Abstract
Background
Few studies have evaluated an individualized home-based exercise prescription during and after cancer treatment.
Objective
The purpose was to evaluate the effectiveness of a home-based exercise training intervention, the PRO-SELF FATIGUE CONTROL PROGRAM on the management of cancer related fatigue.
Interventions/Methods
Participants (N=119) were randomized into one of three groups: Group 1 (EE) received the exercise prescription throughout the study; Group 2 (CE) received their exercise prescription after completing cancer treatment; Group 3 (CC) received usual care. Patients completed the Piper Fatigue Scale, General Sleep Disturbance Scale, Center for Epidemiological Studies-Depression scale, and Worst Pain Intensity Scale.
Results
All groups reported mild fatigue levels, sleep disturbance and mild pain, but not depression. Using multilevel regression analysis significant linear and quadratic trends were found for change in fatigue and pain (i.e., scores increased, then decreased over time). No group differences were found in the changing scores over time. A significant quadratic effect for the trajectory of sleep disturbance was found, but no group differences were detected over time. No significant time or group effects were found for depression.
Conclusions
Our home-based exercise intervention had no effect on fatigue or related symptoms associated with cancer treatment. The optimal timing of exercise remains to be determined.
Implications for practice
Clinicians need to be aware that some physical activity is better than none, and there is no harm in exercise as tolerated during cancer treatment. Further analysis is needed to examine the adherence to exercise. More frequent assessments of fatigue, sleep disturbance, depression, and pain may capture the effect of exercise.
Cancer-related fatigue (CRF) is a common problem in oncology patients and survivors that has received attention during the last two decades. Although the reported prevalence of CRF varies across studies, a consensus exists that it is high both during and after cancer treatment. In addition, numerous studies suggest that increased levels of fatigue are positively associated with sleep disturbance, depression, and pain and that these symptoms have a negative impact on functional status and quality of life (QOL). While the underlying mechanisms of CRF are yet to be established, the effects of exercise on CRF and other patient outcomes has been the subject of numerous studies and suggests that exercise may have a positive effect on some symptoms. 1 In fact, in a series of systematic reviews and meta-analyses in patients with cancer, 2–16 the physiological and psychological benefits of cardiovascular exercise interventions have been enumerated including: improvement in functional capacity, 12, 15–18 physical functioning, 2, 14, 18 and cardiopulmonary fitness; 7, 11, 13, 15, 19–21 increased lean body mass; 2, 8, 13, 22 reduction in fatigue; 2, 7, 9, 12–14, 17, 23–27 improvement in mood and increase in vigor; 2, 9, 11 decrease in depression; 17, 24, 28–32 improvement in sleep; 11, 24 and improvement in QOL. 7, 10, 13, 17–18, 20, 23, 28,32–36
All of these studies are limited by timing, structure, and/or duration of the exercise interventions. Most of the exercise interventions were only conducted during cancer treatment, consisted of predominately supervised aerobic activities performed in a physical activity facility at moderate intensity several times a week for 20 to 30 minutes per session from 5 weeks to 3 months in duration. No studies were found that evaluated an individualized exercise prescription for home-based exercise training during as well as after cancer treatment. Therefore, the primary purpose of this study was to evaluate the effectiveness of a home-based exercise training intervention called the PRO-SELF FATIGUE CONTROL PROGRAM on the management of CRF. Secondary purposes were to investigate the timing of intervention, either during or after completion of cancer treatment on CRF, and to study the effects of the intervention on sleep disturbance, depression, and pain.
The Effect of the Exercise Intervention on CRF
Seven systemic reviews and meta-analyses have reported on the effects of exercise as an intervention for CRF in oncology patients. 2, 9, 11–13, 37–38 In this section, the results of studies of exercise interventions’ effects on CRF are summarized. McNeely et al. (2006) reviewed 14 randomized controlled trials of breast cancer women and survivors, of which six trials assessed the effect of exercise on CRF. Four of the six studies 18–19, 39–40 used the revised Piper Fatigue Scale (PFS). While all six studies showed improvements in fatigue with exercise on either home based or supervised, only two reported statistically significant improvements. These two studies were the ones conducted with breast cancer survivors who were on average 14 months (SD=6) past their completion of treatment 20 or 21 months (SD=18) since their cancer diagnosis. 41
In a more recent study, Schneider, Hsieh, Sprod, Carter, and Hayward 42 used two separate samples to test the effectiveness of an exercise intervention on fatigue and cardiopulmonary function in patients with breast cancer. Women participated in an individualized prescribed exercise program during cancer treatment (n=17) or after cancer treatment (n=96). The intervention included both aerobic and resistance activities. Participants attended individually supervised exercise sessions two or three days/week for six months. The exercise session lasted 60 minutes that included warm-up, 40 minutes of aerobic exercise resistance training and stretches, and 10 minutes cool down. Exercise intensity ranged from 40 to 75% of heart rate reserve. Exercise options included outdoor or treadmill walking or stationary cycling. Intensity was measured by the Borg exertion scale (range 0–10, 0: no exertion, 10: maximal exertion). Self-reported intensity of exercise began at a Borg scale rating of 2 (out of 10) and progressed as tolerated. The patients who received the exercise intervention while in active cancer treatment showed statistically significant improvements in cardiopulmonary function, two subscales of the PFS (i.e., behavioral and sensory), and in total PFS score. The patients who received the intervention after cancer treatment showed improvements in cardiopulmonary function (e.g., improved systolic blood pressure, time on treadmill), all five of the PFS subscales (i.e., behavior, sensory, affective, cognitive, and mood), and in total PFS score.
Somewhat similar results were obtained when these authors reported on 45 males with various cancer diagnoses, who participated in the same study. 43 These men were divided into during treatment and following treatment groups. They received the same six-month individualized exercise prescription as described earlier. Fatigue was significantly reduced (i.e., all five subscales and total PFS score) with the exercise intervention in the men who had completed their cancer treatment, but not in the men who received the intervention during treatment. In a similar, yet retrospective study 44, significant reductions in CRF were found 8-weeks following an individualized comprehensive exercise program that was administered during and after cancer treatment in patients who were receiving cancer treatment (n=13) and in cancer survivors who had completed their cancer treatment (n=26). The intervention included supervised aerobic and resistance exercise, educational classes, and support. The outcome measures were retrieved from medical records. Significant improvement was seen in the total Piper Fatigue Score. The sample included 13 different types of cancer; 15 of the 39 had breast cancer. These patients were a decade older (mean=63 yrs) and had more advanced cancer (6/39 stage IV) than comparable earlier studies.
Unlike the previous findings of positive outcomes of exercise interventions during cancer treatment, McNeely et al. (2006) reported on five home-based exercise intervention studies that had mixed findings. Four of the five studies were conducted during adjuvant cancer treatment and did not find significant improvements in CRF in patients who received the exercise intervention. Similar findings were also reported by a large randomized clinical trial of 245 patients with breast cancer who received a 17-week intervention of supervised aerobic or resistance exercises during their adjuvant chemotherapy. 41, 45 In the follow-up study, Pinto et al. (2008) found an initial improvement in CRF at the end of the 12-week intervention. However, these improvements were not sustained six months later. Of note, at the nine-month follow-up, some of the initial improvements in CRF were regained.
The translation of benefits from exercise interventions to clinical practice occurred rapidly through Oncology Nursing Society’s Putting Evidence into Practice (PEP) Project. Currently, considerable enthusiasm exists for recommending exercise during and after cancer treatment, despite limited data on the most effective timing of the exercise intervention and inconsistent data concerning the relationship of exercise with fatigue management during adjuvant cancer treatment. In addition, studies of the effects of exercise immediately following the completion of cancer treatment are extremely limited.
Exercise Recommendations and Guidelines
Prior to 2000, there were no cancer-specific exercise testing, prescription or training guidelines. As a result, most published intervention studies based their prescriptions on the 1998 American College of Sports Medicine (ACSM) guidelines for healthy adults. 46–47 The American Cancer Society 48 recommended that aerobic activity be performed 3 to 5 days a week for 20 to 60 minutes per session at a moderate level of intensity. According to Schwartz (2008) in her review of physical activity and cancer patients, clinicians should recommend aerobic exercise at least every other day at moderate intensity (50–75% predicted maximum heart rate or 10–14 on the 20 point Borg Scale or symptom limited) for at least 30 minutes per session. Prudent exercise guidelines include starting an exercise program with short duration, low intensity (below their perceived ability) and slowly progressing to continuous longer duration, moderate intensity exercise. 49 The optimum exercise prescription for individual cancer patients has not been fully elucidated.
Conceptual Framework of the Study
CRF was defined as a subjective sensation of unusual whole body tiredness due to cancer.50 The literature suggests that a variety of factors may contribute to CRF. These factors include changes in functional status, activity levels, psychological factors, sleep disturbances, disease, treatment and symptom factors, and diverse inherent or innate factors (e.g., demographic, disease, and treatment variables). 50–60 The most frequently cited theoretical framework for fatigue that addresses these factors in cancer patients is the Integrated Fatigue Model (IFM). 50, 58, 61–62 IFM provided the framework for the current study. CRF is the primary outcome variable of the study and sleep disturbance, depression, and pain were viewed as fatigue-related variables and therefore were secondary outcomes. In addition, innate factors were also considered as possible moderators of CRF. The Piper Fatigue Scale was derived from the IFM and was used to measure the multidimensional characteristics of CRF.
Methods
Study Design
A randomized, single blind, three-arm, controlled trial design was used. Participants were randomly assigned to one of three groups: group 1 (EE) received an individualized exercise prescription and regular follow-up throughout the study period (during and after cancer treatment, n=44), group 2 (CE) received their exercise prescription and regular follow-up after having completed cancer treatment (n=36), group 3 (CC) received usual care (which did not include exercise prescription) both during and after cancer treatment (n=39). All participants completed questionnaires at baseline (T1: the week before the second chemotherapy treatment), at the end of cancer treatment (T2: 4–6 months after T1), and at the end of the study (T3: approximately one year after the start of T1).
Sample and Settings
Participants were recruited from six outpatient settings in the San Francisco Bay Area and stratified by type of cancer before being randomly assigned to one of the three treatment groups from 1999 to 2005. All participants were female age 18 or older who had a confirmed diagnosis of breast, colorectal, or ovarian cancer; were beginning their first chemotherapy treatment; were able to read, write, and understand English; had a Karnofsky Performance Status scale (KPS) score of ≥ 60 63 and were able to provide written informed consent.
Participants were excluded if they were having concurrent radiation therapy, bone marrow transplantation, uncontrolled hypertension, diabetes mellitus, a pain intensity score of ≥ 3 on a 0 to 10 numeric rating scale, a lytic bone lesion, orthopedic limitations, a history of major depression, sleep disorders, chemotherapy within the past year, a diagnosis of AIDS-related malignancy, leukemia, or absolute contraindications to exercise testing as established by the ACSM. 64
The sample size for this study was determined by exploration of power analyses for the proposed tests of the between groups effects in the primary outcome variable of fatigue. The sample sizes required for between groups effects are generally larger than those required for within groups or group by time interaction effects. The previous study provides an estimate of the effect size for baseline adjusted Piper Fatigue Total Scores between subjects in an exercise condition and subjects receiving usual care compared at the end of chemotherapy.24 The results of that study indicate a somewhat larger than medium effect size, f = .30 which provided a sample size calculation of this study. A total sample size of 111 is needed to achieve power of .80 with an alpha of .05. We assessed 252 women for eligibility of the study and 119 participated.
Procedures
The study was approved by the Institutional Review Board at the University and at each recruitment site. We recruited women who had just completed their first chemotherapy treatment. We then measured their fatigue level and associated symptoms within seven days prior to beginning their second chemotherapy treatment. Participants who met the study criteria and were scheduled to begin chemotherapy were told about the study by referring oncologists and nurses at each site and then approached by the research staff. After informed consent, they were scheduled for a symptom-limited cardiopulmonary fitness test in the Clinical & Translational Science Institute, Clinical Research Center (CTSI-CRC) formerly, the General Clinical Research Center (GCRC) at the University Hospital. The participants were given a packet of questionnaires to complete either at the clinic or in their homes that were returned to the research staff when they came in to be tested at Time 1, 2, and 3. Before the cardiopulmonary fitness test, the exercise physiologist obtained height, weight, vital signs, and reviewed medical history to assess contraindications to exercise testing.
The present study started the exercise training with the minimum levels and progressed as the participants tolerated increased activity based on their cardiopulmonary fitness (VO2peak) assessed using symptom-limited treadmill testing. Cardiopulmonary exercise testing was performed three times during the study period (T1, T2, T3) in the exercise physiology lab in the CTSI-CRC by laboratory staff blinded to the participant’s group assignment. A branching treadmill protocol was used, which starts with a comfortable walking speed, after which the speed and grade were increased to achieve a 1–2 metabolic equivalent (MET) increment between stages. The work rate increased every 2 minutes until the subject was unable to continue or until there was an indication to discontinue the test (i.e., electrocardiographic changes, inappropriate blood pressure response). 64 Blood pressure and a continuous 12-lead electrocardiogram monitoring were performed. Ratings of perceived exertion (subjective rating of effort) were measured at each stage on a 6–20 scale. 65 Oxygen consumption (VO2) was determined using an open-circuit spirometry system (Quinton Instrument Co, Bothell, Washington), calibrated against known gases before each test. Respiratory gases were analyzed for volume and fractions of oxygen and carbon dioxide, and VO2 was calculated. Oxygen uptake at the highest tolerated intensity of exercise (VO2peak) was determined and expressed relative to body weight (ml of O2 per kilogram of body weight per minute). All abnormal findings during the test were sent to the patients’ designated primary physician, oncologist, and/or cardiologist asking for further cardiac evaluation, and request official MD clearance, or a release, in order for the subject to continue participation on the study. The exercise physiologists held the subject’s participation until they received either verbal or written, clearance before giving patients an exercise prescription.
Instruments
The Piper Fatigue Scale (PFS) is a 22-item numeric rating scale 66 that evaluates subjective fatigue (behavioral, sensory, affective, cognitive, mood). Each item is rated on a 0 (none) to 10 (a great deal) numeric rating scale (NRS). It was originally developed to measure fatigue in persons with cancer and has excellent reliability and validity estimates. 22, 52, 60–61, 67 Fatigue scores are categorized by mild (1–3), moderate (4–6), and severe (7–10).66 In the current study, the Cronbach’s alpha for the PFS ranged from 0.96 to 0.97.
The General Sleep Disturbance Scale (GSDS) consists of 21 items that evaluate various aspects of sleep disturbance (quality and quantity of sleep, sleep latency, waking up during sleep, daytime sleepiness, and medication use).68 Items are rated on 0 (never) to 7 (every day) NRS. The 21 items are summed to yield a total score that ranges from 0 (no disturbance) to 147 (extreme disturbance). A score ≥ 43 reflects sleep disturbance. 69 The GSDS has well-established reliability and validity in cancer patients. 70–72 In the current study, Cronbach’s alpha ranged from 0.83 to 0.86.
The Center for Epidemiological Studies Depression Inventory (CES-D) is a 20-item self-report instrument that measures the clinical syndrome of depression. 73 Each item is rated on a 4-point scale (0–3). Scores can range from 0 to 60, with higher scores reflecting more depressive symptoms. A score ≥16 indicates a need for a clinical evaluation. 73 The CES-D has well-established reliability and validity estimates across samples of persons with cancer receiving surgery or radiation therapy. 67, 70, 72, 74 In the current study, the Cronbach’s alpha for the CES-D ranged from 0.80 to 0.89.
The Worst Pain Intensity Scale is a single item, completed at T1, T2, and T3, 0 (no pain) to 10 (worst pain imaginable) numeric rating scale (NRS) that patients used to rate their worst pain in the past 24 hours. A descriptive NRS is a valid and reliable measure of pain intensity. 75
The Karnofsky Performance Status (KPS) Scale measures the physical abilities of the patient based on definitions provided using a 0 to 100 scale. 63 Since its development, the scale has been used extensively in oncology to evaluate performance status. A score of 100 indicates that the individual is able to carry on normal activities and has no decrease in performance status. A score of 30 indicates that the individual is severely disabled and needs to be hospitalized. The KPS has well-established inter-rater reliability, concurrent validity, and criterion validity. 63, 76–78
The Demographic Profile (baseline and follow up) Form was completed by the participants at the three time points. Baseline data included age, income, ethnicity, gender, menopausal status, and symptom checklist (symptomatology). The follow-up demographic form was completed at T2 and T3 to document any changes that occurred since baseline in occupational status, KPS, menstrual status, and symptomatology. This instrument was developed and used in previous studies with no difficulties. 79–82
The Medical Record Review Form was used to obtain medical and treatment-related data. It contained 10 items plus a flow sheet that was used to obtain data on the patients’ tumor characteristics, chemotherapy protocol, treatment goals, and response to chemotherapy. Laboratory data and clinical parameters were recorded on the flow sheet. This instrument was completed by research personnel based on medical record reviews and interviews with the patient’s physician or nurse. Data were collected monthly to obtain precise information and to document any changes in the chemotherapy protocol (e.g., treatment delays or dose reductions) since baseline. This form was developed and used previously in our RCTs and pilot studies with no difficulties. 83–84
Intervention Framework
The intervention framework called the PRO-SELF: FATIGUE CONTROL PROGRAM is based on self-care and adult learning theory. 85–86 It includes three components: relevant knowledge about fatigue, self-care skills to manage CRF, and supportive coaching to continue positive behaviors. This framework was used in our earlier RCT’s. 85, 87
The exercise prescription was comprehensive and individualized to the participant’s fitness level, based on the baseline exercise treadmill test. The exercise prescription was adjusted by the exercise physiologist through weekly follow up phone calls to maintain the exercise prescription. It consisted of a mode (cardiovascular/aerobic exercise e.g. walking, jogging, or bicycling), frequency (3–5 times per week), intensity (training heart rate corresponding to 60–80% VO2peak), and duration (20–30 minutes of continuous exercise). In addition to heart rate, the intensity of the exercise was also targeted to achieve the Borg Scale of 12–14 level (“somewhat hard”). Patients were instructed on how to obtain their pulse by the exercise trainer and the pulse was counted at least twice during each exercise session to monitor their exercise intensity. The EE group was given this exercise prescription at T1, and the CE group was given this exercise prescription at T2. The participants were supported in their exercise through weekly telephone calls from the exercise trainers who provided support and encouragement as well as help in problem solving potential barriers to exercise.
Standard-Care/Usual Activities (CC)
All CC patients received standard care (which did not include an exercise prescription). CC patients who reported exercising regularly prior to enrollment in the study were asked not to change their exercise patterns. To prevent a differential treatment effect, the CC patients were telephoned weekly by the research nurse to inquire about their health and general response to cancer treatment. The research nurse did not provide any formal information or counseling regarding fatigue or exercise.
Data Analysis
Data were analyzed using SPSS version 15. 88 Prior to the analysis of the specific study aims, appropriate descriptive statistics were calculated for all variables at each point in time. The three treatment groups were compared on selected demographic, disease, and chemotherapy treatment variables to assess whether the random assignment procedure was successful in creating comparable groups. If differences were found in these variables among the three groups, such variables were used as covariates in the analysis. Two-tailed tests were used. A comparison of demographics was made between patients who dropped out to those who did not to determine if there was a differential bias. All analyses were completed on the randomized groups by intent to treat (i.e., all randomized patients were analyzed based on their group assignment). The multilevel regression analysis was used to compare how fatigue and other study variables had changed over time and by groups. This analysis enables researchers to describe changes within individuals over time and to examine inter-individual effects over time. In addition, this method allows using the estimated values of parameters to draw conclusions about the direction and the magnitude of proposed effects in the population (Singer & Willet, 2003). Statistical significance was preset at p<0.05.
Results
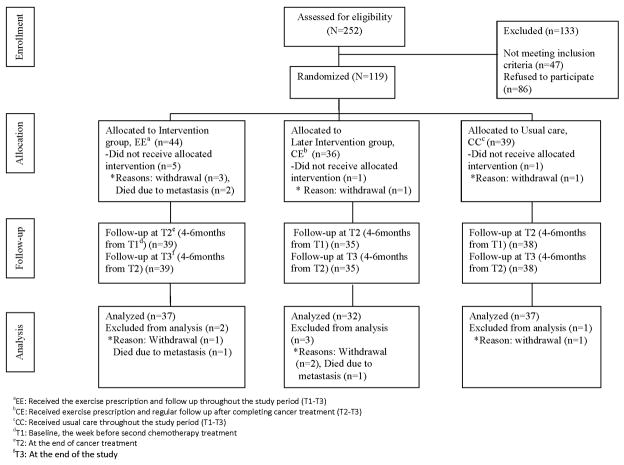
Of the 252 women initially approached and screened, 119 women were determined to be eligible and 133 individuals were either ineligible (n=47) or not interested in the study (n=86). Ineligible reasons (some people provided more than 1 reason, so the total numbers vary) were changing circumstances of their planned cancer care (n=11), treatment plan did not include chemotherapy (n=7), had previously received chemotherapy (n=15), could not exercise due to surgery or cardiac problem (n=6), had bone metastasis or other concurrent cancer (n=6). In addition, 15 potential subjects contacted the investigator after having learned of the study through publicity, but unfortunately their various institutions were not covered by our IRB. Of the eligible women who declined to participate (N=86), reasons reported for not participating were: not interested after learning more about the study (n=25), too busy (n=25), too far (n=10), protocol too much (n=2), wanted EE group only (n=4) or others (n=20). Figure 1 shows the recruitment and dropout of women during the study period by treatment group.
Figure 1.
No significant differences were found in age (p=0.43), income (p=0.26), education (p=0.23), or marital status (p=0.67) between the patients who did and did not drop from the study. The study participants were all female (100%), primarily married/partnered (68.4%), Caucasian (75.2%), and diagnosed with breast (n=112), colon (n=1), or ovarian (n=6) cancer. The average age was 50.5 years (SD=9.35), and education level ranged from 11th grade to beyond master’s study (mean=16.2 years; SD=2.8). The three groups of patients did not differ significantly in any of the demographic, disease, or treatment characteristics on entry into the study (see Table 1).
Table 1.
Comparison of Demographic and Clinical Characteristics at Baseline (T1)
| Variable | All patients (n=119) | EE (n=44) | CE (n=36) | CC (n=39) | P |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age, year | 50.5 (9.4) | 49.4 (8.2) | 50.4 (9.0) | 52.0 (10.8) | .5 |
| Education, year | 16.2 (2.8) | 16.0(2.8) | 16.2 (2.3) | 16.3 (3.2) | .9 |
| Study period Days | |||||
| T1–T2 | 169 (64.6) | 178.3 (71.4) | 156 (62.5) | 170 (58.8) | .4 |
| T2–T3 | 164.8 (60.8) | 168 (66.1) | 160 (62.3) | 165 (55.0) | .9 |
| Karnofsky Performance | 87.63 (9.4) | 89.2 (8.5) | 87.9 (8.9) | 85.6 (10.5) | .23 |
| n (%) | n (%) | n (%) | n (%) | ||
| Marital Status (married) | 80 (68.4) | 33 (75) | 23 (67.6) | 24 (61.5) | .4 |
| Ethnicityb | |||||
| White | 88 (75.2)a | 30 (68.2) | 27 (79.4) | 31 (79.5) | |
| Black | 12 (10.3) | 4 (9.1) | 3 (8.8) | 5(12.8) | .7 |
| Asian | 12 (10.3) | 7 (15.9) | 3(8.8) | 2 (5.1) | |
| Others | 5 (4.3) | 3 (6.8) | 1 (2.9) | 1 (2.6) | |
| Employed (full or part time) | 50 (44.2) | 17 (41.5) | 19 (57.6) | 14 (36.0) | .2 |
| Income (≥40,000) | 94 (83.2) | 35 (83.3) | 30 (90.9) | 29 (76.3) | .3 |
| Menopausal Statusc | |||||
| Premenopausal | 40 (36.7) | 15 (36.6) | 12 (38.7) | 13 (35.1) | |
| Perimenopausal | 19 (17.4) | 9 (22) | 6 (19.4) | 4 (10.8) | .7 |
| Postmenopausal | 50 (45.9) | 17 (41.5) | 13 (41.9) | 20 (54.1) | |
| Sequential chemotherapy then radiation therapy | 59 (49.6) | 21 (47.7) | 18 (50) | 20 (51.3) | .9 |
| Participate regular exercise programd | 75 (64.7) | 29 (67.4) | 24 (70.6) | 22 (56.4) | .4 |
| Cancer Stagee | |||||
| I | 40 (36) | 13 (32.5) | 12 (35.3) | 15 (40.5) | .8 |
| II | 52 (46.8) | 19 (47.5) | 18 (52.9) | 15 (40.5) | |
| III | 19 (17.1) | 8 (20) | 4 (11.8) | 7 (18.9) | |
Due to rounding, percentage may not add up to 100
Two participants in the CE group did not answer the question concerning ethnicity
Four participants in the EE group, two participants in the CE group, and two participants in the CC group did not answer the question concerning menopause
One participant in the EE group and two participants in CE group did not answer the question concerning participation in a regular exercise program
Four participants in the EE group, two participants in the CE group, and two participants in the CC group did not answer the question concerning cancer stage.
Changes in Fatigue over Time
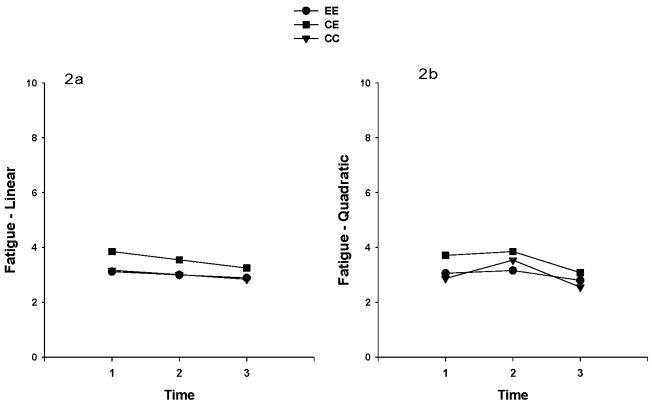
Multilevel regression analysis was used to explore the impact of time and group on levels of fatigue, as measured by the Piper Fatigue Scale. Mean fatigue scores from all participants were at a mild level at the three time points. To increase interpretability of a possible interaction between time and group, many authors (e.g. Singer and Willet) recommend centering the variable representing time so that the model intercept can be interpreted at a meaningful time. 89 Frequently, the first or baseline assessment is selected. With this notion, the fatigue levels were used in these analyses with time (centered at baseline), group, and the interaction between time and group. In addition, “Group” was dummy coded so that the control group was the reference group. A random coefficients model was specified for the analysis by including both random intercepts and slopes for the time variable, in order to address the questions of whether fatigue level varied among groups at baseline, and whether the rate of change in fatigue level varied among groups. The specification for the covariance matrix for the random intercepts and slopes was “unstructured,” and estimation was obtained with restricted maximum likelihood. The unconditional model for fatigue showed that the linear change in fatigue over time was not significant (p=0.084). The linear change in fatigue over time by group showed no significant differences among the groups. However, significant linear (p = .02) and quadratic (p = .004) time effects in fatigue were found, when the quadratic effect was included in the model (See Table 2). However, neither the linear nor the quadratic effects differed among treatment groups. Figure 2 illustrates the linear and quadratic fatigue trajectories by treatment groups (Figures 2a, 2b). Even though there were no statistically significant differences between groups on CRF, the EE patients sustained their exercise activities and maintained a lower average fatigue level for almost a year, whereas, the CE group had worsening average fatigue levels during cancer treatment, but more than recovered when they had completed cancer treatment and/or given their exercise prescription, surpassing their baseline level of fatigue at T3. The contribution of having completed treatment or being given their exercise prescription cannot be determined from our data. Not surprisingly, the usual care (CC) group had increasing average fatigue levels during cancer treatment and their fatigue severity leveled off after completion of treatment and decreased further at the end of the recovery period (T3). This pattern was reported by other investigators. 90
Table 2.
Estimation of Fixed Effects and Covariance Parameters of Fatigue, Sleep Disturbance, Depression, and Pain.
| Parameter estimate | Fatigue | Sleep disturbance | Depression | Pain | |
|---|---|---|---|---|---|
| Initial status | Intercept | 2.87 | 43.56 | 10.60 | 1.31 |
| EE | 0.19 | 2.43 | 1.19 | 0.19 | |
| CE | 0.84 | 8.06 | 2.88 | 0.78 | |
| Rate of Change | Time (linear) | 1.49a | 0.51 | −0.58 | 1.64a |
| Linear time × EE | −1.16 | 1.32 | 3.64 | −0.76 | |
| Linear time × CE | −0.84 | 10.90 | 1.99 | −1.15 | |
| Time (quadratic) | −0.82b | −0.19 | 0.48 | −0.62 | |
| Quadratic × EE | 0.59 | −2.36 | −2.31 | 0.27 | |
| Quadratic × CE | 0.37 | −6.18 | −1.68 | 0.30 | |
| Covariance Parameters | Residual | 1.84c | 128.61c | 34.62c | 3.31c |
| Intercept | 2.21c | 255.47c | 34.61c | 3.25c | |
| Intercept × Linear Slope Cov | −0.03 | −25.82 | 4.63 | −0.66 | |
| Linear Slope | 0.18 | 39.09a | 4.00 | −0.65 |
p<.05,
p<.01,
p<.001
Figure 2.
Figures 2a. 2b. Linear (2a) and quadratic (2b) fatigue trajectories in the three exercise treatment groups
Sleep Disturbance, Depression, and Pain
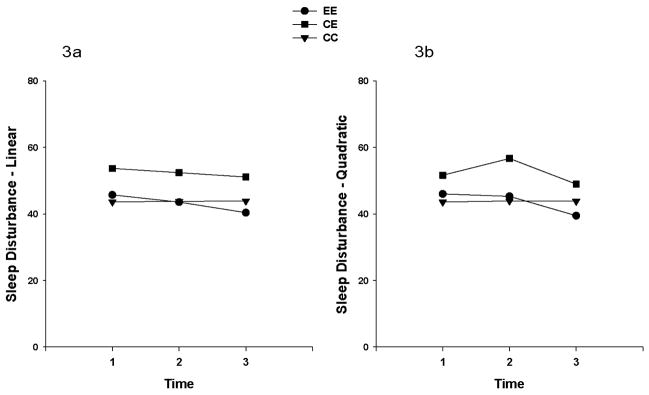
Multilevel regression analysis was done to explore the effects of time and group on levels of sleep disturbance, depression, and pain. The analysis for sleep disturbance showed that the linear change over time did not differ by group. However, a significant quadratic (p=0.043) effect was found for sleep disturbance, when the quadratic effect was included in the model (See Table 2). Neither the linear nor the quadratic effects differed among the groups. Figure 3 illustrates the linear and quadratic sleep disturbance trajectories by treatment groups.
Figure 3.
Figures 3a. 3b. Linear (3a) and quadratic (3b) sleep disturbance trajectories in the three exercise treatment groups
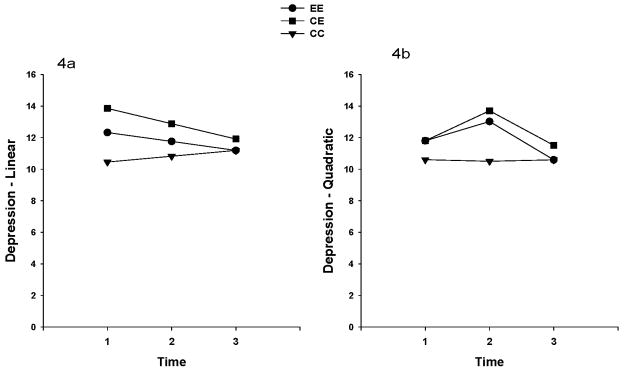
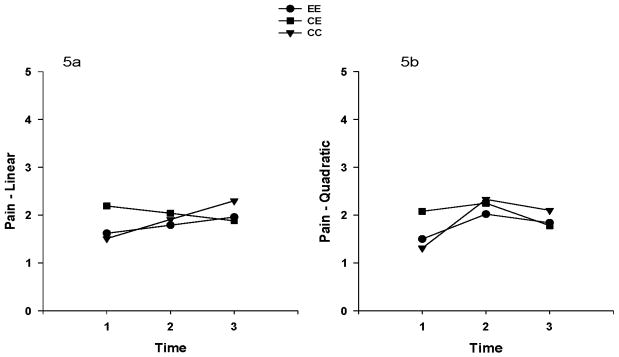
The overall mean depression scores were less than the cutoff score for depression (i.e., 16), and ranged from 10.2 to 13.4 in the total sample for the three time points. The unconditional model for depression showed that the linear change in depression over time was not significant (p=0.43). The linear change in depression over time by group was also not significant. No significant linear or quadratic time effects were found when the quadratic effect was included in the model (See Table 2). Neither the linear nor the quadratic effects differed among the groups. Figure 4 illustrates the linear and quadratic depression trajectories by treatment groups. Overall pain intensity in all groups was mild. The mean score of each group for pain ranged from 1.3 to 2.29 (0 none to10 worst pain) in the total sample for three time points. The unconditional model for pain showed that the linear change in pain over time was not significant (p=0.29). The linear change in pain over time by group was not significant. However, significant linear (p=0.02) and quadratic (p=0.04) time effects in pain were found when the quadratic effect was included in the model (See Table 2). However, neither the linear nor the quadratic effects differed among the groups. Figure 5 illustrates the linear and quadratic pain trajectories by treatment groups.
Figure 4.
Figures 4a. 4b. Linear (4a) and quadratic (4b) depression trajectories in the three exercise treatment groups
Figure 5.
Figures 5a. 5b. Linear (5a) and quadratic (5b) pain trajectories in the three exercise treatment groups
Adherence to Minimum Criteria of Exercise
To maintain the fidelity of the intervention, a research team member called the patients who had received an exercise prescription once a week, and at the major time points (T2 and T3). In this analysis, adherence rates were calculated based on mode, frequency, intensity, and duration of reported exercise at each major time point about past weekly exercise behaviors. The EE groups and later the CE group patients were considered adherent to their exercise prescription if they met the minimum levels of frequency (3 times/week), duration (20 minutes/session), intensity (moderate), and mode of their exercise (aerobic) prescription. The EE group reported an adherence rate of 73% at T2 and 75.7% at T3, and the CE group reported 86.7% adherence at T3.
Discussion
Cancer related fatigue is one of the most devastating symptoms for patients during and after treatment. Exercise, a recommended intervention by the Oncology Nursing Society’s Putting Evidence into Practice guideline, is intended to reduce or manage fatigue. However, in the guideline, exercise as an intervention for CRF has not yet yielded consistently positive outcomes across studies. Meta analyses and systematic reviews have described variations in: dose of exercise (frequency, duration, and intensity); types of exercise (aerobic, and/or resistance/strength); outcome measures (physical functioning, fatigue, vigor, vitality, or peak oxygen consumption); timing of exercise (during or after cancer treatment); and length of the exercise intervention weeks (3 weeks, 12 weeks, or beyond).
The present study was designed to evaluate the effectiveness of an individualized home-based exercise intervention for CRF as well as for the secondary purposes of investigation of the timing of the interventions, and the effects on sleep disturbance, depression, and pain based on the IFM framework. A unique aspect of this study was the evaluation of the effects of exercise that was initiated during or after completion of cancer treatment, compared to a control group. However, no differences were found in the trajectories of fatigue across the study groups. Fatigue and pain intensity levels increased during treatment and then decreased after treatment in all groups. Depression scores did not change over time in any of the groups.
These findings further our understanding of the effects of cardiopulmonary (aerobic) exercise both during and after cancer treatment in a sample of women with selected types of cancer. The present study did not clearly conclude if it is better to perform exercise activities during cancer treatment, or after completion of treatment regarding CRF, since comparisons did not reach the level of statistical significance.
Possible Explanations for the Nonsignificant Fatigue Findings
Several possible explanations could account for our nonsignificant findings: a floor effect for CRF, the exercise prescription, or statistical power. The sample’s mild fatigue levels created a “floor effect”, namely there was a limited opportunity for the intervention to demonstrate a significant reduction in CRF, or related symptoms in this sample. This is the major contributor of the nonsignificant trial. Future research should focus on patients who report higher fatigue prior to and during cancer treatment.9 For example, Heim, Malsburg, Niklas conducted a study of patients with breast cancer during adjuvant chemotherapy who had fatigue scores of ≥4 (on an 11 point scale). 91 Heim et al. tested a multicomponent physical training intervention that resulted in significant reductions in CRF. Unfortunately, this study intervention was confounded by the concurrent enrollment of the patients in a multicomponent “rehabilitation program” which makes interpretation of the effects of exercise difficult.
When our study was initiated, the ACSM 1998 guidelines were used to develop the exercise prescription and to guide the progression of exercise activities to the women’s tolerance levels. As mentioned earlier, at a minimum, the women were prescribed aerobic activities 3x/week, for 20-minute duration, and at a moderate intensity. Participants in the EE group were able to meet ACSM 1998 guidelines 73% of the time from T1–T2, and 76% of the time from T2–T3. Participants in the delayed exercise CE group were able to meet this minimum 87% of the time from T2–T3. In 2006, the updated ACSM guidelines recommended that healthy adults have exercise sessions 5x/week, for 30 minutes duration, at moderate intensity. Even with these more demanding guidelines, women in the EE group met these criteria 24% of the time from T1–T2, and 30% from T2–T3. Furthermore, women in the delayed exercise prescription CE group met the 2006 criteria 17% of the time from T2–T3.
It is interesting to note that the exercise activities in the nonintervention groups (CE and CC) declined during cancer treatment. Forty-four percent of the delayed exercise CE group reported meeting ACSM 1998 guidelines at T1, but by T2 the CE group had decreased to 27%. Similarly, 34% of the control CC group reported meeting our minimum criteria at T1, but this percentage decreased to 31% at T2. This pattern of decreasing regular exercise in nonintervention patient groups during active treatment was reported previously. 92–93 After completion of cancer treatment, the CC group rebounded to 46% at T3, a pattern reported previously. 94
The actual effect size obtained from the level of fatigue changes for the exercise intervention in the present study was weak, f= 0.11, 95 for the comparison of the EE group vs. the CE and CC groups at T2. The effect size was even smaller at T3 (f= 0.07), when the three groups were compared (i.e., EE vs. CE vs. CC). With an effect size this weak, a large sample size would be required to detect a significant difference among the groups, but the investigators had difficulties recruiting the targeted numbers of participants. Originally this study was designed to recruit other types (e.g., colorectal, prostate) of cancer that would have included both genders, but the complexities of the patients’ clinical treatment protocols (use of the hepatic pump for the colorectal patients, and no end to active treatment for the men with prostate cancer) made their inclusion impossible with the study design.
Schmitz et al. (2005) in their meta-analysis reported a small effect size for exercise on fatigue both during and after cancer treatment. This finding indicates that although there may be consistent evidence of the positive effect of activity-based interventions on fatigue, the magnitude of the effect may be too small to be of clinical relevance. Jacobsen 9 in reviewing the effect sizes of earlier meta-analyses of activity based interventions on fatigue 2, 13, 96 made an interesting observation: comparing the findings of Jacobsen et al. with others’ findings they suggest that the effect sizes are reduced when more recent studies are included and the meta-analyses are limited to RCTs. The rationale given by some of these authors of earlier meta-analyses for including nonrandomized studies was to avoid overestimating the magnitude of the effect in the population. 2
Adherence with the exercise prescription
With education, support, and coaching, patients in the present study had a moderate to high adherence rate based on the ACSM’s 1998 minimum criteria of exercise. Their adherence levels were similar to those reported in other exercise intervention studies. However, only one study was found that administered their intervention to their participants for as long as the present study; Courneya, Mackey, Bell et al. tested a supervised exercise post cancer treatment for one year in 52 breast cancer survivors and reported an adherence of 98% for exercise attendance. 20 In another study, Courneya, McKenzie, Reid, Mackey et al. derived three major categories for the types of barriers (or reasons) that patients gave for not adhering to their 17-week supervised exercises (aerobic or resistance) during their chemotherapy. 97 The predominant category was disease and treatment-related (53%), followed by life-related barriers (34%), and motivation-related barriers (13%). The current study’s findings are in agreement with those of Courneya et al., with the early exercise EE group reporting disease and treatment-related barriers to exercise during their cancer treatment (“week of chemotherapy” 14%; “fatigue” 10%); or life-related barriers (“illness e.g., colds or flu” 16%; “family obligations” 13%). Our findings extend those of Courneya et al. 2008, in that the early exercise EE group and the delayed exercise CE group continued for almost six months (T2–T3) beyond their cancer treatment. During that time, a notable shift occurred in reported barriers given by our study participants. The most frequent barriers were predominately life-related for both groups (EE & CE). The EE group reported work (outside employment responsibilities) (14%), vacations (14%), family obligations (8%), and lastly, fatigue (8%). The CE group reported the barriers to exercise to be work (24%), illnesses (cold or flu) (10%), and vacations (8%).
Adverse events
Adverse events were not a factor in the nonsignificant findings of this RCT. During our study period, we telephoned EE and CE patients and asked whether they had experienced any injuries or pain during exercise sessions. Though not many injuries occurred during exercise sessions, those that were reported, included hip pain, sciatica (n=16), arm discomfort (n=4), knee discomfort (n=10), ankle discomfort (n=3), and foot discomfort (n=8). All patients tolerated the symptom-limited treadmill testing without adverse events. Asymptomatic ischemic electrocardiogram changes (i.e., ST segment depression) were detected in ten patients, asymptomatic bigeminy in six patients, and premature ventricular complexes were noted in nine patients. These abnormal findings were sent to each subject’s primary physician, oncologist, and/or cardiologist for further evaluation. With an MD’s release letter, patients continued being tested. A total of eight patients were not cleared to continue with the testing and were discontinued from the study.
Integrated Fatigue Model (IFM)
The model was useful in clarifying proposed relationships among the IFM relevant variables. No significant correlations were found between fatigue, demographic, and treatment variables at T1. At T2, a positive correlation was found between employment and fatigue (r=0.23, p<0.05) and at T3 a negative correlation between income and fatigue (r= −0.32, p<0.01). At each point in time, significant positive correlations were found between fatigue and sleep disturbance (r=0.5–0.63, p<0.01), depression (r=0.62–0.67, p<0.01), and pain (r= 0.25–0.48, p<0.01). In a similar sample, Berger and Higginbotham identified a significant positive relationship between fatigue and sleep disturbance, and an inverse relationship between activity level and fatigue. The current study concurs with their first finding but not with their second. Further development of this theoretical model is warranted to continue to provide structure for research and to guide revisions based on research findings. 98
Limitations
Several limitations are worth noting. When this study was conducted, the benefits of exercise were being reported in the literature and appeared to hold some promise. The present investigators decided that although sedentary patients were preferred, to ask study participants to stop their regular exercise activities was not ethical given the consequences of inactivity and de-conditioning. 13, 47 At the completion of the study, the same home-based exercise prescription as was given to the exercise groups (EE and CE) was provided to the control group based on their symptom limited treadmill test.
Perhaps the PFS being administered only three times in almost a year’s period of time was too infrequent to capture the true effect of exercise on CRF. Clearly using a multidimensional instrument and needing to take measures often is a complex issue. 99 Another limitation was that only self-report of exercise behaviors were obtained and no objective measures were taken (e.g., pedometer, or sleep wrist actigraph). The inclusion of objective measures is recommended for future studies.
A home-based exercise training intervention was successfully implemented in patients both during cancer treatment and beyond. Although there was no reduction in fatigue, the subjects studied experienced only mild fatigue throughout the study. The exercise intervention did not worsen fatigue in any of those in the exercise groups. Further investigation is needed to evaluate fatigue and its related symptoms more often during and after treatment. More frequent assessment of fatigue and related symptoms, higher fatigue levels at baseline, and further analysis in adherence rates may comprehensively capture the effect of exercise. Finally, in light of the ACSM 2006 guidelines, the dose of exercise prescription needs to be increased in subsequent research.
Although this present study did not prove the effectiveness of exercise in the management of fatigue in patients with cancer, it is important to be aware that women with cancer can experience mild fatigue, sleep disturbance, and pain during and after treatment. Clinicians need to assess fatigue and its related symptoms at the beginning of cancer treatment. As a recommendation from the ONS PEP clinical guideline, exercise is one of the interventions that reduces fatigue. Some patients who cannot exercise during their treatment, but initiate exercise activities after cancer treatment (i.e. the CE group) appears to have same merit in managing fatigue.
Acknowledgments
Funding for this study was provided by the National Cancer Institute (CA83316), and the Clinical Translational Research Institute, Clinical Research Center (CTSI-CRC). We would like to express appreciation to earlier research team members: Kathleen Dzubur, Amy Strasner, Elaine McKenna, Sheila Duncan, Karen Bayuk, Mary Lou Ernest, Lynda Frassetto, DeAnne Sheeley, Hsin-Tien Hsu, Lih-Mih Chen, Barbara Piper. We also thank Ambar Munoz for assisting in preparation of the paper and to the patients who gave so generously of their time.
References
- 1.Stricker CT, Drake D, Hoyer KA, Mock V. Evidence-based practice for fatigue management in adults with cancer: exercise as an intervention. Oncol Nurs Forum. 2004 Sep;31(5):963–976. doi: 10.1188/04.ONF.963-976. [DOI] [PubMed] [Google Scholar]
- 2.Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer. 2006 Jul;14(7):699–712. doi: 10.1007/s00520-005-0905-5. [DOI] [PubMed] [Google Scholar]
- 3.Courneya K, Mackey J, McKenzie D. Exercise for breast cancer survivors: research, evidence, and clinical guidelines. Phys Sportsmed. 2002;30(33–53) doi: 10.3810/psm.2002.08.402. [DOI] [PubMed] [Google Scholar]
- 4.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003 Nov;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS, Vallance JKH, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol/Hematol. 2004;51:249–261. doi: 10.1016/j.critrevonc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005 Feb 1;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 7.Holtzman J, Schmitz K, Babes G, et al. Effectiveness of behavioral interventions to modify physical activity behaviors in general populations and cancer patients and survivors. Evid Rep Technol Assess (Summ) 2004 Jun;(102):1–8. doi: 10.1037/e439832005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram C, Courneya KS, Kingston D. The effects of exercise on body weight and composition in breast cancer survivors: an integrative systematic review. Oncol Nurs Forum. 2006 Sep;33(5):937–947. doi: 10.1188/06.ONF.937-950. quiz 948–950. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007 Nov;26(6):660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005 Jun 1;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 11.Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2006;(4):CD005001. doi: 10.1002/14651858.CD005001.pub2. [DOI] [PubMed] [Google Scholar]
- 12.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006 Jul 4;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen L, Courneya KS, Stevinson C, Fossa SD. A systematic review of physical activity in prostate cancer survivors: outcomes, prevalence, and determinants. Support Care Cancer. 2008 Sep;16(9):987–997. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]
- 15.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989 Nov–Dec;38(6):348–351. [PubMed] [Google Scholar]
- 16.Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001 May;33(5):718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002 Dec;34(12):1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise program as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Drouin J. Aerobic exercise training effects on physical function, fatigue and mood, immune status, and oxidative stress in subjects undergoing radiation treatment for breast cancer. Detroit: Wayne State University; 2002. [Google Scholar]
- 20.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003 May 1;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 21.Crowley SA. The effect of a structured exercise program on fatigue, strength, endureance, physical self-efficacy, and functional wellness in women with early stage breast cancer. Ann Arbor: University of Michigan; 2003. [Google Scholar]
- 22.Winningham M, MacVicar M. The effects of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1998;15:447–450. [PubMed] [Google Scholar]
- 23.Mock V, Burke MB, Sheehan P, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 1994 Jun;21(5):899–907. discussion 908. [PubMed] [Google Scholar]
- 24.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997 Jul;24(6):991–1000. [PubMed] [Google Scholar]
- 25.Mock V, Pickett M, Ropka M, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001 May–Jun;9(3):119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 26.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003 May 1;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz AL. Cancer. In: Durstine JL, Moore GE, editors. American College of Sports Medicine’s Exercise management for persons with chronic diseases and disabilities. Human Kinetics; USA: 2003. [Google Scholar]
- 28.Kolden GG, Strauman TJ, Ward A, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002 Sep–Oct;11(5):447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 29.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999 May 15;85(10):2273–2277. [PubMed] [Google Scholar]
- 30.Goodwin P, Esplen MJ, Butler K, et al. Multidisciplinary weight management in locoregional breast cancer: results of a phase II study. Breast Cancer Res Treat. 1998 Mar;48(1):53–64. doi: 10.1023/a:1005942017626. [DOI] [PubMed] [Google Scholar]
- 31.Segar ML, Katch VL, Roth RS, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998 Jan–Feb;25(1):107–113. [PubMed] [Google Scholar]
- 32.Holley S, Borger D. Energy for living with cancer: preliminary findings of a cancer rehabilitation group intervention study. Oncol Nurs Forum. 2001;28(9):1393–1396. [PubMed] [Google Scholar]
- 33.Durak E, Lilly PC. Preliminary Results of exercise in breast cancer: a two-year follow-up survey. J Rehabil Outcomes Measure. 1999;3(4):53–60. [Google Scholar]
- 34.Durak EP, Lilly PC. The application of an exercise and wellness program for cancer patients: a preliminary outcomes report. J Strength Cond Res. 1998;12(1):3–6. [Google Scholar]
- 35.Peters C, Lotzerich H, Niemeier B, Schule K, Uhlenbruck G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994 May–Jun;14(3A):1033–1036. [PubMed] [Google Scholar]
- 36.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001 Feb 1;19(3):657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 37.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008 Sep;134(5):700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 38.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;(2):CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Battaglini C, Bottaro M, Dennehy C, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007 Jan 4;125(1):22–28. doi: 10.1590/S1516-31802007000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005 Jun;14(6):464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 41.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005 May 20;23(15):3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 42.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007 Aug 15;110(4):918–925. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 43.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integr Cancer Ther. 2007 Sep;6(3):235–241. doi: 10.1177/1534735407305871. [DOI] [PubMed] [Google Scholar]
- 44.Hanna LR, Avila PF, Meteer MA, Nicholas DR, Kaminsky LA. The Effects of a Comprehensive Exercise Program on Physical Function, Fatigue, and Mood in Patients With Various Types of Cancer. Oncol Nurs Forum. 2008;35(3):461–469. [Google Scholar]
- 45.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007 Oct 1;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 46.American College of Sports Medicine. ACSM s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; USA: 2000. [Google Scholar]
- 47.Humpel N, Iverson DC. Review and critique of the quality of exercise recommendations for cancer patients and survivors. Support Care Cancer. 2005 Jul;13(7):493–502. doi: 10.1007/s00520-005-0811-x. [DOI] [PubMed] [Google Scholar]
- 48.American Cancer Society. [Accessed July, 2004];Exercise to stay active. 2004 http://www.cancer.org/docroot/MIT/MIT_2_1x_ExerciseToStayActive.asp?sitearea=MIT.
- 49.Schwartz AL. Physical activity. Semin Oncol Nurs. 2008 Aug;24(3):164–170. doi: 10.1016/j.soncn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piper BF. Fatigue. In: Carrieri-Kohlman V, Lindsey AM, West C, editors. Pathophysiological phenomena in nursing: Human responses to illness. 2. Philadelphia: W.B. Saunders; 1993. pp. 279–302. [Google Scholar]
- 51.Aistars J. Fatigue in the cancer patient: a conceptual approach to a clinical problem. Oncol Nurs Forum. 1987 Nov–Dec;14(6):25–30. [PubMed] [Google Scholar]
- 52.Dimeo F, Bertz H, Finke J, Fetscher S, Mertelsmann R, Keul J. An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplant. 1996 Dec;18(6):1157–1160. [PubMed] [Google Scholar]
- 53.Dimeo FC, Tilmann MH, Bertz H, Kanz L, Mertelsmann R, Keul J. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer. 1997 May 1;79(9):1717–1722. [PubMed] [Google Scholar]
- 54.Ferrell BR, Grant M, Funk B, Garcia N, Otis-Green S, Schaffner ML. Quality of life in breast cancer. Cancer Pract. 1996 Nov–Dec;4(6):331–340. [PubMed] [Google Scholar]
- 55.Messias DK, Yeager KA, Dibble SL, Dodd MJ. Patients’ perspectives of fatigue while undergoing chemotherapy. Oncol Nurs Forum. 1997 Jan–Feb;24(1):43–48. [PubMed] [Google Scholar]
- 56.Nail LM, King KB. Symptom distress. Fatigue. Semin Oncol Nurs. 1987 Nov;3(4):257–262. doi: 10.1016/s0749-2081(87)80016-5. [DOI] [PubMed] [Google Scholar]
- 57.Nail LM, Winningham ML. Fatigue. In: Groenwald M, Frogge M, Goodman M, Yarbro C, editors. Cancer Nursing: Principles and Practice. 3. Boston: Jones and Bartlett; 1993. [Google Scholar]
- 58.Piper B. Alterations in energy: The sensation of fatigue. In: Baird S, McCorkle R, Grant M, editors. Cancer Nursing: A comprehensive textbook. Philadelphia: W.B. Saunders; 1991. pp. 894–908. [Google Scholar]
- 59.Winningham ML. The role of exercise in cancer therapy. In: Eisinger M, Watson R, editors. Exercise and disease. Boca Raton, FL: CRC Press; 1992. pp. 63–70. [Google Scholar]
- 60.Young-McCaughan S, Sexton DL. A retrospective investigation of the relationship between aerobic exercise and quality of life in women with breast cancer. Oncol Nurs Forum. 1991 May–Jun;18(4):751–757. [PubMed] [Google Scholar]
- 61.Piper BF, Lindsey AM, Dodd MJ. Fatigue mechanisms in cancer patients: developing nursing theory. Oncol Nurs Forum. 1987 Nov–Dec;14(6):17–23. [PubMed] [Google Scholar]
- 62.Piper BF, Lindsey AM, Dodd MJ, Ferketich S, Paul S, Weller S. The development of an instrument to measure the subjective dimensions of fatigue. In: Funk SG, Tournquist EM, Champagne MT, Copp LA, Weise RA, editors. Key aspects of comfort: Management of pain, fatigue and nausea. New York: Springer; 1989. pp. 199–208. [Google Scholar]
- 63.Karnofsky D. Performance scale. In: Kennealey GT, Mitchell MS, editors. Factors that influence the therapeutic response in cancer: a comprehensive treatise. New York: Plenum Press; 1977. [Google Scholar]
- 64.American College of Sports Medicine. Guidelines for exercise testing and prescription. 5. Philadelphia: Williams & Wilkins; 1995. [Google Scholar]
- 65.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, Illinois: Human Kinetics; 1998. [Google Scholar]
- 66.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998 May;25(4):677–684. [PubMed] [Google Scholar]
- 67.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001 Apr;28(3):465–470. [PubMed] [Google Scholar]
- 68.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 69.Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004 Dec;191(6):2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 70.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006 Sep;33(5):E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 71.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999 May;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 72.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008 Feb;35(2):162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 74.Dodd M, Cho MH, Cooper B, Miaskowski C. The effects of the symptom cluster of fatigue, sleep disturbance, depression, and pain on clinical outcomes in women during and after adjuvant cancer treatment for breast cancer. European Journal of Oncology Nursing. 2009 In print. [Google Scholar]
- 75.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003 Feb;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 76.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984 May 1;53(9):2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 77.Hyde LK, Wolf J, McCracken S, Yesner M. Natural Course of inoperable lung cancer. Chest. 1973 September;64(3):309–312. doi: 10.1378/chest.64.3.309. [DOI] [PubMed] [Google Scholar]
- 78.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984 Mar;2(3):187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 79.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 80.Dodd MJ, Larson P, Miaskowski C, Greenspan D, Dibble S, Hauck W, Paul S, Ignoffo R. Self-care intervention to decrease mucositis morbidity RO1 CA 55555. National Institutes of Health, National Cancer Institute; 1992–1996. [Google Scholar]
- 81.Dodd MJ, Lovejoy N, Larson P, et al. Self-care intervention to decrease chemotherapy morbidity RO1 CA 48312. National Institutes of Health, National Cancer Institute; 1988–1992. [Google Scholar]
- 82.Painter P, Stewart A, Dibble S, Dodd MJ, Ascher N, Gold W. Exercise intervention after kidney transplant RO1 NR 02880. National Institutes of Health, National Institute of Nursing Research; 1993–1996. [Google Scholar]
- 83.Dodd MJ, Painter P, Miaskowski C, et al. Exercise: A feasibility study of an intervention for fatigue in cancer patients: P30 feasibility study. Research Center for Symptom Management, University of California; San Francisco: 1996–1997. [Google Scholar]
- 84.Dodd MJ, Painter P, Miaskowski C, et al. Exercise: An intervention for fatigue in cancer patients. P30 Pilot Study NR 03927. National Institutes of Health, National Institute of Nursing Research; 1997–1998. [Google Scholar]
- 85.Dodd MJ, Dibble SL, Miaskowski C, et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Jul;90(1):39–47. doi: 10.1067/moe.2000.105713. [DOI] [PubMed] [Google Scholar]
- 86.Knowles M. The adult learner: A neglected species. Houston: Gulf; 1978. [Google Scholar]
- 87.Dodd MJ, Miaskowski C. Semin Oncol Nurs. 4. Vol. 16. Nov, 2000. The PRO-SELF Program: a self-care intervention program for patients receiving cancer treatment; pp. 300–308. discussion 308–316. [DOI] [PubMed] [Google Scholar]
- 88.SPSSR 15.0 [computer program] Chicago, IL: SPSS Inc; 2008. [Google Scholar]
- 89.Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 90.Berger AM, Lockhart K, Agrawal S. Variability of patterns of fatigue and quality of life over time based on different breast cancer adjuvant chemotherapy regimens. Oncol Nurs Forum. 2009 Sep;36(5):563–570. doi: 10.1188/09.ONF.563-570. [DOI] [PubMed] [Google Scholar]
- 91.Heim ME, v d Malsburg ML, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007 Sep;30(8–9):429–434. doi: 10.1159/000104097. [DOI] [PubMed] [Google Scholar]
- 92.Courneya K, Friedenreich C. Relationship between exercise during treatment and current quality of life among survivors of breast cancer. J Psychosoc Oncol. 1997;15:35–57. [Google Scholar]
- 93.Schwartz AL. Patterns of exercise and fatigue in physically active cancer survivors. Oncol Nurs Forum. 1998 Apr;25(3):485–491. [PubMed] [Google Scholar]
- 94.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997 Fall;3(3):215–226. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 95.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. New York, NY: Psychology Press, Taylor & Francis Group; 1988. [Google Scholar]
- 96.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 97.Courneya KS, McKenzie DC, Reid RD, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008 Feb;35(1):116–122. doi: 10.1007/s12160-007-9009-4. [DOI] [PubMed] [Google Scholar]
- 98.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000 Oct;27(9):1443–1448. [PubMed] [Google Scholar]
- 99.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006 May;42(7):846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]