Abstract
Porphyrins bearing uracyl motifs at the four meso positions self-organize via homo-complementary hydrogen bonds and π-stacking into nanofibers, nanorods and thin films on mica and glass surfaces depending on deposition conditions.
Self-assembled and self-organized nanoarchitectures of porphyrinoids are of great interest for diverse applications, such as sensors, molecular sieves, catalysts, photonic and electronic materials such as photosensitized solar cells.1 Since porphyrins have tunable photophysical and chemical properties, the functionalities of their materials can be designed. Photosynthetic antenna complexes contain self-assembled arrays of pigments in precise architectures, thus supramolecular porphyrin arrays are also studied as model systems for light harvesting. Assemblies of chromophores formed via non-covalent interactions, such as electrostatics, H-bonding, metal ion coordination, π-stacking, dispersion forces, and combinations of these, can yield robust supramolecular photonic materials.1 The organization of supramolecular systems in solution can be different than on surfaces since both the deposition process, e.g. fluid dynamics, and surface structure/energetics can play pivotal roles.2
There are numerous reports on the fabrication of nanostructured porphyrinic materials on surfaces.2,3 Complex multiporphyrin monomers3b and porphyrins with motifs on the 5,15-positions3c can form nanofibers and nanocrystals, respectively. Nanorods,4 nanotubes,5 nanowires,6 rings,7a and micelles7b are reported. The work of Shelnut et al. 5 shows that appropriate conditions can be found to form nanotubes from simple Sn(iv) porphyrins driven by electrostatic interactions combined with axial coordination. The diverse supramolecular structures of Kobuke8 and Balaban et al. 3 are driven by self-complementary coordination and H-bond interactions. Long alkyl chains can also organize porphyrins into nanorods or fibers.9 Since these assemblies rely on strong interporphyrin interactions and/or complex molecular topologies, the formation of robust nanofibers primarily mediated by weak H-bonds and π-π interactions between simple porphyrins is unexpected (Fig. 1). This study provides further insights into the design principles, processing, and extent of electron and energy transfer in supramolecular porphyrin materials.
Fig. 1.
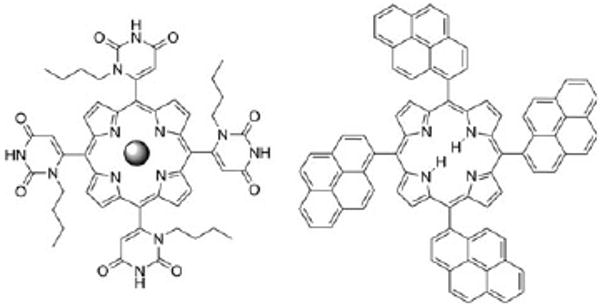
Left: 5,10,15,20-tetrakis(1-butyl-6-uracyl)porphyrin; the sphere represents Zn(II) (ZnTUrP) or free base (TUrP). Right: 5,10,15,20-tetrakis(1-pyrenyl)porphyrin (TPyrP). The uracyl and the pyrenyl groups are orthogonal to the porphyrin plane and the mixture of the four atropisomers (αααα, αααβ, ααββ and αβαβ, as synthesized) was used.
Thermodynamic equilibriums between different supramolecular structures or aggregates in weakly self-organized systems change throughout the deposition process because of the changing of environmental conditions. Concentration, temperature, moisture, solvent, evaporation rate, fluid dynamics, and surface energetics combine to dictate the solid state structure of these materials on surfaces.2,3 Here, we illustrate these concepts by the self-organization of 5,10,15,20-tetrakis(1-butyl-6-uracyl) porphyrinato zinc(II) (ZnTUrP)10 wherein the uracyl moieties serve as homo-complementary H-bonding donor acceptor motifs, and by the free base 5,10,15,20-tetrakis-(1-pyrenyl)porphyrin (TPyrP)11 wherein organization is effected by the π-stacking of the pyrenyl moieties (Fig. 1).‡ The morphology of self-organized ZnTUrP on glass and mica surfaces ranges from thin films to nanorods and nanofibers depending on deposition conditions, while the TPyrP forms nanoparticles only.
H-bonds: the uracyl moieties at the four meso porphyrin positions present motifs that can form self-complementary double H-bonds (ca. 15-21 kJ mol−1, Fig. 2).10 Though zinc metalloporphyrins have a propensity to aggregate by π-stacking with about the same energy,12 the orthogonal uracyl moieties project the N-butyl group above/below the porphyrin plane to diminish π-stacking interactions. The atropisomers do not significantly influence the H-bond energetics and kinetics of self-assembly in solution.10
Fig. 2.
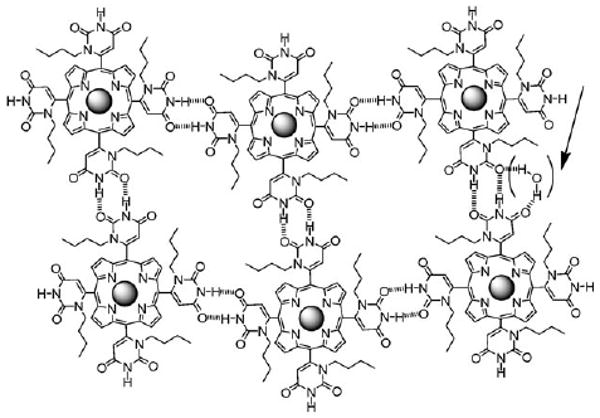
Possible arrangement of porphyrins in the materials mediated by H-bonds. The arrow indicates possible participation of water in the self-organization. The uracyl moieties remain orthogonal to the porphyrin.
The morphology of the deposited materials found by casting solutions of ZnTUrP onto surfaces depends on a variety of environmental conditions. Tetrahydrofuran (THF) is a good solvent for these porphyrins and weakly hydrogen bonds with the uracyl N–H. Atomic force microscopy (AFM) studies reveal that the porphyrin materials cast from wet THF (ca. 5% water) onto mica and glass consistently result in 5–20 nm thin films with varying degrees of surface coverage, whereas when deposited from dry THF, ZnTUrP forms nanofibers and nanorods (Fig. 3). Deposition from 10 μM solutions yields similar structures but with less surface coverage than from 100 μM solutions.
Fig. 3.
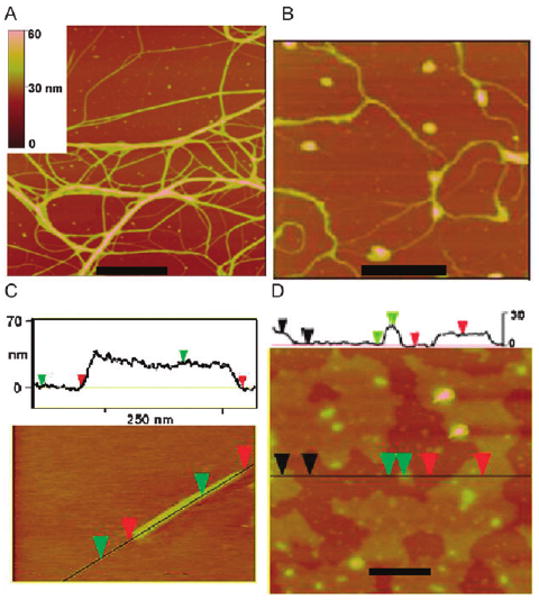
ZnTUrP nanostructures: (A) nanofibers on mica from 100 μM in dry THF; (B) nanofibers on mica from 10 μM in dry THF; (C) a nanorod on glass from 10 μM in dry THF; (D) a film cast on mica from a 10 μM solution of 5% water in THF; scale bars = 200 nm. ESI† shows films cast on glass from a 100 μM solution of 5% water in THF are 14–20 nm, other AFM studies and histograms.
Since the porphyrin with the substituents is ca. 0.5 nm thick, and most adsorbed porphyrins lay flat on surfaces,1c,2 the films likely represent 10 to 40 layers. The formation of the thin films is driven primarily by equatorial H-bond interactions. The water in the solvent competes with the H-bond sites, likely participates in the organization of the films (Fig. 2),10 and slows the evaporation rates to allow film formation. Thus, films represent an equilibrium architecture. The water in the solvent also interacts with the substrates to modulate the adsorption of the porphyrinic materials.
Cast from dry THF solutions, ZnTUrP self-organizes into nanofibers and rods with somewhat more surface density on mica compared to glass. The results suggest that 3-D structures are due to both H-bonds along the porphyrin plane and π-stacking interactions in the axial direction. The 1-butyl groups can modulate the π–π interactions differently depending on their conformations in the different solvents arising from hydrophobic effects. On mica nanorods and nanofibers are formed from both 10 μM and 100 μM solutions of ZnTUrP, independent of solvent evaporation rate (Fig. 3). On mica, the nanorods are measured to be 6–25 nm high by 200–600 nm long, while the fiber heights range from 2–50 nm and can be many μm long. On glass substrates, no fibers or rods were observed from the 100 μm solution. Deposition of the 10 μm ZnTUrP solution yields many bundles of fibers with heights ranging from 2–45 nm, again independent of solvent evaporation rate (Fig. 3). Examination of many samples indicates that many fibers possess substructures, but there is no clear indication of a fundamental structural unit in the height histograms.† From uracyl NH to uracyl NH, the porphyrin is ca. 1.8 nm wide and this is the minimum height measured. Alternatively this height can represent ca. three porphyrins stacked. These results indicate that the fibers and rods are kinetically trapped products wherein π–π and H-bond interactions cooperate to dictate growth rate. The rigid attachment of the H-bond moieties to the macrocycle limits the molecular and supramolecular dynamics, thereby allowing the formation of rods and fibers. When similar H-bonding groups are attached via flexible alkane linkers, neither rods nor fibers are observed under a variety of conditions.3
As expected for aggregates of porphyrin materials on surfaces,1,2 the films, nanofibers, and nanorods exhibit ca. 5 nm red shifts and broadening in the electronic absorption and emission spectra compared to the starting solutions.† Energy transfer from the ZnTUrP antenna to < 10% free base doped into these materials is observed in fluorescence experiments. No detectable change in the optical properties or morphologies after months of storage under ambient conditions indicates robustness of these materials.
Under identical conditions free base TUrP self-organizes into rods and fibers on mica substrate but to a much smaller extent that ZnTUrP. Since Zn(II) coordination increases electronic density on p-pyridyl moieties2 we suspect that ZnTUrP may be forming stronger H-bonds compared to TUrP. Although in other metalloporphyrin nanostructures the coordinated metal ion plays an important role in molecule organization5 there is no UV-visible or NMR evidence for the coordination of the uracyl moieties into the axial position on the Zn center. Thus, axial coordination is not required for formation of the nanostructured assemblies.
π–π interactions: in contrast to the H-bond motifs, self-organization of TPyrP is mediated only by π–π interactions between the orthogonal meso pyrene subunits and the atropisomers inhibit porphyrin π-stacking. TPyrP is of interest because of the coupling of the two different chromophores into one molecule, e.g. porphyrins bearing fluorenyl moieties13 or 2-pyrene11 imparts new photonic properties. As expected for hydrophobic TPyrP, the presence of water in toluene has no observable effect on the morphology of the deposited materials. A variety of deposition methods show that the surface structure on glass, mica, and HOPG substrates is dictated by the four pyrene units and that films or nanoparticles form depending solely on solvent evaporation rate. meso Tetraphenylporphyrin (TPP) results in amorphous aggregates using the same deposition conditions. Therefore, the known propensity of pyrene to form aggregates can be exploited to drive the formation of nanostructured materials on surfaces.
On mica, deposition from both 5 μM and 20 μM toluene solutions result in small nanoparticles independent of evaporation rate or solvent. Conversely, on glass, slow evaporation of 5 μM solutions of TPyrP results in 2–9 nm thick films, and faster evaporation results in 65 nm tall by 400–600 nm wide islands (Fig. 4). Since TPyrP is ca. 0.8 nm wide, the films are a minimum of three molecules. Under slower evaporation conditions, the domain size increases to greater than 100 nm in height and by several microns in length. We found no organization of TPyrP on HOPG. The atropisomers inhibit TPyrP organization driven by surface energetics and structure as observed for flat porphyrins.1c
Fig. 4.
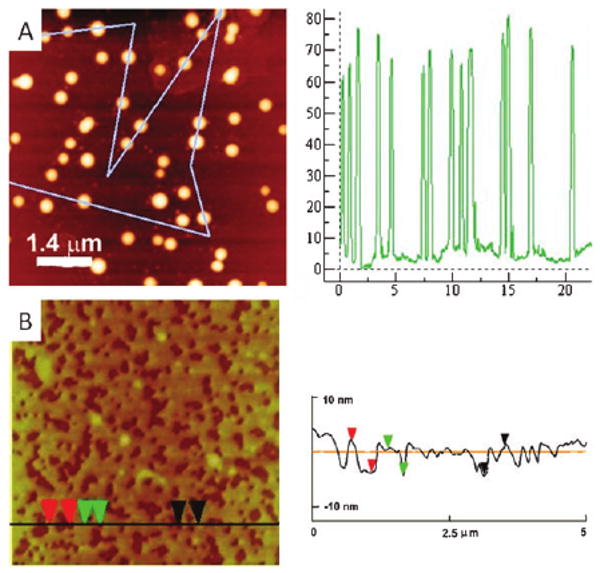
TPyrP drop cast on glass: (A) nanoparticles from a 20 μM solution with evaporation under ambient conditions, (B) films from a 5 μM solution with slow evaporation; heights of line traces on right.
The algorithms to control supramolecular structure in solution and in crystals are reasonably well established. However, the inherently non-equilibrium processes for self-assembly and self-organization on surfaces require additional considerations in terms of the varying concentrations, fluid dynamics, surface interactions, and supramolecular dynamics. Complex material architectures on surfaces need not to be the result of complex molecular structures or strong intermolecular forces that form in solution and deposit intact onto surfaces. Simple porphyrins can be used to form hierarchical structures on surfaces by control of the kinetics and thermodynamics of intermolecular interactions.
Acknowledgments
Supported by the U.S. National Science Foundation (NSF CHE-0554703, 0847997), Hunter College science infrastructure is supported by the NSF, the National Institutes of Health, including the RCMI program (G12-RR-03037) and the City University of New York.
Footnotes
Experimental: ZnTUrP10 and TPyrP11 were synthesized according to previous studies. ZnTUrP was dissolved in THF, and drop cast on mica or ozone cleaned glass. The solutions were allowed to dry at RT overnight in a glass cell culture dish with the top covered.
The rates of evaporation were slowed by the addition of a few drops of THF in the glass dish. When completely dried, samples were carefully rinsed with dry THF to remove amorphous aggregates at the edge of the deposited area. After drying at RT, the samples were probed by AFM (Veeco Multimode).
Electronic supplementary information (ESI) available: UV-visible and fluorescence spectra, AFM, histograms, TUrP and TPP data, experimental details. See DOI: 10.1039/b919468a
References
- 1.(a) Drain CM, Bazzan G, Milic T, Vinodu M, Goeltz JC. Isr J Chem. 2005;45:255–269. [Google Scholar]; (b) Doan SC, Shanmugham S, Aston DE, McHale JL. J Am Chem Soc. 2005;127:5885–5892. doi: 10.1021/ja0430651. [DOI] [PubMed] [Google Scholar]; (c) Drain CM, Batteas JD, Smeureanu G, Patel S. In: Encyclopedia of Nanoscience and Nanotechnology. Schwartz JA, Contescu CI, Putyera K, editors. Marcel Dekker, Inc; New York: 2004. pp. 3481–3502. [Google Scholar]
- 2.Milic T, Garno JC, Batteas JD, Smeureanu G, Drain CM. Langmuir. 2004;20:3974–3983. doi: 10.1021/la0359023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Balaban TS, Linke-Schaetzel M, Bhise AD, Vanthuyne N, Roussel C, Anson CE, Buth G, Eichhöfer A, Foster K, Garab G, Gliemann H, Goddard R, Javorfi T, Powell AK, Rösner H, Schimmel T. Chem–Eur J. 2005;11:2267–2275. doi: 10.1002/chem.200400664. [DOI] [PubMed] [Google Scholar]; (b) Hameren Rv, Schön P, Buul AMv, Hoogboom J, Lazarenko SV, Gerritsen JW, Engelkamp H, Christianen PCM, Heus HA, Maan JC, Rasing T, Speller S, Rowan AE, Elemans JAAW, Nolte RJM. Science. 2006;314:1433–1436. doi: 10.1126/science.1133004. [DOI] [PubMed] [Google Scholar]; (c) Lee SJ, Hupp JT, Nguyen ST. J Am Chem Soc. 2008;130:9632–9633. doi: 10.1021/ja801733t. [DOI] [PubMed] [Google Scholar]; (d) Balaban TS, Berova N, Drain CM, Hauschild R, Huang X, Kalt H, Lebedkin S, Lehn JM, Nifiatis F, Pescitelli G, Prokhorenko VI, Riedel G, Smeureanu G, Zeller J. Chem-Eur J. 2007;13:8411–8427. doi: 10.1002/chem.200601691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Schwab AD, Smith DE, Bond-Watts B, Johnston DE, Hone J, Johnson AT, Paula JCd, Smith WF. Nano Lett. 2004;4:1261–1265. [Google Scholar]; (b) Schwab AD, Smith DE, Rich CS, Young ER, Smith WF, Paula JCd. J Phys Chem B. 2003;107:11339–11345. [Google Scholar]
- 5.(a) Wang Z, Medforth CJ, Shelnutt JA. J Am Chem Soc. 2004;126:15954–15955. doi: 10.1021/ja045068j. [DOI] [PubMed] [Google Scholar]; (b) Wang Z, Medforth CJ, Shelnutt JA. J Am Chem Soc. 2004;126:16720–16721. doi: 10.1021/ja044148k. [DOI] [PubMed] [Google Scholar]; (c) Kojima T, Harada R, Nakanishi T, Kaneko K, Fukuzumi S. Chem Mater. 2007;19:51–58. [Google Scholar]
- 6.Li C, Ly J, Lei B, Fan W, Zhang D, Han J, Meyyappan M, Thompson M, Zhou C. J Phys Chem B. 2004;108:9646–9649. [Google Scholar]
- 7.(a) Jeukens CRLPN, Lensen MC, Wijnen FJP, Elemans JAAW, Christianen PCM, Rowan AE, Gerritsen JW, Nolte RJM, Maan JC. Nano Lett. 2004;4:1401–1406. [Google Scholar]; (b) Yuasa M, Oyaizu K, Yamaguchi A, Kuwakado M. J Am Chem Soc. 2004;126:11128–11129. doi: 10.1021/ja0486216. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kobuke Y. Struct Bonding. 2006;121:49–104. [Google Scholar]; (b) Kobuke Y. Eur J Inorg Chem. 2006:2333–2351. [Google Scholar]
- 9.(a) Jintoku H, Sagawa T, Takafuji M, Ihara H. Org Biomol Chem. 2009;7:2430–2434. doi: 10.1039/b818358a. [DOI] [PubMed] [Google Scholar]; (b) Iavicoli P, Simon-Sorbed M, Amabilino DB. New J Chem. 2009;33:358–365. [Google Scholar]
- 10.Shi X, Barkigia KM, Fajer J, Drain CM. J Org Chem. 2001;66:6513–6522. doi: 10.1021/jo010108c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knor G. Inorg Chem Commun. 2001;4:160–163. [Google Scholar]
- 12.Hunter CA, Sanders JKM. J Am Chem Soc. 1990;112:5525–5534. [Google Scholar]
- 13.Drouet S, Paul-Roth CO, Simonneaux G. Tetrahedron. 2009;65:2975–2981. [Google Scholar]


