Abstract
Purpose
Oxidant stress may be an effect of antiretroviral therapy (ART) or chronic HIV infection. Plasma F2-isoprostanes (F2-IsoP) reflect lipid peroxidation and oxidant stress and have been described in ART-associated toxicities. We explored factors associated with F2-IsoP in HIV-infected adults.
Methods
HIV-infected adults enrolled in this cross-sectional study were (a) on ART including zidovudine or stavudine but not non-nucleoside reverse transcriptase inhibitors (NNRTI), (b) on ART including NNRTI, or (c) not on ART. Plasma F2-IsoP levels were quantified by GC/MS, and clinical and laboratory data were collected at enrollment.
Results
Among 285 participants, 24% were female, 37% were African American, and 194 (68%) were on ART; 44 (23%) of whom were receiving efavirenz, 45 (23%) nevirapine, and 85 (44%) protease inhibitors. Median F2-IsoP was lower in those on NNRTI than those on ART without NNRTI (p = .02). In a multivariable model, factors independently associated with increased F2-IsoP were female sex (p =.002), higher BMI (p = .01), and heavy smoking (p =.004). There was a trend toward lower F2-IsoP among nevirapine users (p = .054).
Conclusions
Among HIV-infected adults, oxidant stress status differs by sex, BMI, smoking status, and perhaps specific ART. Prospective studies should better define relationships between oxidant stress and complications of HIV infection and its therapy.
Keywords: antiretroviral therapy, F2-isoprostanes, highly active, HIV, lipid peroxidation, oxidative stress
Access to potent antiretroviral therapy (ART) decreases morbidity and mortality due to human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS)1 and has made HIV infection a manageable chronic illness. Unfortunately many patients who are prescribed chronic ART experience drug toxicities that include metabolic, hepatic, neurological, and cardiovascular complications.2 Some adverse effects of ART including body fat changes (lipodystrophy), and peripheral neuropathy may not be completely reversible despite stopping the causative drug. The effects of chronic ART toxicity on treatment durability and adherence are likely substantial, but there are considerable gaps in our understanding of the underlying pathogenesis of ART toxicities. As patients may require life-long therapy, it is critical that we elucidate mechanisms of ART-associated complications in order to prevent and manage them.
Some ART-associated toxicities such as lipoatrophy, hepatic steatosis, lactic acidosis, and peripheral neuropathy appear to be mediated at least in part by nucleoside reverse transcriptase inhibitor (NRTI)-induced mitochondrial damage.3–5 A putative mechanism of these toxicities includes inhibition of mitochondrial DNA polymerase-γ, with resultant mitochondrial DNA depletion and tissue-specific mitochondrial dysfunction.3 Other metabolic toxicities including dyslipidemia and insulin resistance appear to be predominately associated with protease inhibitor (PI) exposure, likely through effects on lipid metabolism and cellular glucose uptake, respectively.2 These toxicities presumably contribute to an increased risk of cardiovascular disease observed in PI-treated persons.6,7 Although specific mechanisms of ART-associated toxicities are largely unknown, all of these processes may be mediated at a fundamental level by free radical-induced cellular damage, also referred to as oxidant stress.8
Oxidant stress is an underlying pathogenic mechanism during various human conditions including cardiovascular and neurodegenerative diseases.9, 10 Production of reactive oxygen species (ROS) during HIV infection may result from viral activation of inflammatory cytokines, especially TNF-α, and activated macrophages.11 The pathogenesis of immunodeficiency during chronic HIV infection includes apoptosis of CD4 T cells that is influenced by ROS production.12 An increased level of ROS may also increase blood-brain barrier permeability and contribute to HIV-associated dementia.13
Oxidant stress has been documented during HIV infection by demonstrating changes in plasma and/or cellular markers such as glutathione, cystine, serum malondialdehyde, zinc, and selenium. Oxidant stress associated with viral infection is correlated with decreased plasma glutathione and cystine, decreased plasma antioxidants,14 and increased malondialdehyde, an aldehyde produced by peroxide breakdown.15 Another marker of oxidant stress is 8-hydroxyguanine, an oxidation-modified DNA base that is present in higher concentrations in lymphocytes from HIV-infected individuals than from uninfected controls.16
The F2-isoprostanes (F2-IsoP) are prostaglandin-like compounds produced by peroxidation of cell membrane lipids by free radicals and are accurate and reliable markers of in vivo lipid peroxidation and systemic oxidant stress in humans.17 Plasma F2-IsoP levels may be elevated with heavy cigarette smoking18 and during chronic diseases that include atherosclerosis,10 scleroderma,19 Alzheimer’s disease,20 non-alcoholic fatty liver disease and chronic hepatitis C,21 and end-stage renal disease.22 Elevated plasma F2-IsoP levels have also been described in patients with chronic HIV infection, in particular among individuals with ART-associated lipoatrophy or hyperlactatemia,23 during therapeutic control of HIV-1 viremia,24 and potentially with some specific ART drugs.24 Factors that could potentially contribute to oxidant stress during chronic ART include ongoing HIV replication or chronic exposure to medications that increase oxidant stress. Results from an earlier, smaller study suggested higher F2-IsoP levels in persons receiving the non-NRTI (NNRTI) efavirenz24 and, in unpublished exploratory analyses, in those receiving the NRTI zidovudine. The present study was designed in an attempt to determine the factors associated with increased plasma F2-IsoP levels in a larger group of HIV-infected subjects and also to verify and further distinguish differences between particular ART drugs, with the hypothesis that efavirenz- and/or zidovudine-treated subjects would have higher plasma F2-IsoP levels than untreated subjects or those treated with other ART regimens.
METHODS
Study Population
This cross-sectional study enrolled a convenience sample of ambulatory HIV-infected adults from the Comprehensive Care Center in Nashville, Tennessee, from 2003 to 2005. Based on results from a previous study,24 we sought to identify subjects based on the use of particular thymidine analogue NRTIs or the use of different NNRTIs. Thus, eligible participants were (a) presently on ART that included zidovudine or stavudine but not an NNRTI for at least 30 days; (b) presently on ART that included an NNRTI (efavirenz or nevirapine) for at least 30 days; or (c) presently not on ART for at least 3 months and not on an NNRTI for at least 6 months. Because of the ubiquity of thymidine analogue NRTI use during the study period, subjects included in group b could be receiving thymidine analogue NRTIs in addition to the NNRTIs of interest. Target enrollment was 300 individuals, to include 100 in each group, and 50 each receiving zidovudine, stavudine, efavirenz, and/or nevirapine. Participants on ART were all considered adherent based on primary provider opinion. No other adherence assessments were performed. Patients receiving HMG-CoA reductase inhibitors were excluded. The study was approved by the Vanderbilt Institutional Review Board, and all participants provided written, informed consent.
Clinical Assessments
Study personnel collected clinical data at the time of study enrollment in a standardized fashion. A questionnaire was administered to assess ART and current antioxidant medication usage. Smoking status was self-reported as a history of use or current use and quantified as cigarettes/day on average. Smoking was categorized in a predetermined fashion as non-current smoker, current non-heavy smoker (<20 cigarettes/day), or current heavy smoker (≥20 cigarettes/day). Antioxidant use was self-reported as use of any “alternative therapies and/or dietary supplements, including antioxidants” and categorized as current use or no current use. Data on specific agents or doses were not routinely collected. Body mass index (BMI) was calculated as weight (kg)/height (m)2. The presence of lipoatrophy was graded by study personnel using a severity scale and was considered present if at least mild fat wasting was noted in both the face and peripheral extremities.
Laboratory Data
Laboratory data were collected at the time of study enrollment. Assays for plasma HIV-1 RNA, CD4 T cells, and nonfasting cholesterol were performed at a commercial laboratory (LabCorp, Louisville, Kentucky, USA). Plasma F2-IsoP levels were quantified from EDTA plasma. Whole-blood specimens were placed on ice promptly after collection and were centrifuged at 400 × g for 10 minutes at 4°C; plasma aliquots were stored at −70°C within 1 to 2 hours of collection. Plasma levels of 15-F2t-isoprostane (8-iso-PGF2alpha) were quantified by gas chromatographic/negative ion chemical ionization mass spectrometry employing stable isotope dilution as described previously.25 The assay has an intraday variability of less than 10%, and normal F2-IsoP in plasma of healthy volunteers is 35 pg/mL ± 1 SD (6 pg/mL).25 Plasma NNRTI concentrations were determined by high-performance liquid chromatography (HPLC) using slightly modified assays that have been previously reported.26
Statistical Analyses
Participant characteristics are presented using median and interquartile range (IQR) for continuous variables and frequencies for categorical variables. F2-IsoP levels were compared using the Mann-Whitney U test, Kruskal-Wallis test, or Spearman correlation for continuous variables, and chi-square or Fisher exact tests for categorical variables. Linear regression was used to assess independent association between F2-IsoP level as a continuous variable and covariates of interest, which were chosen a priori based on their known or potential effects on F2-IsoP. Among 307 participants, 264 had complete data. Missing continuous covariates included in the regression model (plasma HIV-1 RNA, CD4 T cells, BMI, and cholesterol) were imputed using multiple imputation methods.27 Factors included in the model were age, sex, race (White vs. non-White), self-reported use of antioxidant supplements (yes vs. no), BMI, CD4 T-cell count, log10 HIV-1 RNA level, total cholesterol, self-reported current smoking status (non-smoker vs. non-heavy smoker [<20 cigarettes/day] and vs. heavy smoker [≥20 cigarettes/day]), use of specific ART drugs (zidovudine vs. stavudine; nevirapine vs. efavirenz), and drug classes (PI vs. no PI).
F2-IsoP level was natural log-transformed to provide normality in the regression residuals. Beta coefficients were back-transformed indicating percent increase in F2-IsoP by one unit increase in corresponding covariate. Nonlinear cubic splines were used to assess the nonlinear association between log F2-IsoP and BMI. All two-way interactions were included to evaluate the effect of combined use of drugs or drug classes, and a global test indicated no statistically significant overall effect of all two-way interactions (p = .25); thus the interaction terms were excluded from the final model. The final model was validated using a bootstrap method,27 indicating a robustness of the final model (estimated shrinkage = 20%). All analyses were performed using R statistical software (version 2.3.1; available at: http://www.r-project.org).
RESULTS
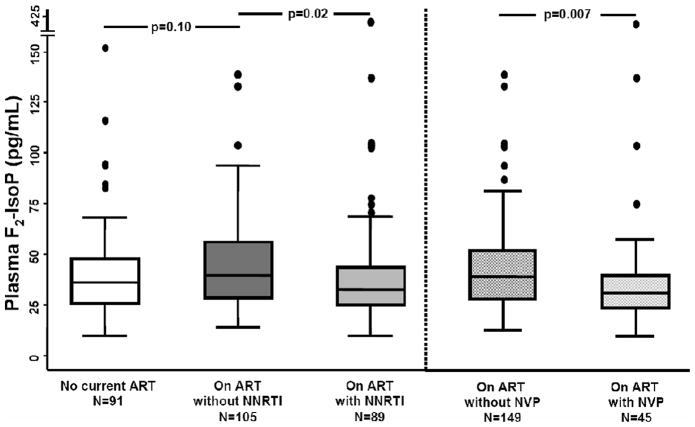
Plasma F2-IsoP assay results were available for 285 study participants. The median age was 41 years; 24% were female, 37% were self-reported African American, and 68% were receiving ART (Table 1). Eighty-nine (46%) of those on ART were receiving an NNRTI (44 on efavirenz, 45 on nevirapine), 85 (44%) were receiving a PI, and 110 (57%) were receiving a thymidine analogue NRTI without an NNRTI (61 on zidovudine, 49 on stavudine). Other NRTI use included lamivudine/emtricitabine in 153 (79%); abacavir in 56 (29%); tenofovir in 42 (22%); and didanosine in 7 (4%). Current cigarette smoking was reported by 150 (53%), and 82 (29%) reported smoking at least 20 cigarettes (one pack) per day. Ten persons (4%) reported regular use of antioxidant supplements. Among all study participants, median plasma F2-IsoP was 37 pg/mL (IQR 27–48 pg/mL), and the mean (SD) was 42 (31) pg/mL plasma. Median plasma F2-IsoP values differed by treatment group (Kruskal-Wallis p = .05): 40 pg/mL (29–56 pg/mL) in those on ART without an NNRTI, 36 pg/mL (26–48 pg/mL) for those not on ART, and 33 pg/mL (26–44 pg/mL) for those on ART that included an NNRTI (Figure 1).
Table 1.
Demographic, clinical, and treatment status of study participants, total and by various treatment categories
| Total study group (n =285) |
No current ART (n =91) |
Currently receiving ART (n =194) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total ART group |
Thymidine analogue nucleoside reverse transcriptase inhibitor (tNRTI) group |
Non-nucleoside reverse transcriptase inhibitor (NNRTI) group |
|||||||
| Total (n =169) | Zidovudine (n =103) |
Stavudine (n =66) |
Total (n =89) | Efavirenz (n =44) |
Nevirapine (n =45) |
||||
| Age in years | 41 (22–68) | 38 (23–58) | 42 (23–67) | 42 (23–67) | 42 (23–67) | 42 (23–60) | 43 (24–59) | 40 (23–59) | 44 (23–68) |
| Female sex | 69 (24) | 33 (36) | 36 (19) | 31 (18) | 20 (19) | 11 (17) | 16 (18) | 7 (16) | 9 (20) |
| Race/ethnicity | |||||||||
| White, non- Hispanic | 163 (57) | 46 (51) | 117 (60) | 98 (58) | 58 (56) | 40 (61) | 54 (61) | 32 (73) | 22 (49) |
| Black, non- Hispanic | 106 (37) | 41 (45) | 65 (34) | 61 (36) | 38 (37) | 23 (35) | 30 (34) | 10 (23) | 20 (44) |
| Othera | 16 (6) | 4 (4) | 12 (6) | 10 (6) | 7 (7) | 3 (4) | 5 (5) | 2 (5) | 3 (7) |
| ART | |||||||||
| Protease inhibitor | 85 (30) | – | 85 (44) | 71 (42) | 31 (30) | 40 (61) | 24 (27) | 11 (25) | 13 (29) |
| Indinavir | 13 (5) | – | 13 (7) | 13 (8) | 3 (3) | 10 (15) | 1 (1) | 0 | 1 (2) |
| Ritonavirb | 51 (18) | – | 51 (26) | 38 (22) | 19 (18) | 19 (29) | 18 (20) | 11 (25) | 7 (16) |
| Saquinavir | 2 (1) | – | 2 (1) | 2 (1) | 1 (1) | 1 (2) | 0 | 0 | 0 |
| Amprenavir | 6 (2) | – | 6 (3) | 5 (3) | 4 (4) | 1 (1) | 1 (1) | 1 (2) | 0 |
| Nelfinavir | 13 (5) | – | 13 (7) | 12 (7) | 3 (3) | 9 (14) | 5 (6) | 0 | 5 (11) |
| Lopinavir/ritonavir | 32 (11) | – | 32 (16) | 20 (12) | 8 (8) | 12 (18) | 16 (18) | 10 (23) | 6 (13) |
| Atazanavir | 18 (6) | – | 18 (9) | 18 (11) | 12 (12) | 6 (9) | 0 | 0 | 0 |
| NNRTI | 89 (31) | – | 89 (46) | 64 (38) | 45 (44) | 19 (29) | 89 (100) | 44 (100) | 45 (100) |
| Efavirenz | 44 (15) | – | 44 (23) | 26 (15) | 17 (17) | 9 (14) | 44 (49) | 44 (100) | – |
| Nevirapine | 45 (16) | – | 45 (23) | 38 (22) | 28 (27) | 10 (15) | 45 (51) | – | 45 (100) |
| tNRTI | 169 (59) | – | 169 (87) | 169 (100) | 103 (100) | 66 (100) | 64 (72) | 26 (54) | 39 (87) |
| Zidovudine | 103 (36) | – | 103 (53) | 103 (61) | 103 (100) | – | 45 (51) | 17 (39) | 28 (62) |
| Stavudine | 66 (23) | – | 66 (34) | 66 (39) | – | 66 (100) | 19 (21) | 9 (20) | 11 (22) |
| Plasma NNRTI level (ng/mL) | – | – | – | – | – | – | – | 1525 (1033–2438) | 5792 (4829–7670) |
| HIV RNA (log10 copies/mL) | 3.0 (1.8–4.0) | 4.2 (3.6–4.6) | 2.3 (1.7–3.2) | 2.4 (1.7–3.2) | 2.3 (1.7–3.2) | 2.5 (1.7–3.3) | 2.1 (1.7–2.8) | 1.8 (1.5–2.7) | 2.3 (1.7–2.9) |
| HIV RNA <400 copies/mL | 112 (39) | 5 (2.9) | 107 (55) | 90 (53) | 54 (52) | 36 (55) | 56 (63) | 32 (73) | 24 (44) |
| CD4+ lymphocytes (cells/mm3) | 428 (268–622) | 432 (296–555) | 425 (252–648) | 435 (253–672) | 423 (253–688) | 451 (256–660) | 434 (260–624) | 390 (187–525) | 534 (327–752) |
| Total cholesterol (mg/dL) | 181 (152–210) | 163 (186–137) | 192 (162–219) | 187 (158–212) | 184 (152–219) | 191 (168–208) | 200 (163–223) | 191 (162–222) | 206 (170–228) |
| Body mass index (kg/m2) | 26 (23–30) | 26 (23–31) | 26 (22–29) | 26 (23–30) | 26 (23–30) | 26 (22–30) | 26 (23–30) | 26 (23–29) | 28 (24–30) |
| Current smoker | 150 (53) | 49 (54) | 101 (52) | 88 (52) | 54 (53) | 34 (52) | 39 (44) | 22 (50) | 17 (38) |
| Heavy smoker (≥20 cigarettes/day) | 78 (52) | 28 (58) | 50 (50) | 42 (48) | 21 (39) | 21 (62) | 19 (49) | 10 (45) | 9 (53) |
| Plasma F2-isopostane (pg/mL) | 37 (27–48) | 36 (26–48) | 38 (27–50) | 37 (27–51) | 33 (24–46) | 39 (30–58) | 33 (26–44) | 38 (29–46) | 32 (24–40) |
Note: Values shown are median (interquartile range) or n (%). ART = antiretroviral therapy; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-NRTI; tNRTI = thymidine analogue NRTI.
“Other” includes Hispanic (n = 8), Asian (n = 5), and other self-reported race/ethnicities (n = 3).
Includes full dose as well as pharmacologic boosting doses with protease inhibitors other than lopinavir.
Figure 1.
Box plots of plasma F2-isoprostanes (F2-IsoP) in study participants receiving no antiretroviral therapy (ART; white box), receiving ART without a non-nucleoside reverse transcriptase inhibitor (NNRTI; dark grey shaded boxes), receiving ART with an NNRTI (light grey shaded boxes), and receiving ART that did not include nevirapine (NVP) and receiving ART that included NVP (shaded cross-hatched boxes). Horizontal lines represent median, interquartile range, and 5th and 95th percentile values. Outliers are shown by individual markers. P values shown are from unadjusted pair-wise Mann-Whitney U tests. The Kruskal-Wallis p value for comparison of first three groups = .05. Sample sizes for each group are shown.
By univariate analysis (Table 2), higher plasma F2-IsoP levels were significantly associated with female sex (p = .004), heavy cigarette smoking (p = .003), and higher BMI (p = .004). Lower F2-IsoP levels were associated with antioxidant use (p = .01). Among individuals on ART, lower F2-IsoP levels were associated with current NNRTI use (p = .02). The latter association was seen with nevirapine (p = .007; Figure 1) but not with efavirenz (p = .94). Current stavudine use was associated with higher F2-IsoP (p = .04) and zidovudine use with lower F2-IsoP levels (p = .03) compared with persons on ART not including these agents. Plasma F2-IsoP levels did not differ based on current use of abacavir or other NRTI. There were no significant univariate associations between F2-IsoP and current use of PIs, CD4 T count, HIV-1 RNA level, total cholesterol, plasma NNRTI concentration, or the presence of clinical lipoatrophy.
Table 2.
Univariate analysis of factors potentially associated with plasma F2-isoprostane levels
| Median (IQR) F2-IsoP (pg/mL) or Spearman correlation coefficient | Unadjusted p valuea | |
|---|---|---|
| Sex | ||
| Female (n = 69) | 42 (32–62) | .004 |
| Male (n = 216) | 36 (25–46) | |
| Smoking | ||
| Heavy smoker (≥20 cigarettes/day; n = 78) | 42 (32–54) | .003 |
| Non-heavy smoker (<20 cigarettes/day; n = 72) | 32 (25–51) | .83 |
| Non-smoker (n = 135) | 36 (26–46) | reference |
| Body mass index (kg/m2) | 0.17 | .004 |
| Current nevirapine useb | ||
| Yes (n = 45) | 32 (24–40) | .007 |
| No (n = 149) | 39 (29–52) | |
| Age | 0.05 | .41 |
| CD4 T cell count | −0.03 | .62 |
| Race/ethnicity | ||
| White, non-Hispanic (n = 163) | 39 (27–48) | .17 |
| Non-white (n = 122) | 36 (25–49) | |
| Antioxidant use | ||
| Yes (n = 10) | 24 (20–31) | .01 |
| No (n = 275) | 38 (27–49) | |
| Log10 HIV-1 RNA | −0.07 | .28 |
| Total cholesterol | 0.11 | .07 |
| Current efavirenz useb | ||
| Yes (n = 44) | 38 (29–46) | .94 |
| No (n = 150) | 38 (26–52) | |
| Current stavudine useb | ||
| Yes (n = 66) | 39 (30–58) | .04 |
| No (n = 128) | 36 (26–46) | |
| Current zidovudine useb | ||
| Yes (n = 103) | 33 (24–46) | .03 |
| No (n = 91) | 39 (30–57) | |
| Current protease inhibitor useb | ||
| Yes (n = 85) | 40 (28–52) | .13 |
| No (n = 109) | 36 (26–45) | |
| Lipoatrophyc | ||
| Yes (n = 50) | 39 (26–46) | .97 |
| No (n = 221) | 37 (27–49) | |
Note: ART = antiretroviral therapy; NNRTI = non-nucleoside reverse transcriptase inhibitor; IQR = interquartile range; F2-IsoP = F2-isoprostanes.
P values shown are from the Mann-Whitney U test or Spearman correlation.
Comparisons are for users of specific ART compared to nonusers among individuals on ART.
Clinically defined as at least mild fat wasting of the face and peripheral extremities. 271 individuals had lipoatrophy assessments and plasma F2-IsoP levels.
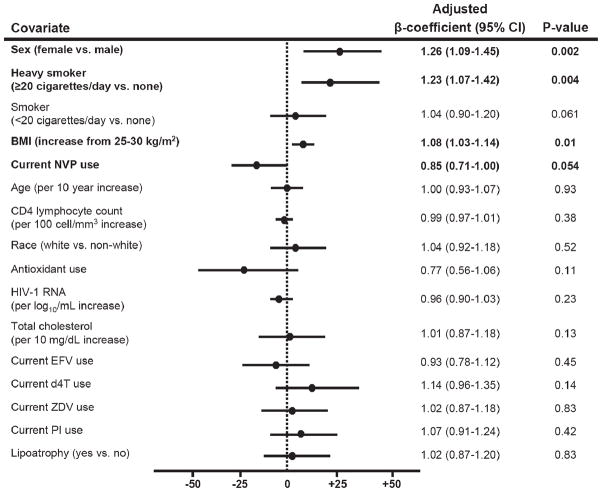
In a multivariable model that adjusted for demographic and clinical variables (Figure 2) including use of stavudine or zidovudine, and efavirenz or nevirapine, factors independently associated with higher F2-IsoP were female sex (26% relative increase in F2-IsoP [95% CI 9%–45%]; p=.002), higher BMI (8% increase in F2-IsoP [3%–14%] for an increase in BMI from 25 to 30 kg/m2; p = .01), and heavy smoking (23% increase in F2-IsoP [7%–42%]; p = .004), while nevirapine use tended to be associated with lower F2-IsoP levels (15% decrease in F2-IsoP [0%–29%]; p = .054). After adjusting for other factors, associations between F2-IsoP and current antioxidant, stavudine, or zidovudine use were no longer apparent. In a secondary multivariable model that included study groups rather than individual drugs, use of NNRTIs was independently associated with lower F2-IsoP referent to persons receiving ART without an NNRTI (p = .01; Table 3).
Figure 2.
Plots of adjusted β-coefficient values from multivariate linear regression modeling shown as percent change in F2-IsoP for a unit change in covariate. As body mass index (BMI) was included in the model as a nonlinear effect using restricted cubic spline, the β-coefficient shown for BMI corresponds to percent change in F2-IsoP as BMI increase from 25 to 30 kg/m2. Missing values for BMI (n = 4), total cholesterol (n = 5), HIV-1 RNA (n = 8), and CD4 lymphocyte count (n = 9) were imputed using multiple imputation methods.27 ART = antiretroviral therapy; CI = confidence interval; NNRTI = non-nucleoside reverse transcriptase inhibitor; F2-IsoP = F2-isoprostanes; NVP = nevirapine; EFV = efavirenz; d4T = stavudine; ZDV = zidovudine; PI = protease inhibitor.
Table 3.
Univarate and multivariate analysis of ART treatment status and plasma F2-isoprostane levels
| Current treatment status | Median (IQR) F2-IsoP (pg/mL) or Spearman correlation coefficient | Unadjusted p valuea | Adjusted β-coefficient (95% CI) | Adjusted p valueb |
|---|---|---|---|---|
| No ART (n = 91) | 36 (26–48) | .10 | 0.88 (0.74–1.04) | .13 |
| ART with NNRTI (n = 89) | 33 (26–44) | .02 | 0.83 (0.72–0.95) | .01 |
| ART without NNRTI (n = 105) | 40 (29–56) | reference | reference | reference |
Note: ART = antiretroviral therapy; CI = confidence interval; NNRTI = non-nucleoside reverse transcriptase inhibitor; IQR = interquartile range; F2-IsoP = F2-isoprostanes.
Unadjusted p values shown are from the Mann-Whitney U test.
Because preliminary studies had identified an association between plasma F2-IsoP and NNRTI use,24 we determined plasma NNRTI concentrations in this population. Among individuals receiving nevirapine, there was no correlation between plasma nevirapine concentration and F2-IsoP. However, in a secondary multivariable model that included individual drugs and nevirapine and efavirenz concentrations, the statistical association between nevirapine use and lower F2-isoP strengthened (p = .007; data not shown).
There was no statistically significant association between total cholesterol and F2-IsoP. However, there was a modest association between total cholesterol and F2-IsoP levels among individuals on ART without an NNRTI (Spearman’s rho = 0.23; p = .02) but not among individuals receiving an NNRTI or those not on ART (p = .6 for both). Not surprisingly, among individuals on ART without an NNRTI, 58% were receiving a PI, while only 27% of those on an NNRTI were also receiving a PI.
DISCUSSION
Among HIV-infected adults in this cross-sectional study, increased systemic oxidant stress as assessed by plasma F2-IsoP was associated with female sex, heavy smoking, and greater BMI, with estimated increases in plasma F2-IsoP of 26%, 23%, and 8%, respectively. These findings are consistent with previous studies of HIV-negative populations that also identified increased F2-IsoP among persons with increased BMI28 and heavy smokers.18 Increased BMI and cigarette smoking are well-established cardiovascular risk factors, and inflammation and oxidant stress are believed to play a modifying if not causative role in these relationships. These factors continued to be strongly associated with oxidant stress despite adjusting for other HIV-specific factors such as CD4 T-cell count, HIV RNA level, and ART use. This supports the importance of traditional risk factors as major determinants of cardiovascular disease in the HIV-infected population. Previous studies in HIV-negative populations also identified increased F2-IsoP in females,28–30 but others have reported lower F2-IsoP in healthy premenopausal females.31 The relationship between sex hormones, oxidant stress, and complications of HIV infection and its treatment, particularly cardiovascular complications, warrants further study.
We did not find an association between PI use and oxidant stress, despite the fact that prospective cohort studies have identified an epidemiological link between PI use and cardiovascular outcomes in HIV-infected persons.7 This finding suggests that PI-associated metabolic effects may influence cardiovascular risk through mechanisms that are independent of lipid peroxidation. Alternatively, our ability to identify a true association between PI use and oxidant stress may have been limited by our cross-sectional study design, the relatively small number of individuals on PIs, and the fact that we did not capture cumulative duration of PI exposure. We also saw no significant associations between either stavudine or zidovudine use and F2-IsoP after adjusting for other factors in our multivariable model. As with PIs, our ability to detect true associations between stavudine or zidovudine and oxidant stress may have been limited by the lack of information on duration of drug exposure. In contrast to a previous study,23 we did not find an association between plasma F2-IsoP and clinically defined lipoatrophy. Subjects included in the present study were not selected based on lipoatrophy status and were defined subjectively. Prospective studies using objective assessments will be needed to definitively determine relationships between plasma F2-IsoP and lipoatrophy.
In the present study, current NNRTI use, and in particular nevirapine, tended to be associated with lower oxidant stress as assessed by plasma F2-IsoP, even after adjusting for other clinical covariates (including nonfasting total cholesterol and concomitant ART including stavudine and PI use). Although this observation must be interpreted cautiously, it is particularly intriguing in light of data suggesting a more favorable lipid profile and lower levels of other cardiovascular risk markers in nevirapine-treated individuals compared with those receiving efavirenz or PI-containing ART.32–37 Patients with dyslipidemia on PI-containing regimens have favorable changes in lipid profiles following nevirapine substitution including increases in high-density lipoprotein (HDL) levels and apo-lipoprotein A1 (apoA1) levels, as well as decreased low-density lipoprotein (LDL)38, 39 and total cholesterol levels.34 Similar effects of nevirapine on lipid profiles when compared to PIs have been seen in treatment-naïve patients who initiate ART.40 When compared to efavirenz, nevirapine has been reported to cause greater increases in HDL cholesterol,41 decreases in triglycerides,42 or both.43 Proposed mechanisms for the differences include enhanced synthesis or impaired clearance of HDL particles and/or apoA1 by nevirapine41 and/or stimulation of lipogenic pathways in adipose tissue by efavirenz.44 Although nevirapine concentration did not directly correlate with plasma F2-isoP levels, including NNRTI concentration in our model appeared to strengthen the relationship between nevirapine use and F2-IsoP (data not shown).
Lipid metabolism is mediated in part by oxidant stress, and elevated plasma F2-IsoP as a marker of oxidant stress has been linked to coronary artery disease.10 We speculate that the somewhat lower plasma F2-IsoP in patients on nevirapine may reflect the beneficial effect of this drug on lipids and/or lipid-independent effects on oxidant stress. Subjects were not required to be fasting at the time of enrollment, which may have influenced total cholesterol measurements, but data from other HIV-infected populations have shown no difference in urinary F2-IsoP metabolites based on fasting status (authors’ unpublished data). A prospective study that includes thorough assessments of fasting lipid profiles and other markers of cardiovascular disease could better determine whether there is any differential effect of nevirapine on oxidant stress and whether this modifies cardiovascular risk. Although we did not find a significant association between total cholesterol and F2-IsoP, in secondary analyses there was a modest association between total cholesterol and F2-IsoP levels among the group on ART without an NNRTI that was not present among persons receiving an NNRTI or those not on ART. Because most of these individuals (58%) were receiving a PI (compared with 27% of the ART group receiving an NNRTI), perhaps this reflects a differential relationship between PI use, lipid peroxidation, and dyslipidemia.
This is the largest study to date to examine F2-IsoP as a marker of oxidant stress in HIV-infected individuals. In a previous cross-sectional study by our group involving 120 HIV-infected adults, we identified a weak correlation between plasma HIV RNA and F2-IsoP, with a greater proportion of persons with plasma HIV RNA <400 copies/mL on ART having higher F2-IsoP levels compared with persons receiving no ART.24 A previous study of 59 HIV-infected adults identified an association between lipoatrophy, symptomatic hyperlactatemia (with or without acidemia), and increased plasma F2-IsoP.23 We did not find an association between F2-IsoP in cryopreserved plasma and development of NRTI-associated peripheral neuropathy in a case-control study of 164 clinical trial participants.45 In addition to its larger size, the present study population was more demographically diverse and included participants with heterogeneous ART regimens including many not receiving ART. In contrast to our previous finding, the present study found no association between plasma HIV-1 RNA and F2-IsoP among all study participants or in subgroup analyses based on whether or not individuals were on ART.
This study has several limitations, particularly those inherent to cross-sectional analyses. We cannot determine causality of factors associated with F2-IsoP. Data regarding previous ART regimens and duration of ART exposure were not collected. Although the multivariable analysis adjusted for PI class use, the contribution of individual PIs to oxidant stress and toxicity was not assessed due to relatively small numbers of persons on any single PI. At the time of the study, NRTI-only regimens were still common (23% of those on ART), limiting our data related to more contemporary ART regimens. Nevertheless, we were able to evaluate many individuals receiving NNRTIs or PIs, and the identification of factors associated with oxidant stress remains relevant and applicable to contemporary therapies. Because plasma for NNRTI assays was collected at random times, true pharmacokinetic parameters and drug metabolism could not be precisely accounted for in our analysis. Finally, information on dietary antioxidant intake was not obtained nor did we quantify markers of oxidant stress or inflammation other than F2-IsoP.
We believe the results presented here have identified important HIV- and non-HIV-related factors contributing to oxidant stress that require more detailed study. An important complication of ART appears to be accelerated cardiovascular risk, which is likely influenced by oxidant stress status. Prospective studies are underway to better define contributions of ART and other factors to oxidant stress and complications of HIV infection and its therapy. Further clarification is needed regarding the role of F2-IsoP and other biomarkers of oxidant stress in HIV-infected individuals, as are prospective studies to assess potential benefits of interventions to decrease oxidant stress in this population.
Acknowledgments
We acknowledge Stephanie Sanchez and Kedria Reed-Walker for assisting with laboratory assays; Ravi Misra, MD, and the Vanderbilt ACTC staff (Vicki Bailey, RN, Brenda Jackson, RN, Michael Morgan, FNP, Janet Nicotera, RN, and Fred Nicotera) for assisting with subject recruitment; and the staff, providers, and study participants from the Comprehensive Care Center who made this study possible. We also acknowledge Dr. Jason Morrow, who died in 2008, and whose collaborative spirit, mentorship, and friendship will be dearly missed.
These studies were supported in part by research grants from Boehringer-Ingelheim Pharmaceuticals and the Bristol-Myers Squibb Company. Additional support was provided by NIH/NCCAM grant K23 AT002508 (T.H.), the Vanderbilt-Meharry Center for AIDS Research grant P30 AI54999 (A.S., D.W.H.), and the Vanderbilt University School of Medicine Emphasis Program (L.A.D.). The funding agencies were not involved in data collection, analyses, or manuscript preparation.
D.W.H. has received research grants from Bavarian Nordic, Boehringer-Ingelheim, the Bristol-Meyers Squibb Company, Gilead Sciences, Merck, Tanox, and Tibotec. He is a Scientific Advisory Board member for GlaxoSmithKline. E.P.A. is a consultant with the Bristol-Myers Squibb Company. The other authors have no potential conflicts to declare.
Footnotes
Results from this study were presented in part at the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; November 13–16, 2005; Dublin, Ireland (Abstract 77), and at the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA (Poster 798).
References
- 1.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. JAMA. 2006;296(7):844–854. doi: 10.1001/jama.296.7.844. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354(9184):1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC) Lab Invest. 2001;81(11):1537–1544. doi: 10.1038/labinvest.3780367. [DOI] [PubMed] [Google Scholar]
- 5.Day L, Shikuma C, Gerschenson M. Mitochondrial injury in the pathogenesis of antiretroviral-induced hepatic steatosis and lactic acidemia. Mitochondrion. 2004;4 (2–3):95–109. doi: 10.1016/j.mito.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med. 2002;33(2):192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 7.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 8.Day BJ, Lewis W. Oxidative stress in NRTI-induced toxicity: evidence from clinical experience and experiments in vitro and in vivo. Cardiovasc Toxicol. 2004;4(3):207–216. doi: 10.1385/ct:4:3:207. [DOI] [PubMed] [Google Scholar]
- 9.Montine KS, Quinn JF, Zhang J, et al. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128(1–2):117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Shishehbor MH, Zhang R, Medina H, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. 2006;41(11):1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil L, Perez D, Tapanes R, Perez J, Grune T. Does mitochondrial dysfunction during antiretroviral therapy in human immunodeficiency virus infection suggest antioxidant supplementation as a beneficial option? Redox Rep. 2005;10(3):113–119. doi: 10.1179/135100005X38905. [DOI] [PubMed] [Google Scholar]
- 12.Dobmeyer TS, Findhammer S, Dobmeyer JM, et al. Ex vivo induction of apoptosis in lymphocytes is mediated by oxidative stress: role for lymphocyte loss in HIV infection. Free Radic Biol Med. 1997;22(5):775–785. doi: 10.1016/s0891-5849(96)00403-0. [DOI] [PubMed] [Google Scholar]
- 13.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004;4(2–3):119–129. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Stehbens WE. Oxidative stress in viral hepatitis and AIDS. Exp Mol Pathol. 2004;77(2):121–132. doi: 10.1016/j.yexmp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc Res. 2000;47(3):410–418. doi: 10.1016/s0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 16.Jaruga P, Jaruga B, Gackowski D, et al. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic Biol Med. 2002;32(5):414–420. doi: 10.1016/s0891-5849(01)00821-8. [DOI] [PubMed] [Google Scholar]
- 17.Morrow JD, Roberts LJ. The isoprostanes: unique bio-active products of lipid peroxidation. Prog Lipid Res. 1997;36(1):1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 19.Stein CM, Tanner SB, Awad JA, Roberts LJ, II, Morrow JD. Evidence of free radical-mediated injury (isoprostane overproduction) in scleroderma. Arthritis Rheum. 1996;39(7):1146–1150. doi: 10.1002/art.1780390711. [DOI] [PubMed] [Google Scholar]
- 20.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44(3):410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 21.Konishi M, Iwasa M, Araki J, et al. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J Gastroenterol Hepatol. 2006;21(12):1821–1825. doi: 10.1111/j.1440-1746.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikizler TA, Morrow JD, Roberts LJ, et al. Plasma F2- isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58(3):190–197. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- 23.McComsey GA, Morrow JD. Lipid oxidative markers are significantly increased in lipoatrophy but not in sustained asymptomatic hyperlactatemia. J Acquir Immune Defic Syndr. 2003;34(1):45–49. doi: 10.1097/00126334-200309010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hulgan T, Morrow J, D’Aquila RT, et al. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37(12):1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 25.Morrow JD, Roberts LJ., II Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 26.Turner ML, Reed-Walker K, King JR, Acosta EP. Simultaneous determination of nine antiretroviral compounds in human plasma using liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784(2):331–341. doi: 10.1016/s1570-0232(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE., Jr . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 28.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Dietrich M, Norkus EP, et al. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 30.Gross M, Steffes M, Jacobs DR, Jr, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51(1):125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 31.Ide T, Tsutsui H, Ohashi N, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22(3):438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 32.Barreiro P, Soriano V, Blanco F, Casimiro C, de la Cruz JJ, Gonzalez-Lahoz J. Risks and benefits of replacing protease inhibitors by nevirapine in HIV-infected subjects under long-term successful triple combination therapy. AIDS. 2000;14(7):807–812. doi: 10.1097/00002030-200005050-00006. [DOI] [PubMed] [Google Scholar]
- 33.Fisac C, Virgili N, Ferrer E, et al. A comparison of the effects of nevirapine and nelfinavir on metabolism and body habitus in antiretroviral-naive human immunodeficiency virus-infected patients: a randomized controlled study. J Clin Endocrinol Metab. 2003;88(11):5186–5192. doi: 10.1210/jc.2002-021830. [DOI] [PubMed] [Google Scholar]
- 34.Negredo E, Ribalta J, Paredes R, et al. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. AIDS. 2002;16(10):1383–1389. doi: 10.1097/00002030-200207050-00010. [DOI] [PubMed] [Google Scholar]
- 35.Petit JM, Duong M, Masson D, et al. Serum adiponectin and metabolic parameters in HIV-1-infected patients after substitution of nevirapine for protease inhibitors. Eur J Clin Invest. 2004;34(8):569–575. doi: 10.1111/j.1365-2362.2004.01379.x. [DOI] [PubMed] [Google Scholar]
- 36.Tebas P, Yarasheski K, Henry K, et al. Evaluation of the virological and metabolic effects of switching protease inhibitor combination antiretroviral therapy to nevirapine-based therapy for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20(6):589–594. doi: 10.1089/0889222041217374. [DOI] [PubMed] [Google Scholar]
- 37.Young J, Weber R, Rickenbach M, et al. Lipid profiles for antiretroviral-naive patients starting PI- and NNRTI-based therapy in the Swiss HIV cohort study. Antivir Ther. 2005;10(5):585–591. [PubMed] [Google Scholar]
- 38.van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15(18):2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 39.Parienti JJ, Massari V, Rey D, Poubeau P, Verdon R. Efavirenz to nevirapine switch in HIV-1-infected patients with dyslipidemia: a randomized, controlled study. Clin Infect Dis. 2007;45(2):263–266. doi: 10.1086/518973. [DOI] [PubMed] [Google Scholar]
- 40.Clotet B, van der Valk M, Negredo E, Reiss P. Impact of nevirapine on lipid metabolism. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S79–84. doi: 10.1097/00126334-200309011-00012. [DOI] [PubMed] [Google Scholar]
- 41.van Leth F, Phanuphak P, Stroes E, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1(1):e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward DJ, Curtin JM. Switch from efavirenz to nevirapine associated with resolution of efavirenz-related neuropsychiatric adverse events and improvement in lipid profiles. AIDS Patient Care STDS. 2006;20(8):542–548. doi: 10.1089/apc.2006.20.542. [DOI] [PubMed] [Google Scholar]
- 43.Manfredi R, Calza L, Chiodo F. An extremely different dysmetabolic profile between the two available nonnucleoside reverse transcriptase inhibitors: efavirenz and nevirapine. J Acquir Immune Defic Syndr. 2005;38(2):236–238. doi: 10.1097/01.qai.0000143037.70120.fc. [DOI] [PubMed] [Google Scholar]
- 44.El Hadri K, Glorian M, Monsempes C, et al. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J Biol Chem. 2004;279(15):15130–15141. doi: 10.1074/jbc.M312875200. [DOI] [PubMed] [Google Scholar]
- 45.Hulgan T, Hughes M, Sun X, et al. Oxidant stress and peripheral neuropathy during antiretroviral therapy: an AIDS clinical trials group study. J Acquir Immune Defic Syndr. 2006;42(4):450–454. doi: 10.1097/01.qai.0000226792.16216.1c. [DOI] [PubMed] [Google Scholar]