Abstract
BACKGROUND
Previous studies have suggested injury to the anterior talofibular ligament may be linked to altered kinematics and the development of osteoarthritis of the ankle joint. However, the effects of ATFL injury on the in vivo kinematics of the ankle joint are unclear.
HYPOTHESIS
Based on the orientation of the ATFL fibers, we hypothesized that ATFL deficiency would lead to increased anterior translation and increased internal rotation of the talus relative to the tibia.
STUDY DESIGN
Controlled laboratory study.
METHODS
The ankles of 9 patients with unilateral ATFL injuries were compared as they stepped onto a level surface. Kinematic measurements were made as a function of increasing load. Using magnetic resonance imaging and orthogonal fluoroscopy, the in vivo kinematics of the tibiotalar joint were measured in the ATFL deficient and intact ankles from the same individuals.
RESULTS
A statistically significant increase in internal rotation, anterior translation, and superior translation of the talus was measured in ATFL deficient ankles as compared to intact, contralateral controls. For example, at 100% body weight, ATFL deficient ankles demonstrated a statistically significant increase in anterior translation of 0.9 ± 0.5mm (p = 0.008). At 100% body weight, the ATFL deficient ankle was internally rotated relative to the intact ankle by 5.7 ± 3.6° (p = 0.008). There was a slight increase of 0.2 ± 0.2mm in the superior translation of the ATFL deficient ankle compared to the intact ankle at 100% body weight (p = 0.02).
CONCLUSIONS
ATFL deficiency increases anterior translation, internal rotation, and superior translation of the talus.
CLINICAL RELEVANCE
Altered kinematics may contribute to the degenerative changes observed with chronic lateral ankle instability. These findings might help to explain the degenerative changes frequently observed on the medial talus in patients with chronic ATFL insufficiency and provide a baseline for improving ankle ligament reconstructions aimed at restoring normal joint motion.
Keywords: lateral ankle instability, anterior talofibular ligament, osteoarthritis, tibiotalar joint, ankle kinematics, ankle biomechanics
Introduction
Lateral ankle ligament sprains are one of the most common injuries in sports and recreation19, 23, 37, 44, 51 and are among the most frequently treated injuries in emergency departments 14, 21. Although many patients have good clinical outcomes after conservative treatment, a significant number of patients experience chronic pain, instability, loss of range of motion, poor proprioception, and develop osteoarthritis 18, 20, 21, 23, 28, 32, 33, 42, 44, 48, 49, 51. Between 10 and 40% of patients with lateral ankle sprains have been thought to experience chronic symptoms1, 8, 11, 15, 22, 35, 39, 45, 49, 51.
Many investigators have reported a relationship between chronic lateral ankle instability and the development of degenerative changes 20, 23, 51, 52. For example, one study reported that of 36 patients with chronic lateral ankle instability for at least 10 years, 72% had evidence of arthritic changes in the tibiotalar joint20. More recent patient studies attributed 13-16% of ankle arthritis to ligamentous injuries51, 52, 85% of whom had sustained injury to the lateral ankle ligaments51. Many investigators have hypothesized that altered kinematics are a factor contributing to the development of osteoarthritis after lateral ankle ligament injuries 18, 20, 22, 23, 44, 48, 51, 52.
While these studies suggest a relationship between altered kinematics and the development of osteoarthritis, the effect of lateral ankle injuries on the in vivo weight-bearing kinematics of the ankle joint are unclear. Previous studies have measured the effects of rupture of the lateral ankle ligaments on joint biomechanics in cadavers 5, 16, 38, 44, 46. However, it is difficult to reproduce the complex in vivo loading environment on cadavers38. Other studies have used skin markers and videographic analyses to study the altered biomechanics after lateral ankle ligament injury 10. Although these studies provide valuable data on altered biomechanics after lateral ankle stability, it is difficult to measure directly the motion of the tibiotalar joint using skin markers.
Therefore, the objective of our study was to quantify the effects of lateral ankle instability on the motion of the tibiotalar joint under in vivo weight-bearing loading. Using a combination of a 3D magnetic resonance model and dual-orthogonal fluoroscopes, ankles with lateral instability were compared in vivo to contralateral controls under physiological loading conditions. We hypothesized that lateral ankle instability leads to increased anterior translation and increased internal rotation of the talus due to the orientation of the fibers of the anterior talofibular ligament (ATFL).
Methods
Patient Recruitment
A total of nine subjects (5 males, 4 females) were studied with IRB approval from our institution. All subjects had a body mass index of less than 30, and were aged 19-57 years. Subjects recruited in this study had unilateral ankle sprains which had been treated conservatively for at least 6 months. All subjects reported symptoms of chronic lateral ankle instability, including pain, feelings of instability, and recurrent ankle sprains. Patients with cartilaginous lesions (visualized during study MRI or after the study during surgery) were excluded. All subjects were required to have a healthy, normal contralateral ankle, confirmed by both physical examination and MRI, or they were excluded from study.
Each subject was clinically examined by a board-certified orthopaedic foot and ankle surgeon, who serves as a mentor to our foot and ankle fellows and has vast experience in foot and ankle. The surgeons defined the diagnosis of chronic instability as a history of recurrent ankle sprains and a sensation of instability in the setting of positive physical exam findings, including a positive anterior drawer or talar tilt tests. To corroborate the clinical findings of instability, a fellowship-trained, board-certified musculoskeletal radiologist confirmed that all subjects had unilateral ATFL injury using magnetic resonance (MR) imaging. For patients that underwent surgery after the study, these findings were confirmed intraoperatively. Though the anterior talofibular ligament (ATFL) is the most frequently involved ligament in lateral ankle injuries, additional injury to the calcaneofibular ligament (CFL) is common 9, 14, 22, 28, 34, 51. Patients with isolated ATFL injuries are difficult to recruit, so 4 patients included in this study had combined ATFL/CFL injuries.
Imaging
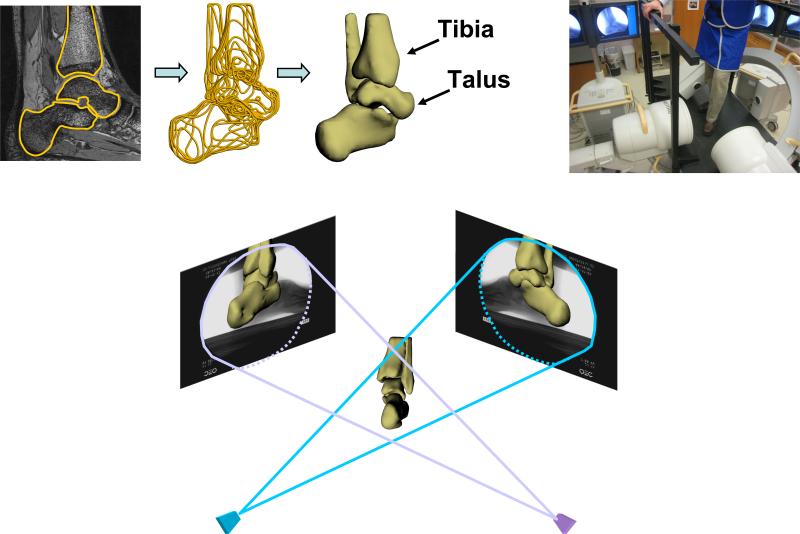
Subjects had both ankles MR imaged at the Center for Advanced Magnetic Resonance Development in the Department of Radiology using a 3.0T magnet (Trio, Siemens, Germany) and a dedicated 8 channel receive-only foot and ankle coil (Invivo, Orlando FL). For each subject, both ankles were imaged separately using a 3D double-echo steady state sequence (DESS, Flip angle 25 degrees, TE 6 msec, TR 17 msec) with a 15cm by 15cm field of view. The images produced had a resolution of 512 by 512 pixels and a slice thickness of 0.7 mm. Each ankle was scanned twice, once with water excitation and once without. In each sagittal image, the anatomy of the tibia and talus were outlined using solid modeling software (Rhinoceros, McNeel and Associates, Seattle WA). Comparison of water excitation and non-water excitation images allowed for accurate outlining of complex bone and soft tissue interfaces. Each contour was then placed in the appropriate plane in space, and the curves were used to generate a 3D surface model of the tibiotalar joint (Figure 1).
Figure 1.
3D models of the tibia and talus were created from high-resolution MR images (upper left). The patient then stepped onto a level surface with increasing body weight while being imaged using orthogonal fluoroscopy (upper right). The 3D models of the tibia and talus were manipulated in 6 degrees-of-freedom until their projection matched that of the orthogonal fluoroscopic images (bottom).
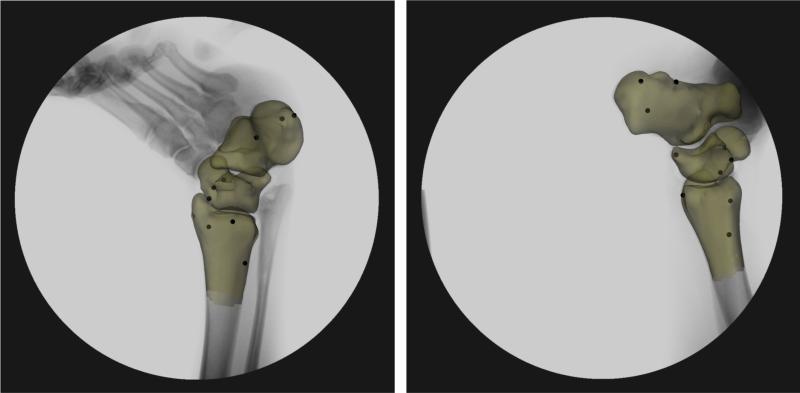
Next, each patient was imaged using two fluoroscopes (Pulsera, Philips, The Netherlands) positioned orthogonally above a platform. The fluoroscopes were wired to take images simultaneously with a resolution of 1024 by 1024 pixels. Subjects stood on the platform and stepped onto a level surface within the beam of both fluoroscopes. Subjects repeated this step for each ankle, increasing the load from 25 to 100 percent of their body weight as measured by a force plate. One fluoroscope obtained an anterolateral image, the other an anteromedial image (Figure 1).
Kinematics Measurements
Each pair of fluoroscopic images was imported into solid modeling software, where they were positioned in a 3D environment to reproduce the orthogonal orientation of the fluoroscopes during testing. Using a pixel density gradient matrix, edge detection software was used to outline the bone structures on the fluoroscopic images 12. The 3D MR model of the tibia and talus was imported into this virtual dual-orthogonal fluoroscopic system. The 3D model was viewed from two directions corresponding to the location of the image source of the fluoroscopes. Then, the position of the tibial and talar models were individually manipulated in six degrees-of-freedom until their projections, as viewed from the two orthogonal directions, matched the outlines on the fluoroscopic images (Figure 1). Thus, the 3D models were used to reproduce the 3D motion of the tibiotalar joint during in vivo weight-bearing loading.
In order to quantify the motion at the tibiotalar joint, a Cartesian coordinate system was constructed for each subject based on the 3D anatomy of the tibia and talus. In order to reduce variability, the coordinate systems were created on both the intact and injured joints simultaneously. 12 Because the same coordinate system was drawn on both the intact and deficient ankles, the motion of the two joints was directly compared using the same anatomic coordinate systems. First, point clouds of approximately 5,000 points were created from the 3D models of each talus and tibia. The point cloud of the deficient ankle was then aligned to the position of the intact ankle using an iterative closest point technique 12, 31. In this fashion, the position of the intact ankle under minimal load (approximately 10N) was defined as neutral for both ankles. Next, the coordinate axes were drawn on the 3D models. To define the proximal-distal axis, a cylinder was fit to the shaft of the tibia. The medial-lateral axis was defined by a segment connecting the most medial and lateral extremes of the tibia. Finally, the anterior-posterior axis was positioned orthogonal to these two axes. To determine the origin of the axis, the talus was visualized in the sagittal plane and a circle was fitted to the curve of the talar dome. The center of this circle served to define the origin in the sagittal plane. The medial-lateral component of the origin was defined by the geometric center of the surface area of the talar dome.
The coordinate systems were used to measure the six degrees-of-freedom kinematics of the tibiotalar joint. Measurements included anteroposterior, mediolateral, and superoinferior translations as well as internal-external rotation, dorsiflexion-plantarflexion, and inversion-eversion. These rotations were represented by an Euler angle sequence, where plantarflexion-dorsiflexion is measured about a fixed axis in the tibia, internal rotation about a fixed axis in the talus, and inversion-eversion about an axis perpendicular to the other two.
Validation Study
To validate the accuracy and repeatability of this system to measure kinematics, a pilot study on a cadaveric ankle was conducted. First, the ankle was MR imaged with the protocol described above to construct a 3D model of the ankle joint. Next, sets of spherical, radio-opaque markers13 with a diameter of 2.38 mm and a tolerance of 0.002 mm were implanted into the tibia, calcaneus, and talus. These markers were used to measure the motion of the ankle as it was moved from neutral to maximal dorsiflexion and maximal plantarflexion. Orthogonal images in each position were recorded using the two fluoroscopes. The orthogonal fluoroscopic images and 3D computer model of the ankle were imported into solid modeling software. The 3D models of the tibia, talus, and calcaneus were manipulated in six degrees-of-freedom to create a reproduction of the ankle in space as described above (Figure 2). Five independent matching trials were performed with the ankle in neutral, dorsiflexion, and plantarflexion.
Figure 2.
Radio-opaque markers were implanted into a cadaver for a validation study. The accuracy of the system in measuring tibiotalar kinematics was within 0.04 ± 0.11mm and 0.2±0.1°.
The motions of the marker sets were tracked using edge detection algorithms based on the gradient of pixel intensity 12, 13 within each fluoroscopic image. The center of each bead on the two orthogonal fluoroscopic images was then used to calculate the position of each marker in 3D space. From these data, the relative motion of the ankle joint measured using the combined fluoroscopic and MR imaging techniques was compared to the motion measured using the marker sets. Orthogonal coordinate systems were created on the ankle, and the difference in translation and rotation between the markers and model-based matching technique were calculated. The average error in displacement across each of the five trials for each position was 0.04 ± 0.11mm, and the average error in rotation was 0.2 ± 0.1°. Therefore, we are confident in our ability to measure the changes in motion as attributed to lateral ankle instability.
Data Analysis
The kinematics data for each ankle (anteroposterior, mediolateral, and superoinferior translations as well as internal-external rotation, dorsiflexion-plantarflexion, and inversion-eversion) was averaged as a function of percent body weight. At each loading level, the motion of the ATFL deficient ankle was compared to the corresponding contralateral intact ankle using the Wilcoxon Signed-Rank Test. This nonparametric alternative to the paired t-test does not require that the data be normally distributed and therefore, in general, is more robust than the paired t-test for a small sample size 17. Differences were considered statistically significant where p < 0.05.
Results
Anteroposterior Translation
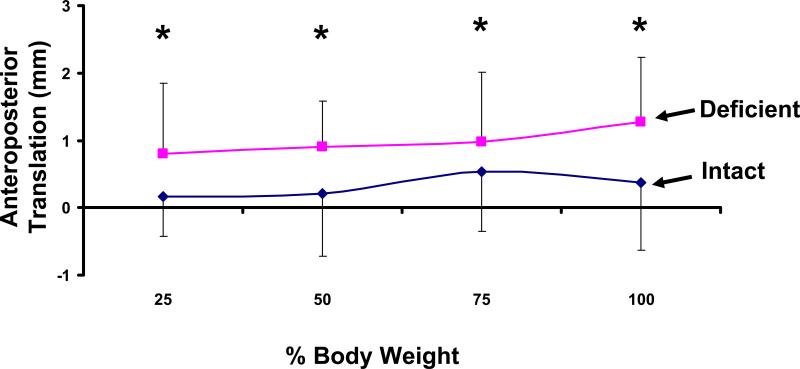
On average, the talus of both ATFL deficient and intact ankles translated anteriorly under the applied load (Figure 3). In the intact ankles, the talus translated anteriorly from a mean of 0.2 ± 0.6mm at 25% body weight to 0.4 ± 1.0mm at 100% body weight. A similar trend was detected in the ATFL deficient ankles. However, there were statistically significant increases in anterior translation compared to the intact ankle at each loading level. For example, at 100% body weight the injured talus translated anteriorly by 1.3 ± 0.9mm. This represents an increase of 0.9 ± 0.6mm compared to the intact ankle (p = 0.008). All 9 of the injured ankles translated anteriorly relative to the intact ankle.
Figure 3.
Weight-bearing loads caused an anterior translation in both the intact and ATFL deficient ankles. Statistically significant increases were observed in the deficient ankle compared to the intact ankle (* p < 0.05).
Mediolateral Translation
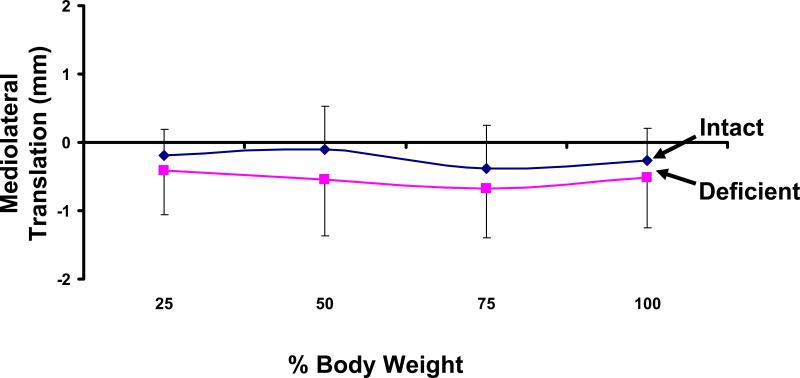
With weight-bearing loading, there was a slight lateral translation of the talus in both the intact and ATFL deficient ankles with weight-bearing loading (Figure 4). In the intact ankle, the lateral position of the talus ranged from 0.1 ± 0.6mm at 50% body weight to 0.4 ± 0.6mm at 75% body weight. Similar values were found for the deficient ankle, ranging from 0.4 ± 0.6mm at 25% body weight to 0.7 ± 0.7mm at 75% body weight. No statistically significant differences were detected between the intact and unstable ankles.
Figure 4.
Weight-bearing loads caused a lateral translation of the talus in both the intact and ATFL deficient ankles. No statistically significant differences were detected.
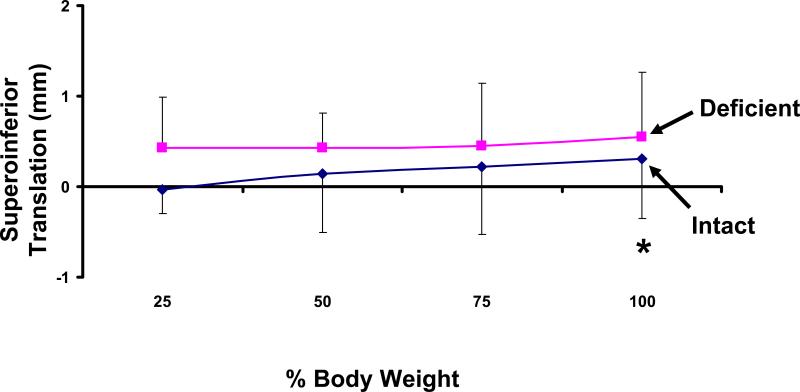
Superoinferior Translation
With increasing loading, the talus displaced superiorly relative to the tibia in both the intact and injured ankles (Figure 5). In the intact ankle the superior displacement ranged from 0.0 ± 0.3mm at 25% body weight to 0.3 ± 0.7mm at 100% body weight. A similar trend was observed in the injured ankle, where superior translation ranged from 0.4 ± 0.6mm at 25% body weight to a maximum of 0.6 ± 0.7mm at 100% body weight. There was a statistically significant increase in the superior translation of the talus in the injured ankle compared to the intact ankle at 100% body weight (p = 0.02). Eight ankles translated superiorly, and one ankle was within 0.1mm of the contralateral side.
Figure 5.
Weight-bearing loads caused a superior translation of the talus relative to the tibia. At 100% body weight, a statistically significant increase in superior translation was observed in the ATFL deficient ankle (*p < 0.05).
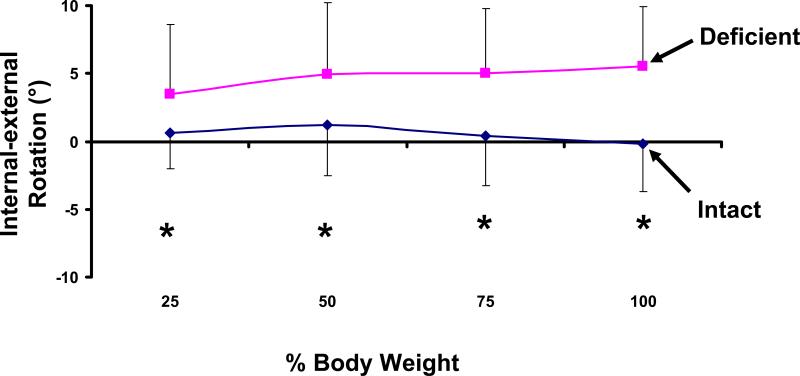
Internal-External Rotation
The internal rotation of the talus relative to the tibia in the intact ankles did not change dramatically with increasing load (Figure 6). At 25% body weight, the talus was a mean of 0.6 ± 2.7° internally rotated, compared to 0.2 ± 3.5° of external rotation at 100% body weight. The talus in the deficient ankles rotated internally with increasing body weight, ranging from a minimum of 3.5 ± 5.1° at 25% body weight to a maximum of 5.5 ± 4.4° at 100% body weight. There was a statistically significant increase in the internal rotation of the talus at each loading level. For example, at 100% body weight, the injured talus was rotated internally by 5.7 ± 3.7° compared to the intact talus (p = 0.008). All 9 of the injured ankles rotated internally relative to the intact ankle.
Figure 6.
ATFL deficient caused a statistically significant internal rotation of the ATFL deficient ankle relative to the intact ankle (*p < 0.05).
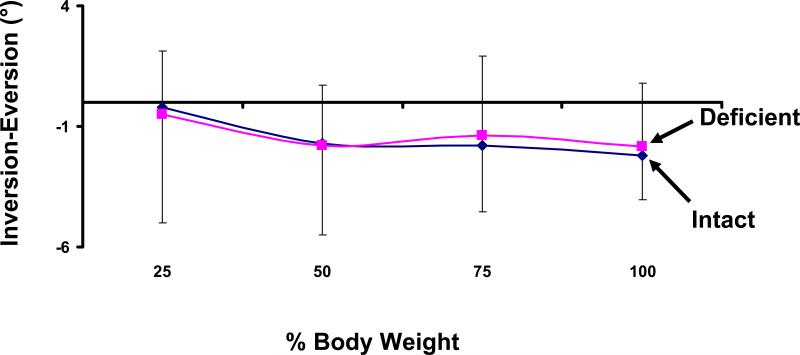
Inversion-Eversion
On average, the talus in both ATFL deficient and ATFL intact ankles showed increasing eversion with increased load (Figure 7). Intact ankles everted from 0.4 ± 1.3° to 2.5 ± 2.7° as the applied body weight increased from 25% to 100%. Similarly, the ATFL intact ankles everted from 0.6 ± 2.3° to 2.2± 2.2°. The differences in inversion between intact and injured ankles were less than 0.4° under this loading. No statistically significant differences in inversion were detected between ATFL deficient and intact ankles.
Figure 7.
Weight-bearing loads caused an eversion of the talus. No statistically significant differences were observed between intact and ATFL deficient ankles.
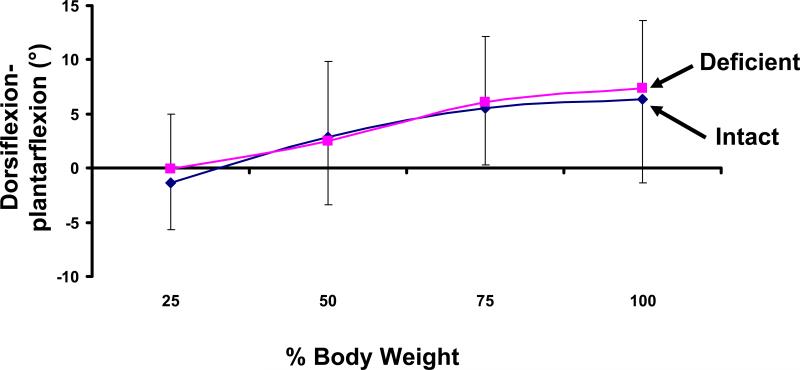
Dorsiflexion-Plantarflexion
Both the intact and injured ankles dorsiflexed with increasing load (Figure 8). In the intact ankle, the talus was 1.3 ± 4.3° plantarflexed at 25% body weight and dorsiflexed to 6.4 ± 7.7° at 100% body weight. Similarly, the deficient ankle ranged from 0.0 ± 5.0° of plantarflexion at 25% to 7.3 ± 6.2° of dorsiflexion at 100% body weight. No statistically significant differences in plantarflexion-dorsiflexion were observed between injured and intact ankles.
Figure 8.
Increasing weight-bearing loading caused an increase in dorsiflexion of the talus. No statistically significant differences were observed between the intact and ATFL deficient ankles.
Discussion
In this study, we used a combination of a 3D magnetic resonance and orthogonal fluoroscopic imaging to investigate the in vivo tibiotalar joint motion of patients with lateral ankle instability under physiological loading conditions. Six degrees-of-freedom kinematics were compared between the injured ankle and the intact, normal contralateral ankle. We found that ATFL deficiency increased anterior translation and increased internal rotation of the talus relative to the tibia. In addition, an increase in the superior translation of the talus was observed. All 9 of the ankles translated anteriorly and rotated internally. Regarding superior translation, 8 ankles translated superiorly, and one ankle was within 0.1mm of the contralateral side. The strength of these trends is reflected by the smaller p values for anterior translation and internal rotation, compared to superior translation.
Previous studies have focused on the role of the ATFL in resisting anterior translation and inversion 24-27, 32, 42. However, some studies have reported that the ATFL resists internal rotation of the talus 38, 43, 47. For example, Omori et al applied a 100N compressive load to cadaveric ankles and showed that transecting the ATFL and CFL caused increases in inversion and an increase in internal rotation only in 10° plantarflexion 38. The function for the ATFL in the current study is somewhat different than that reported in previous studies in that we did not detect a large difference in inversion, but measured significant differences in internal rotation. However, our findings are in agreement with a cadaveric study by Stormont et al, who reported that under a compressive load of 670N, the ATFL and CFL did not contribute significantly to the stability of the ankle in resisting inversion. Rather, the articular surfaces were thought to constrain inversion. In their study, the ATFL also provided resistance to the internal rotation of the talus. Our finding that injury to the lateral ligaments has a greater affect on the internal rotation compared to inversion may be further explained by the differences between in vivo and in vitro loading conditions. As noted previously 38, the levels of loading applied in vivo might be larger than those applied during cadaveric studies. Furthermore, the active musculature present during in vivo loading might provide additional stability to help prevent the inversion of the talus during weight-bearing.
The findings of increased anterior translation and internal rotation in ATFL deficient ankles may be explained by the orientation of the ATFL (Figure 9). The ATFL originates on the anterior border of the lateral malleolus and courses anteromedially and inserts on the talar body just anterior to the lateral malleolar articular surface 50. The orientation of the ATFL suggests that it restricts the anterior translation of the lateral side of the talus, thereby countering the anterior translation of the talus that we observed with increasing weight-bearing. In addition, because the medial constraints are intact, the lateral side of the talus is preferentially unstable, which could explain our observation of increased internal rotation of the talus. Further support for the role of the ATFL in internal rotational stability can be found in measurements made by Taser et al50. They reported that as the ATFL runs from the fibula to the talus, it angles 25° distally from the horizontal plane and 47° medially from the sagittal plane 50. These data suggest that the ATFL insufficiency might result in larger changes in rotational stability as compared to inversion stability.
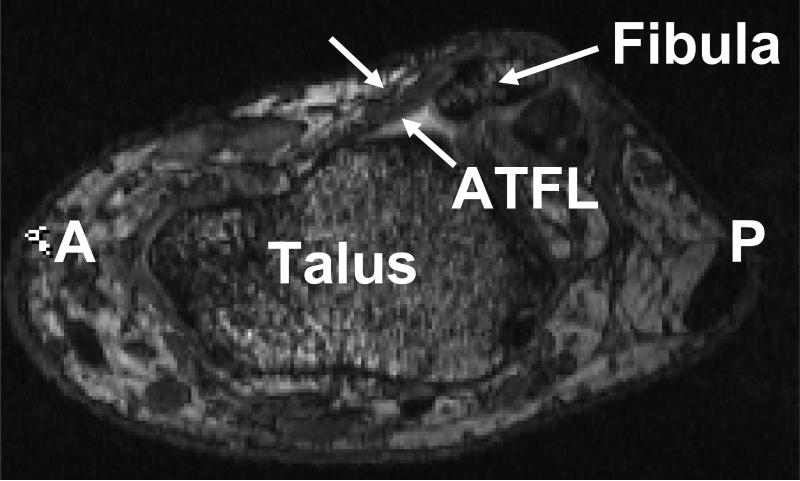
Figure 9.
The oblique orientation of the anterior talofibular ligament in the transverse plane suggests that it resists the anterior translation and internal rotation of the talus. (A = Anterior, P = Posterior)
Previous authors have reported an association between lateral ankle instability and the early onset of post-traumatic osteoarthritis20, 48. Many investigators have hypothesized that a change in kinematics and cartilage loading may explain osteoarthritic lesions on the medial talus in prolonged ankle instability. Harrington observed increased wear on the medial portion of the tibiotalar joint in patients who had experienced lateral ankle sprains ten years previously 20. It was hypothesized that these changes were due to increased stresses on the medial portion of the joint as a result of abnormal kinematics 20. Taga et al reported that patients with chronic ankle instability had an increased incidence of cartilage lesions, particularly in the anteromedial portion of the joint 48. They hypothesized increased anterior translation of the talus in chronic ankle instability might contribute to the increased incidence of cartilage lesions in the joint 48. Hinterman et al 22 noted that 72% of patients with lateral ankle injuries had cartilage damage, primarily on the medial portion of the talus. They hypothesized that the damage might be due to increased contact stresses in this region 22. Whether the development of osteochondral defects is in fact a result of altered kinematics or due to other factors (e.g., initial trauma to the cartilage) remains unclear.
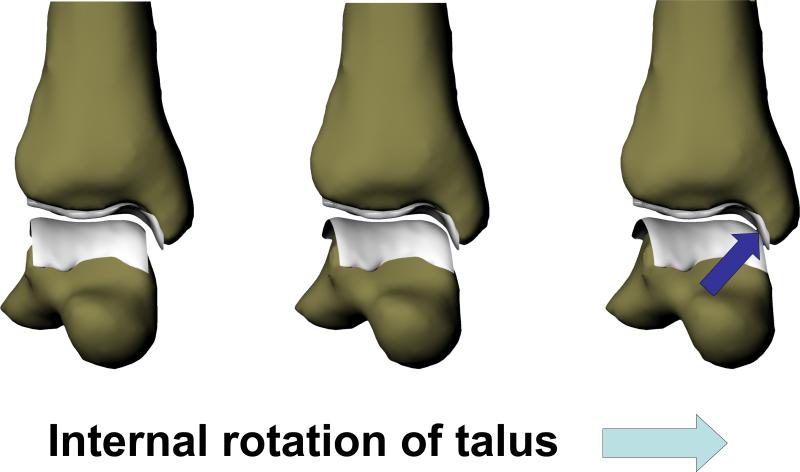
The findings of altered anterior translation, internal rotation, and superior translation of the talus in the current study might support the hypothesis that altered kinematics contribute to the degeneration of the tibiotalar joint that is observed in patients 20, 22, 48. Previous authors have suggested that increased anterior translation of the talus might increase wear on tibiotalar joint48. There may also be a relationship between the increased internal rotation of the talus detected in this study and the development of osteoarthritis. Increased internal rotation may lead to increased cartilage loading on the medial side of the talus, due to the curved surface of the cartilage on the medial portion of the tibia (Figure 10). Furthermore, increased rotation might cause increased shear stresses in the cartilage and predispose the joint to degenerative changes 2-4. These findings might help to explain the increased degeneration observed on the medial talus in patients with lateral ankle instability 20, 22, 48. Finally, our observation of increased superior translation of the talus relative to the tibia might indicate that there are increased compressive strains in the cartilage of the ATFL deficient joint. Future studies will address the effects of altered motion on in vivo contact patterns in these patients.
Figure 10.
Increased internal rotation may lead to increased cartilage loading on the medial side of the talus, due to the curved surface of the cartilage on the medial portion of the tibia.
The kinematic data elucidated by this study may also have important clinical applications to reconstruction of the lateral ankle ligaments. Understanding how ATFL deficiency affects the motion of the tibiotalar joint may provide direction in the design and evaluation of reconstructive techniques. A major goal of either repair or reconstruction is to restore normal motion to the joint, and hence prevent degenerative changes to the cartilage 30, 44, 48, 49. Currently, the appropriate surgical treatment for ankle ligament injuries remains controversial 11, 21, 28-30, 34, 36, 40, 41, 44, with more than 80 different reconstruction and repair techniques described in the literature 7. Many authors advocate anatomic reconstruction, arguing that tenodesis does not restore normal anatomy and normal motion 6, 30. A recent long-term follow-up study by Krips et al advocated an anatomic reconstruction over tenodesis30. However, radiographic evidence of degenerative changes was observed in 60% of patients in the anatomic reconstruction group and 80% of patients in the Evans tenodesis group30. The ability of current surgical techniques to prevent the development of osteoarthritis remains unclear. The findings of this study may help surgeons to focus their efforts on reconstructive techniques that counter the increased internal rotation and anterior translation found with lateral ankle instability.
There are some limitations with this study. This study only examined quasi-static weight-bearing motion of the joint. Future studies should to evaluate the dynamic motion of the tibiotalar joint during gait. Furthermore, the independent contributions of ATFL, CFL, and other soft tissue injuries to the kinematics of lateral ankle injuries could not be elucidated in this study because of the small patient population. Future studies might investigate the differences between these types of injuries. Finally, future studies might also investigate ATFL-deficient subjects who do not have ankle instability to see if they show the same changes in kinematics as those with ankle instability.
In summary, this study combined a 3D magnetic resonance model and dual-orthogonal fluoroscopes to compare chronically unstable ankles in vivo to contralateral controls. We found that under weight-bearing loading, ATFL deficient ankles showed a significant increase in anterior translation, internal rotation, and superior translation of the talus compared to intact controls. These data may provide insight into the mechanisms behind the development of osteoarthritis and provide baseline data for evaluating the surgical treatment of these injuries.
Acknowledgments
This work was supported by the NIH (R03AR055659). The authors also gratefully acknowledge the financial support of the Division of Orthopaedics and the Department of Radiology. We would also like to thank Farshid Guilak, Ph.D. for his helpful comments regarding this work. The technical support of Johanna Bischof, Libby Pennington, Ermias Abebe, Gary Utturkar, and Dean C Taylor M.D. is greatly appreciated.
References
- 1.Ajis A, Younger AS, Maffulli N. Anatomic repair for chronic lateral ankle instability. Foot Ankle Clin. 2006 Sep;11(3):539–545. doi: 10.1016/j.fcl.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Anderst WJ, Tashman S. The association between velocity of the center of closest proximity on subchondral bones and osteoarthritis progression. J Orthop Res. 2008 Jul 16; doi: 10.1002/jor.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006 Jan;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004 Mar;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 5.Aydogan U, Glisson RR, Nunley JA. Extensor retinaculum augmentation reinforces anterior talofibular ligament repair. Clin Orthop Relat Res. 2006 Jan;442:210–215. doi: 10.1097/01.blo.0000183737.43245.26. [DOI] [PubMed] [Google Scholar]
- 6.Baumhauer JF, O'Brien T. Surgical Considerations in the Treatment of Ankle Instability. J Athl Train. 2002 Dec;37(4):458–462. [PMC free article] [PubMed] [Google Scholar]
- 7.Becker HP, Rosenbaum D, Zeithammel G, et al. Tenodesis versus carbon fiber repair of ankle ligaments: a clinical comparison. Clin Orthop Relat Res. 1996 Apr;(325):194–202. doi: 10.1097/00003086-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Bell SJ, Mologne TS, Sitler DF, Cox JS. Twenty-six-year results after Brostrom procedure for chronic lateral ankle instability. Am J Sports Med. 2006 Jun;34(6):975–978. doi: 10.1177/0363546505282616. [DOI] [PubMed] [Google Scholar]
- 9.Bennett WF. Lateral ankle sprains. Part I: Anatomy, biomechanics, diagnosis, and natural history. Orthop Rev. 1994 May;23(5):381–387. [PubMed] [Google Scholar]
- 10.Brown C, Padua D, Marshall SW, Guskiewicz K. Individuals with mechanical ankle instability exhibit different motion patterns than those with functional ankle instability and ankle sprain copers. Clin Biomech (Bristol, Avon) 2008 Jul;23(6):822–831. doi: 10.1016/j.clinbiomech.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.de Vries JS, Krips R, Sierevelt IN, Blankevoort L. Interventions for treating chronic ankle instability. Cochrane Database Syst Rev. 2006;(4):CD004124. doi: 10.1002/14651858.CD004124.pub2. [DOI] [PubMed] [Google Scholar]
- 12.DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006 Aug;34(8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 13.Defrate LE, van der Ven A, Boyer PJ, Gill TJ, Li G. The measurement of the variation in the surface strains of Achilles tendon grafts using imaging techniques. J Biomech. 2006;39(3):399–405. doi: 10.1016/j.jbiomech.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 14.DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004 Jan;23(1):1–19. v. doi: 10.1016/S0278-5919(03)00095-4. [DOI] [PubMed] [Google Scholar]
- 15.Frigg A, Magerkurth O, Valderrabano V, Ledermann HP, Hintermann B. The Effect of Osseous Ankle Configuration on Chronic Ankle Instability. Br J Sports Med. 2007 Feb 2; doi: 10.1136/bjsm.2006.032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii T, Kitaoka HB, Watanabe K, Luo ZP, An KN. Comparison of modified brostrom and Evans procedures in simulated lateral ankle injury. Med Sci Sports Exerc. 2006 Jun;38(6):1025–1031. doi: 10.1249/01.mss.0000222827.56982.40. [DOI] [PubMed] [Google Scholar]
- 17.Glantz SA. Primer of biostatistics. McGraw-Hill; New York: 2002. [Google Scholar]
- 18.Gross P, Marti B. Risk of degenerative ankle joint disease in volleyball players: study of former elite athletes. Int J Sports Med. 1999 Jan;20(1):58–63. doi: 10.1055/s-2007-971094. [DOI] [PubMed] [Google Scholar]
- 19.Grossman MG, ElAttrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005 Apr;21(4):418–423. doi: 10.1016/j.arthro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Harrington KD. Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. J Bone Joint Surg Am. 1979 Apr;61(3):354–361. [PubMed] [Google Scholar]
- 21.Haywood KL, Hargreaves J, Lamb SE. Multi-item outcome measures for lateral ligament injury of the ankle: a structured review. J Eval Clin Pract. 2004 May;10(2):339–352. doi: 10.1111/j.1365-2753.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 22.Hintermann B, Boss A, Schafer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002 May-Jun;30(3):402–409. doi: 10.1177/03635465020300031601. [DOI] [PubMed] [Google Scholar]
- 23.Hirose K, Murakami G, Minowa T, Kura H, Yamashita T. Lateral ligament injury of the ankle and associated articular cartilage degeneration in the talocrural joint: anatomic study using elderly cadavers. J Orthop Sci. 2004;9(1):37–43. doi: 10.1007/s00776-003-0732-9. [DOI] [PubMed] [Google Scholar]
- 24.Hollis JM, Blasier RD, Flahiff CM. Simulated lateral ankle ligamentous injury. Change in ankle stability. Am J Sports Med. 1995 Nov-Dec;23(6):672–677. doi: 10.1177/036354659502300606. [DOI] [PubMed] [Google Scholar]
- 25.Hollis JM, Blasier RD, Flahiff CM, Hofmann OE. Biomechanical comparison of reconstruction techniques in simulated lateral ankle ligament injury. Am J Sports Med. 1995 Nov-Dec;23(6):678–682. doi: 10.1177/036354659502300607. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard TJ. Ligament laxity following inversion injury with and without chronic ankle instability. Foot Ankle Int. 2008 Mar;29(3):305–311. doi: 10.3113/FAI.2008.0305. [DOI] [PubMed] [Google Scholar]
- 27.Jones AP, Sidhom S, Sefton G. A minimally invasive surgical technique for augmented reconstruction of the lateral ankle ligaments with woven polyester tape. J Foot Ankle Surg. 2007 Nov-Dec;46(6):416–423. doi: 10.1053/j.jfas.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson J, Lansinger O. Lateral instability of the ankle joint. Clin Orthop Relat Res. 1992 Mar;(276):253–261. [PubMed] [Google Scholar]
- 29.Kerkhoffs GM, Handoll HH, de Bie R, Rowe BH, Struijs PA. Surgical versus conservative treatment for acute injuries of the lateral ligament complex of the ankle in adults. Cochrane Database Syst Rev. 2002;(3):CD000380. doi: 10.1002/14651858.CD000380. [DOI] [PubMed] [Google Scholar]
- 30.Krips R, Brandsson S, Swensson C, van Dijk CN, Karlsson J. Anatomical reconstruction and Evans tenodesis of the lateral ligaments of the ankle. Clinical and radiological findings after follow-up for 15 to 30 years. J Bone Joint Surg Br. 2002 Mar;84(2):232–236. doi: 10.1302/0301-620x.84b2.12143. [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Seon JK, Shin VI, Kim GH, Jeon M. Anatomical evaluation of CT-MRI combined femoral model. Biomed Eng Online. 2008;7:6. doi: 10.1186/1475-925X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofvenberg R, Karrholm J, Ahlgren O. Ligament reconstruction for ankle instability. A 5-year prospective RSA follow-up of 30 cases. Acta Orthop Scand. 1994 Aug;65(4):401–407. doi: 10.3109/17453679408995479. [DOI] [PubMed] [Google Scholar]
- 33.Lofvenberg R, Karrholm J, Lund B. The outcome of nonoperated patients with chronic lateral instability of the ankle: a 20-year follow-up study. Foot Ankle Int. 1994 Apr;15(4):165–169. doi: 10.1177/107110079401500401. [DOI] [PubMed] [Google Scholar]
- 34.Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999 Jan;27(1):61–71. doi: 10.2165/00007256-199927010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Nyska M, Shabat S, Simkin A, Neeb M, Matan Y, Mann G. Dynamic force distribution during level walking under the feet of patients with chronic ankle instability. Br J Sports Med. 2003 Dec;37(6):495–497. doi: 10.1136/bjsm.37.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvie-Harris DJ, Gilbart M. Treatment modalities for soft tissue injuries of the ankle: a critical review. Clin J Sport Med. 1995 Jul;5(3):175–186. doi: 10.1097/00042752-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997 Oct;13(5):564–574. doi: 10.1016/s0749-8063(97)90181-x. [DOI] [PubMed] [Google Scholar]
- 38.Omori G, Kawakami K, Sakamoto M, Hara T, Koga Y. The effect of an ankle brace on the 3-dimensional kinematics and tibio-talar contact condition for lateral ankle sprains. Knee Surg Sports Traumatol Arthrosc. 2004 Sep;12(5):457–462. doi: 10.1007/s00167-004-0493-9. [DOI] [PubMed] [Google Scholar]
- 39.Peters JW, Trevino SG, Renstrom PA. Chronic lateral ankle instability. Foot Ankle. 1991 Dec;12(3):182–191. doi: 10.1177/107110079101200310. [DOI] [PubMed] [Google Scholar]
- 40.Pijnenburg AC, Van Dijk CN, Bossuyt PM, Marti RK. Treatment of ruptures of the lateral ankle ligaments: a meta-analysis. J Bone Joint Surg Am. 2000 Jun;82(6):761–773. doi: 10.2106/00004623-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Povacz P, Unger SF, Miller WK, Tockner R, Resch H. A randomized, prospective study of operative and non-operative treatment of injuries of the fibular collateral ligaments of the ankle. J Bone Joint Surg Am. 1998 Mar;80(3):345–351. doi: 10.2106/00004623-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Ringleb SI, Udupa JK, Siegler S, et al. The effect of ankle ligament damage and surgical reconstructions on the mechanics of the ankle and subtalar joints revealed by three-dimensional stress MRI. J Orthop Res. 2005 Jul;23(4):743–749. doi: 10.1016/j.orthres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Rosenbaum D, Becker HP, Wilke HJ, Claes LE. Tenodeses destroy the kinematic coupling of the ankle joint complex. A three-dimensional in vitro analysis of joint movement. J Bone Joint Surg Br. 1998 Jan;80(1):162–168. doi: 10.1302/0301-620x.80b1.8014. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum D, Bertsch C, Claes LE. NOVEL Award 1996: 2nd prize Tenodeses do not fully restore ankle joint loading characteristics: a biomechanical in vitro investigation in the hind foot. Clin Biomech (Bristol, Avon) 1997 Apr;12(3):202–209. doi: 10.1016/s0268-0033(97)00017-x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt R, Benesch S, Friemert B, Herbst A, Claes L, Gerngross H. Anatomical repair of lateral ligaments in patients with chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2005 Apr;13(3):231–237. doi: 10.1007/s00167-004-0562-0. [DOI] [PubMed] [Google Scholar]
- 46.Siegler S, Udupa JK, Ringleb SI, et al. Mechanics of the ankle and subtalar joints revealed through a 3D quasi-static stress MRI technique. J Biomech. 2005 Mar;38(3):567–578. doi: 10.1016/j.jbiomech.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Stormont DM, Morrey BF, An KN, Cass JR. Stability of the loaded ankle. Relation between articular restraint and primary and secondary static restraints. Am J Sports Med. 1985 Sep-Oct;13(5):295–300. doi: 10.1177/036354658501300502. [DOI] [PubMed] [Google Scholar]
- 48.Taga I, Shino K, Inoue M, Nakata K, Maeda A. Articular cartilage lesions in ankles with lateral ligament injury. An arthroscopic study. Am J Sports Med. 1993 Jan-Feb;21(1):120–126. doi: 10.1177/036354659302100120. discussion 126-127. [DOI] [PubMed] [Google Scholar]
- 49.Takao M, Oae K, Uchio Y, Ochi M, Yamamoto H. Anatomical reconstruction of the lateral ligaments of the ankle with a gracilis autograft: a new technique using an interference fit anchoring system. Am J Sports Med. 2005 Jun;33(6):814–823. doi: 10.1177/0363546504272688. [DOI] [PubMed] [Google Scholar]
- 50.Taser F, Shafiq Q, Ebraheim NA. Anatomy of lateral ankle ligaments and their relationship to bony landmarks. Surg Radiol Anat. 2006 Aug;28(4):391–397. doi: 10.1007/s00276-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 51.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006 Apr;34(4):612–620. doi: 10.1177/0363546505281813. [DOI] [PubMed] [Google Scholar]
- 52.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of Ankle Osteoarthritis. Clin Orthop Relat Res. 2008 Oct 2; doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]