407. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 34.Kabat GC, Miller AB, Jain M, et al. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br J Cancer. 2007;97:118–122. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Torres C, Leets I, Taylor P, et al. Heme, ferritin and vegetable iron absorption in humans from meals denatured of heme iron during the cooking of beef. J Nutr. 1986;116:1720–1725. doi: 10.1093/jn/116.9.1720. [DOI] [PubMed] [Google Scholar]
- 36.Schricker BRM. D.D. Effects of Cooking and Chemical Treatment on Heme and Nonheme Iron in Meat. Journal of Food Science. 1983;48:1340–1343. [Google Scholar]
- 37.Igene JO, King JA, Pearson AM, Gray JI. Influence of heme pigments, nitrite and nonheme iron development of warned-over flavor (WOF) in cooked meat. Journal of Agricultural and Food Chemistry. 1979;27:838–844. [Google Scholar]
- 38.Bogovski P, Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27:471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- 39.Knekt P, Jarvinen R, Dich J, et al. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer. 1999;80:852–856. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Ward MH, Cross AJ, Divan H, et al. Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis. 2007;28:1210–1216. doi: 10.1093/carcin/bgm009. [DOI] [PubMed] [Google Scholar]
- 41.Sinha R, Cross A, Curtin J, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res. 2005;49:648–655. doi: 10.1002/mnfr.200500018. [DOI] [PubMed] [Google Scholar]
- 42.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 43.Diet History Questionnaire Version 1.0. Applied Research Program, National Cancer Institute, National Institutes of Health; 2000. [Google Scholar]
- 44.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 45.Willett WC. Nutritional Epidemiology. 2nd Edition. New York: Oxford University Press; 1998. [Google Scholar]
- 46.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 47.Fleiss JL, Cohen J, Everitt BS. Large-sample standard errors of kappa and weighted kappa. Psychological Bulletin. 1969;72:323–327. [Google Scholar]
- 48.Cicchetti DV, Allison T. A new procedure for assessing reliability of scoring EEG sleep recordings. American Journal of EEG Technology. 1971;11:101–109. [Google Scholar]
- 49.Yoon H, Benamouzig R, Little J, et al. Systematic review of epidemiological studies on meat, dairy products and egg consumption and risk of colorectal adenomas. Eur J Cancer Prev. 2000;9:151–164. [PubMed] [Google Scholar]
- 50.Sinha R, Chow WH, Kulldorff M, et al. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999;59:4320–4324. [PubMed] [Google Scholar]
- 51.Martinez ME, Jacobs ET, Ashbeck EL, et al. Meat Intake, Preparation Methods, Mutagens, and Colorectal Adenoma Recurrence. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm179. [DOI] [PubMed] [Google Scholar]
- 52.Gunter MJ, Probst-Hensch NM, Cortessis VK, et al. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis. 2005;26:637–642. doi: 10.1093/carcin/bgh350. [DOI] [PubMed] [Google Scholar]
- 53.Wurzelmann JI, Silver A, Schreinemachers DM, et al. Iron intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:503–507. [PubMed] [Google Scholar]
- 54.Herrinton LJ, Friedman GD, Baer D, et al. Transferrin saturation and risk of cancer. Am J Epidemiol. 1995;142:692–698. doi: 10.1093/oxfordjournals.aje.a117698. [DOI] [PubMed] [Google Scholar]
- 55.Knekt P, Reunanen A, Takkunen H, et al. Body iron stores and risk of cancer. Int J Cancer. 1994;56:379–382. doi: 10.1002/ijc.2910560315. [DOI] [PubMed] [Google Scholar]
- 56.Nelson RL. Iron and colorectal cancer risk: human studies. Nutr Rev. 2001;59:140–148. doi: 10.1111/j.1753-4887.2001.tb07002.x. [DOI] [PubMed] [Google Scholar]
- 57.Cross AJ, Gunter MJ, Wood RJ, et al. Iron and colorectal cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study. Int J Cancer. 2006;118:3147–3152. doi: 10.1002/ijc.21780. [DOI] [PubMed] [Google Scholar]