Abstract
Aims
To summarize the changes that occur in the properties of bladder afferent neurons following spinal cord injury.
Methods
Literature review of anatomical, immunohistochemical, and pharmacologic studies of normal and dysfunctional bladder afferent pathways.
Results
Studies in animals indicate that the micturition reflex is mediated by a spinobulbospinal pathway passing through coordination centers (periaqueductal gray and pontine micturition center) located in the rostral brain stem. This reflex pathway, which is activated by small myelinated (Aδ) bladder afferent nerves, is in turn modulated by higher centers in the cerebral cortex involved in the voluntary control of micturition. Spinal cord injury at cervical or thoracic levels disrupts voluntary voiding, as well as the normal reflex pathways that coordinate bladder and sphincter function. Following spinal cord injury, the bladder is initially areflexic but then becomes hyperreflexic due to the emergence of a spinal micturition reflex pathway. The recovery of bladder function after spinal cord injury is dependent in part on the plasticity of bladder afferent pathways and the unmasking of reflexes triggered by unmyelinated, capsaicin-sensitive, C-fiber bladder afferent neurons. Plasticity is associated with morphologic, chemical, and electrical changes in bladder afferent neurons and appears to be mediated in part by neurotrophic factors released in the spinal cord and the peripheral target organs.
Conclusions
Spinal cord injury at sites remote from the lumbosacral spinal cord can indirectly influence properties of bladder afferent neurons by altering the function and chemical environment in the bladder or the spinal cord.
Keywords: afferent neurons, micturition, neuropeptides, neuroplasticity, neurotrophic factors, urinary bladder, voltage-gated ion channels
INTRODUCTION
The functions of the lower urinary tract (LUT)—to store and periodically release urine—are dependent upon neural circuits located in the brain, spinal cord, and peripheral ganglia.1–7 This dependence on central nervous system (CNS) control distinguishes the LUT from many other visceral structures (e.g., the gastrointestinal tract and cardiovascular system) that maintain a certain level of function even after elimination of extrinsic neural input.
The dependence of LUT functions on complex central neural networks renders these functions susceptible to a variety of neurologic disorders, including spinal cord injury.2,7 Spinal cord injury rostral to the lumbosacral level eliminates the voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention and then to a slow development of automatic micturition and neurogenic detrusor overactivity (NDO) that is mediated by spinal reflex pathways. However, voiding is commonly inefficient owing to simultaneous contractions of the bladder and urethral sphincter (detrusor-sphincter-dyssynergia, DSD).
The recovery of reflex bladder activity after spinal cord injury is dependent on the reorganization of reflex pathways in the spinal cord, as well as alterations in the properties of bladder afferent neurons.3,5,8 This review will summarize the morphologic, electrophysiologic, and chemical changes in bladder afferent neurons after spinal cord injury and the molecular mechanisms that might underlie these changes.
AFFERENT INNERVATION OF THE LOWER URINARY TRACT
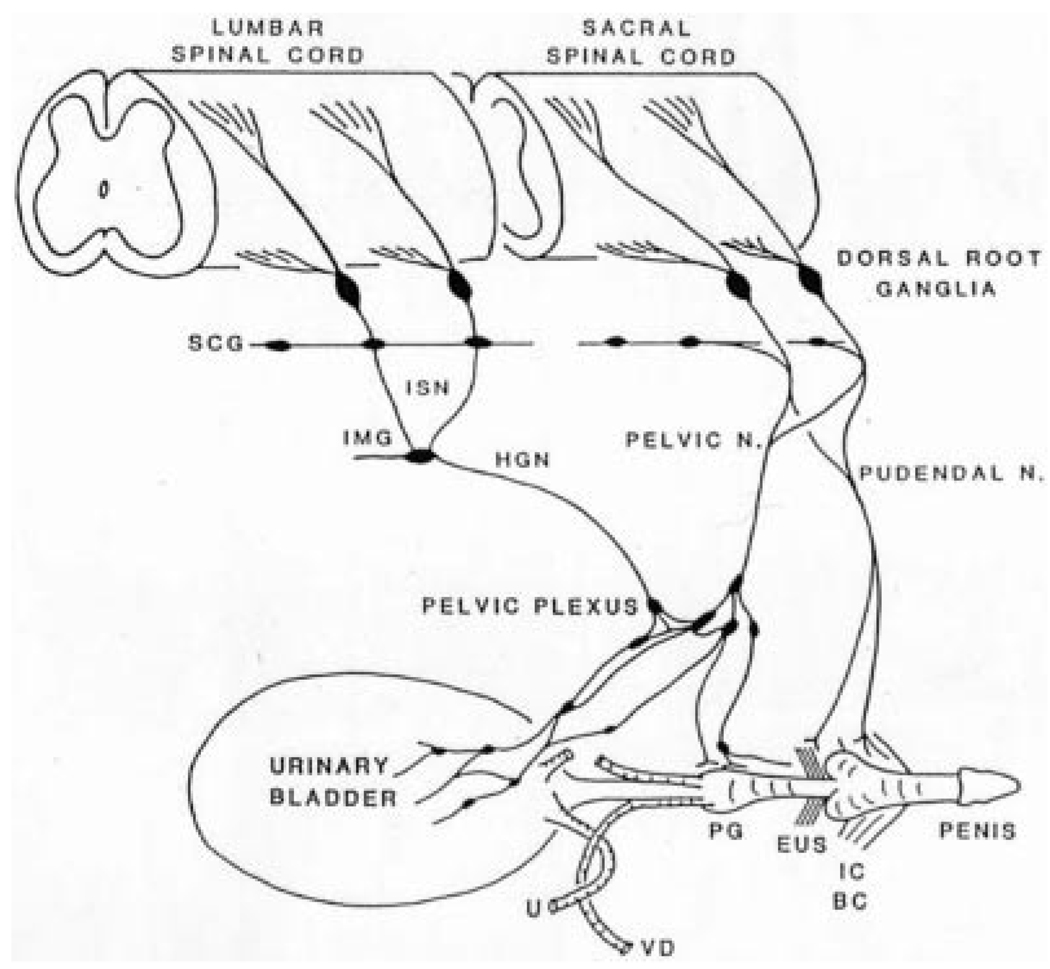
The LUT, which consists of (1) a reservoir (the urinary bladder) and (2) an outlet (the bladder neck, urethra, and striated muscles of the external urethral sphincter [EUS]), is regulated by three sets of peripheral nerves: sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and somatic nerves (pudendal nerves) (Fig. 1).5,6 The nerves consist of efferent and afferent axons originating at thoracolumbar and sacral spinal levels. Afferent innervation arises from neurons located in the dorsal root ganglia (DRG) (Fig. 1).9
Fig. 1.
Diagram showing the sympathetic, parasympathetic, and somatic innervation of the urogenital tract of the male cat. Sympathetic preganglionic pathways emerge from the lumbar spinal cord and pass to the sympathetic chain ganglia (SCG) and then via the inferior splanchnic nerves (ISN) to the inferior mesenteric ganglia (IMG). Preganglionic and postganglionic sympathetic axons then travel in the hypogastric nerve (HGN) to the pelvic plexus and the urogenital organs. Parasympathetic preganglionic axons, which originate in the sacral spinal cord, pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. Sacral somatic pathways are contained in the pudendal nerve, which provides an innervation to the penis, the ischiocavernosus (IC), bulbocavernosus (BC), and external urethral sphincter (EUS) muscles. The pudendal and pelvic nerves also receive postganglionic axons from the caudal SCG. These three sets of nerves contain afferent axons from the lumbosacral dorsal root ganglia. U, ureter; PG, prostate gland; VD, vas deferens.
Afferent axons, identified primarily by neuropeptide immunoreactivity/calcitonin-gene-related peptide (CGRP), pituitary adenylate cyclase-activating polypeptide (PACAP), or substance P are distributed throughout the bladder wall10–12 from the serosal layer to the lamina propria, including a dense suburothelial plexus that gives rise to axons extending into the urothelium.5,13 Sacral afferents are more abundant in the muscularis than in the suburothelium and have a more uniform distribution throughout the fundus and trigone regions, whereas the lumbar afferents are localized in the trigone and are more abundant in the suburothelium than in the muscularis.13,14 In human and animal bladders, afferent axons containing tachykinins and CGRP immunoreactivity are also located around blood vessels and in close proximity to intramural ganglion cells where they may make synaptic connections and participate in local reflex networks within the bladder wall.11,15 Afferent nerves arising in the DRG on one side of the spinal cord appear to be distributed bilaterally in the bladder wall.16
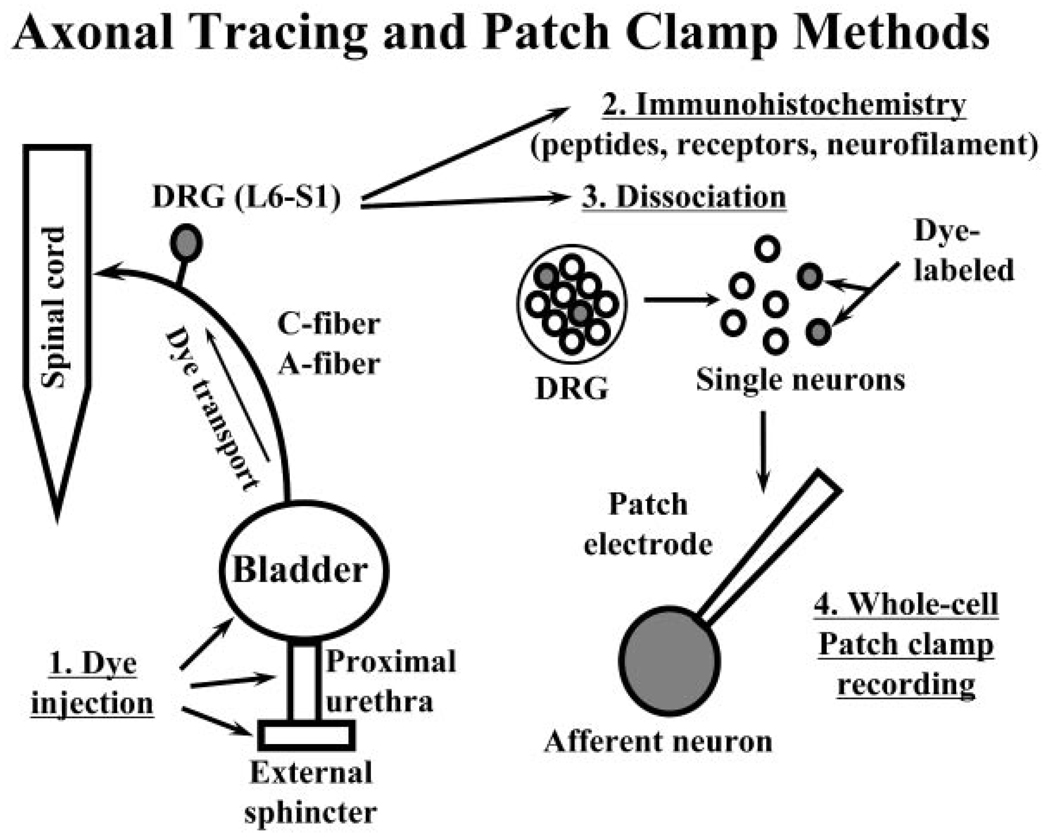
Retrograde axonal tracing methods have identified DRG cells innervating the bladder, urethra, and external urethral sphincter (Fig. 2). Relatively small numbers, less than 3% of the total population of neurons in an individual DRG, innervate different parts of the LUT (e.g., less than 3,000 sacral afferent neurons innervate the bladder of the cat).9,17 The neurons are small to medium size and are distributed randomly throughout the DRG.
Fig. 2.
Diagram illustrating the method for studying identified lower urinary tract afferent neurons in the L6-S1 dorsal root ganglia (DRG) using immunohistochemistry or patch-clamp techniques. (1) DRG neurons are labeled by axonal transport of fluorescent dyes injected into the bladder, urethra, or external sphincter several days prior to the experiment. (2) DRGs are removed for histologic experiments or (3) enzymatically dissociated to liberate single neurons. (4) Individual dye-labeled neurons are studied with whole cell patch-clamps methods.
When different axonal tracers are injected into multiple pelvic organs, for example, the bladder and colon, a small percentage (5–17%) of DRG neurons are double labeled,18–20 indicating that some sensory neurons can innervate multiple target organs. This pattern of innervation may contribute to the phenomenon of cross-sensitization of afferent pathways and provide a mechanism by which pathology in one organ can influence sensations in an adjacent organ.18,21
CENTRAL AFFERENT PATHWAYS
Central projections of afferent neurons innervating the LUT, which have been labeled by transganglionic transport of tracers, project to discrete regions of the dorsal horn (Figs. 3A and 4) that contain interneurons (Fig. 3C) and efferent neurons controlling the LUT (Fig. 4). Afferent pathways from the urinary bladder of the cat9,17 and rat22,23 project into Lissauer’s tract in the lumbosacral spinal cord and then pass rostrocaudally, giving off collaterals at regular intervals in the rostrocaudal axis (Fig. 4), which extend through lamina I laterally and medially around the dorsal horn into deeper laminae (laminae V–VII and X) at the base of the dorsal horn. The lateral pathway (lateral collateral pathway, LCP), which is the most prominent projection, terminates in the region of the sacral parasympathetic nucleus and also sends some axons medially to the dorsal commissure. Bladder afferents have not been detected in the center of the dorsal horn (laminae III–IV) or in the ventral horn (Fig. 4). Afferent axons from the pelvic viscera of the cat passing through sympathetic nerves to the rostral lumbar segments have similar sites of termination in laminae I, V–VII, and X.24 Although afferents are distributed primarily to the ipsilateral side of the spinal cord, an estimated 10–20% also project to the opposite side of the cord.25,26
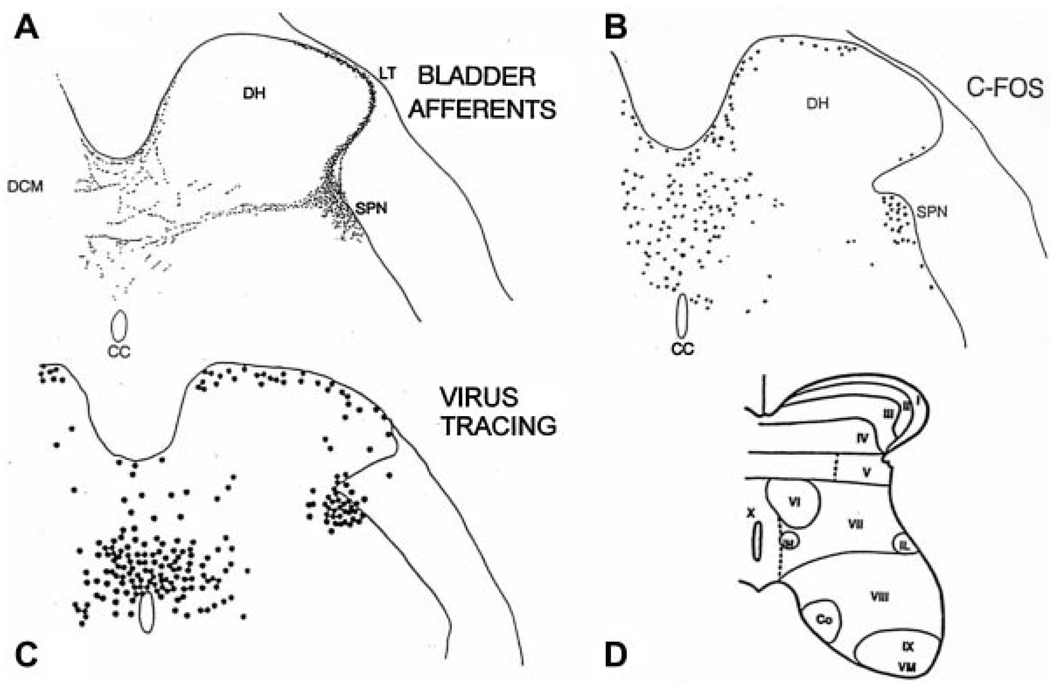
Fig. 3.
Comparison of the distribution of bladder afferent projections to the L6 spinal cord of the rat (A) with the distribution of c-fos positive cells in the L6 spinal segment following chemical irritation of the lower urinary tract of the rat (B), and the distribution of interneurons in the L6 spinal cord labeled by transneuronal transport of pseudorabies virus injected into the urinary bladder (C). Afferents labeled by WGA-HRP injected into the urinary bladder. c-fos immunoreactivity is present in the nuclei of cells. DH, dorsal horn; SPN, sacral parasympathetic nucleus; CC, central canal. D: Drawing shows the laminar organization of the cat spinal cord.
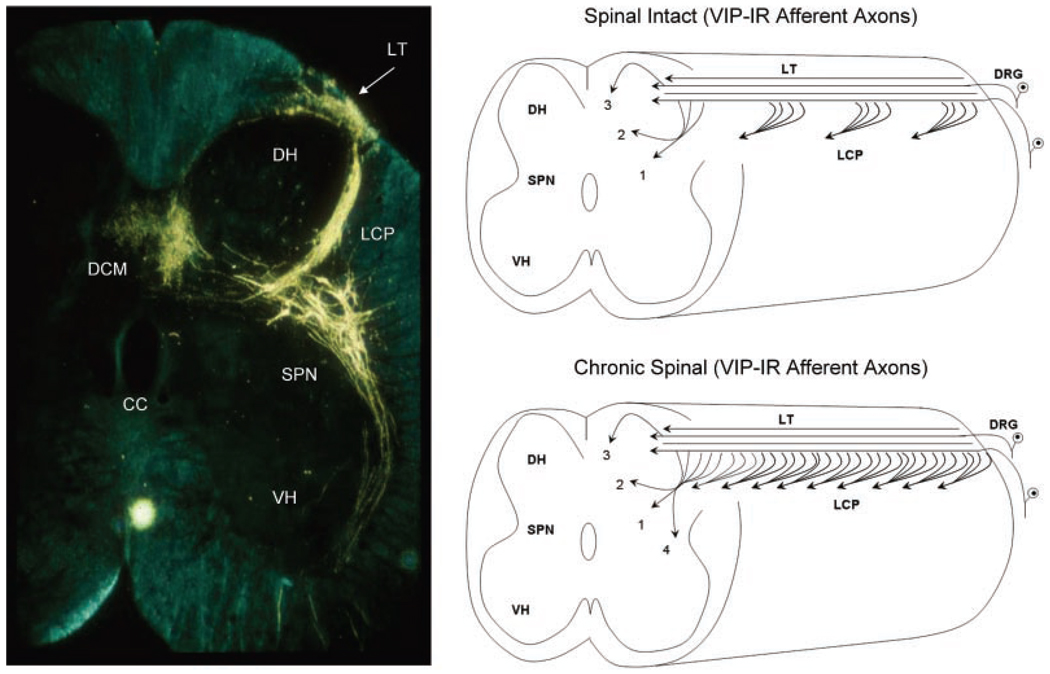
Fig. 4.
Transverse section of the S2 spinal cord of the cat showing primary afferent axons and preganglionic neurons after application of horseradish peroxidase (HRP) to the pelvic nerve. Afferents enter Lissauer’s tract (LT) and then send collaterals through lamina 1 laterally around the dorsal horn (DH) in a large bundle (the lateral collateral pathway, LCP) into the area of the sacral parasympathetic nucleus (SPN). A smaller group of afferents extend medially into the dorsal gray commissure (DCM). Axons of preganglionic neurons in the SPN project into the ventral horn (VH). Diagram on the top right side shows that the distribution of VIP-immunoreactive (VIP-IR) afferent axons in the sacral spinal cord of the cat is similar to the distribution of pelvic visceral afferent axons in the LCP, lamina V (1) and in the DCM (2, 3). In addition, the VIP-IR axons and pelvic afferent axons in the LCP, which arise as collaterals from longitudinal axons in LT, occur in bundles distributed at regular intervals along the rostrocaudal axis of the cord. After chronic spinal cord injury (lower left diagram) the VIP-IR afferent pathways expand and reorganize, leading to a continuous band of axons in the LCP and more extensive projections into region of the SPN (4).
Pudendal nerve afferent pathways from the EUS of the cat have central terminations that overlap in part with those of bladder afferents in lateral laminae I, V–VII, and X.9,27 These afferents differ markedly from other populations of pudendal nerve afferents innervating sex organs or cutaneous and subcutaneous tissues of the perineum that terminate in the deeper layers of the dorsal horn (laminae II–IV).27,28 Pudendal afferent projections in the LCP have a periodic distribution in the rostrocaudal axis similar to the distribution of pelvic nerve afferents shown in Figure 4.
The spinal neurons involved in processing afferent input from the LUT have been identified by the expression of the immediate early gene, c-fos (Fig. 3B). In the rat, noxious or non-noxious stimulation of the bladder and urethra increases the levels of Fos protein primarily in the dorsal commissure, the superficial dorsal horn, and in the area of the sacral parasympathetic nucleus.29–31 Noxious stimulation induces c-fos expression in a greater number of spinal neurons, particularly in the dorsal commissure (Fig. 3B). Some of these interneurons (Fig. 3C) send long projections to the brain, whereas others make local connections in the spinal cord and participate in segmental spinal reflexes.8,30
HISTOLOGIC AND CHEMICAL PROPERTIES OF AFFERENT NERVES
Light and electron microscopy have revealed that the visceral nerves innervating the LUT are composed primarily of small myelinated (Aδ-fiber) and unmyelinated (C-fiber) axons.32,33 DRG neurons give rise to the myelinated Aδ-fiber and unmyelinated C-fiber axons, which can be distinguished by immunohistochemical staining for the 200 kDa neurofilament protein, which is exclusively expressed in myelinated Aδ-fiber DRG neurons but not in unmyelinated C-fiber neurons.34 Approximately two thirds of bladder afferent neurons identified in rats by axonal tracing methods (Fig. 2) are neurofilament-poor (i.e., C-fiber neurons), while the remaining one third of cells exhibit intense neurofilament immunoreactivity (Aδ-fiber neurons).35 Approximately 80% of neurofilament-poor C-fiber-dissociated bladder afferent neurons are sensitive to capsaicin (Fig. 5).35
Fig. 5.
Comparison of the electrophysiologic characteristics of C-fiber (top traces) and Aδ-fiber (lower traces) bladder afferent neurons isolated from the lumbar dorsal root ganglion (DRG) of the rat. The left panels are voltage responses and action potentials evoked by 20 msec depolarizing current pulses. Asterisks with dashed lines indicate thresholds for spike activation (20 and 29 mV in the top and bottom traces, respectively). C-fiber neurons have higher thresholds and longer duration action potentials. The second panels show that C-fiber and Aδ-fiber afferent neurons have tetrodotoxin (TTX)-resistant and -sensitive action potentials, respectively. The third panel shows the firing patterns during long duration (600 msec) depolarizing currents pulses. C-fiber neurons fire phasically (1–2 spikes), whereas Aδ-fiber neurons fire tonically (12–15 spikes). The fourth panel shows that C-fiber neurons are capsaicin sensitive, whereas Aδ-fiber neurons are capsaicin insensitive.
Afferent neurons innervating the LUT exhibit immunoreactivity for various neuropeptides, such as substance P, calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating polypeptide (PACAP), leucine enkephalin, corticotrophin-releasing factor, and vasoactive intestinal polypeptide (VIP),9,19,36–39 as well as growth-associated protein-43 (GAP-43), nitric oxide synthase,40 glutamic acid, and aspartic acid.41 These substances have been identified in many species and at one or more locations in the afferent pathways, including (1) afferent neurons in the lumbosacral DRG, (2) afferent nerves in the peripheral organs, and (3) afferent axons and terminals in the lumbosacral spinal cord.6,42–44 The majority (>70%) of bladder DRG neurons in rats appear to contain multiple neuropeptides—CGRP, substance P, or PACAP being the most common. In cats, VIP is also contained in a large percentage of bladder DRG neurons and is coexpressed with other peptides.36
In the spinal cord of rats and cats, peptidergic afferents are present in Lissauer’s tract, in lamina I where they are very prominent on the lateral edge of the dorsal horn (LCP), and in the region of the parasympathetic nucleus.38,42,44 This distribution is similar to that of the central projections of bladder afferent neurons labeled by axonal tracers (Fig. 4).9,23 VIP, which is a marker for C-fiber afferent projections in the cat sacral spinal cord, exhibits a periodic distribution in the LCP on the lateral edge of the dorsal horn similar to the distribution of bladder afferent axons (Fig. 4). Chronic treatment with C-fiber afferent neurotoxins, capsaicin, or resiniferatoxin reduces peptidergic afferent staining in the bladder wall of animals and humans, indicating that the majority of peptidergic bladder afferent nerves are capsaicin-sensitive C-fibers.5,37
Bladder afferent neurons and axons, especially C-fiber afferents, also express various receptors,5,6,39 including the transient receptor potential vanilloid receptor 1 (TRPV1, the capsaicin receptor); transient receptor potential ankyrin 1 receptor (TRPA1); transient receptor potential cation channel subfamily M (TRPM8, a cold receptor); tropomyosin-related kinase A (TrkA), which responds to nerve growth factor (NGF); α and β estrogen receptors;45 tropomyosin-related kinase B (TrkB), which responds to brain-derived neurotrophic factor (BDNF); glial cell line-derived neurotrophic factor (GDNF) receptors, which respond to GDNF (GFRα1) and artemin (GFRα3);46 p75 neurotrophin receptor;47 isolectin B4 binding sites (IB4);48 muscarinic receptors; endothelin receptors; and purinergic receptors (P2X2, P2X3 P2Y) receptors, which can be activated by adenosine triphosphate (ATP).49–52 Many of these receptors have been detected not only in axons in the bladder, but also in the lumbosacral spinal cord in the same locations as the projections of bladder afferent axons.
C-fiber afferents innervating the LUT of the rat have been subdivided into two subpopulations based on lectin-binding: (1) IB4-negative, peptidergic and (2) IB4-positive, nonpeptidergic.48,49,53 The IB4 negative, peptidergic subgroup represents the largest population (70–80%) of C-fiber afferents. IB4-binding has also been used to identify different types of somatic C-fiber afferents.49,54 One type that does not exhibit IB4 binding is NGF-dependent, which expresses TrkA receptors and contains neuropeptides,54 whereas a second type that does bind IB4 expresses the GDNF family of growth factor receptors (GFRα) and is thought to be largely nonpeptidergic.49 Bladder afferent neurons have a lower percentage of IB4-positive cells (approximately 30%) than somatic afferent neurons innervating the skin (43%).49 The smaller number of IB4-positive bladder afferents is also reflected in the smaller number of GFRα receptor positive neurons. GFRα1 is present in 15.4%, GFRα3 in 8.4%, and GFRα2 in only 1% of lumbosacral bladder DRG neurons.46 The total percentage of GFRα positive bladder neurons is similar to the percentage of IB4 positive bladder neurons.
The expression of multiple receptors in bladder afferent nerves indicates that sensory mechanisms in the bladder are likely to be complex and involve the summation of a variety of chemical and mechanical signaling mechanisms, many of which may interact to produce excitation, while others may produce the opposite effect and suppress afferent firing. Activation of TRPV1, TRPA1, TRPM8, TrkA, P2X, nicotinic, muscarinic, or endothelin receptors by intravesical administration of receptor agonists in in vivo experiments, or by direct application to nerves in in vitro preparations, can enhance afferent nerve activity, release afferent transmitters, or stimulate reflex bladder activity.4,50,52,55–70 On the other hand, some putative transmitters/neuromodulators, such as nitric oxide, nicotinic, and muscarinic agonists, also have inhibitory effects.65,68–71 The complex chemical modulation of bladder afferent activity may be related not only to the expression of multiple receptors on afferent nerves but also to effects on non-neural cells (urothelial cells and myofibroblasts) that can interact with afferent nerves via chemical messengers.5,13,72
ELECTROPHYSIOLOGIC PROPERTIES OF BLADDER AFFERENT NEURONS
The most important afferents for initiating micturition are the small myelinated (Aδ) and unmyelinated (C) fibers passing in the pelvic nerve to the sacral spinal cord. Aδ bladder afferents in the cat respond in a graded manner to passive distension as well as active contraction of the bladder and exhibit pressure thresholds in the range of 5–15 mm Hg, which are similar to the pressures at which humans report the first sensation of bladder filling.2,5,6 These fibers also code for noxious stimuli in the bladder. On the other hand, C-fiber bladder afferents in the cat have high thresholds and commonly do not respond to even high levels of intravesical pressure.73 However, activity in some of these afferents is unmasked or enhanced by chemical irritation of the bladder mucosa. Thus the C-fiber afferents in the cat have specialized functions, such as the signaling of inflammatory or noxious events in the LUT. Nociceptive and mechanoceptive information is also carried in the hypogastric nerves to the thoracolumbar segments of the spinal cord.74
In the rat and mouse, Aδ and C-fiber bladder afferents consist of both mechanosensitive and chemosensitive subpopulations and are not distinguishable on the basis of stimulus modality.66,75–77 C-fiber afferents that respond only to bladder filling have also been identified in the rat bladder and appear to be volume receptors that are activated by stretch of the mucosa.6
C-fiber afferents are sensitive to the neurotoxins capsaicin and resiniferatoxin,6,13,37,52,77,78 as well as to other substances, such as nitric oxide, ATP, prostaglandins, and neurotrophic factors released in the bladder by afferent nerves, urothelial cells, and inflammatory cells. Activation of TRPV1 receptors in C-fiber afferents releases tachykinins and induces bladder contractions, as well as bladder inflammation.37,79,80
The properties of lumbosacral DRG cells innervating the LUT in the rat have been studied with patch-clamp recording techniques in combination with axonal tracing methods to identify the different populations of neurons (Fig. 2).20,53,81–87 Capsaicin-sensitive C-fiber bladder afferent neurons exhibit high threshold tetrodotoxin-resistant sodium channels, action potentials, and phasic firing (1–2 spikes) in response to prolonged depolarizing current pulses (Fig. 5). Approximately 90% of the bladder C-fiber afferent neurons also are excited by ATP, which induces a depolarization and firing by activating P2X3 or P2X2/3 receptors.52 A-fiber afferent neurons resistant to capsaicin exhibit low threshold tetrodotoxin-sensitive sodium channels, action potentials, and tonic firing (multiple spikes) to depolarizing current pulses (Fig. 5).
C-fiber bladder afferent neurons also express a slowly decaying A-type K+ current that controls spike threshold and firing frequency.87 Suppression of this K+ current induces hyperexcitability of bladder afferent neurons. These properties of DRG cells are consistent with the different properties of A-fiber and C-fiber afferent receptors in the bladder.
ROLE OF AFFERENT NEURONS IN THE NORMAL CONTROL OF THE LOWER URINARY TRACT
Mechanosensitive afferents in the bladder are activated during bladder filling and transmit information to the brain about the degree of bladder distension, and in turn, the amount of urine stored in the bladder.5 Studies in healthy volunteers have shown that the first sensation of filling occurs when about 40% of bladder capacity is reached, but this sensation is indistinct and easily disregarded. The first desire to void is reported at approximately 60% of capacity and has been defined by the International Continence Society (ICS) standardization committee as “the feeling during filling cystometry that would lead the patient to pass urine at the next convenient moment, but voiding can be delayed if necessary.” At more than 90% of capacity, people report a strong desire to void, which is defined by ICS as a “persistent desire to void without fear of leakage.” Based on studies in animals that examined the effects of C-fiber afferent neurotoxins on voiding, and studies in humans after transection of sympathetic or parasympathetic nerves, it appears that the normal sensations of bladder filling are dependent on Aδ afferents carried in the pelvic nerves to the sacral spinal cord.5 These afferents are also essential for the generation of storage and voiding reflexes. Studies in anesthetized and decerebrate cats and rats revealed that reflex activation of the bladder is mediated by a spinobulbospinal pathway passing through the pontine micturition center (PMC, Barrington’s nucleus) at the level of the inferior colliculus.88,89 The reflex pathway is activated by Aδ bladder afferents traveling in the pelvic nerve to the sacral spinal cord (Fig. 6).
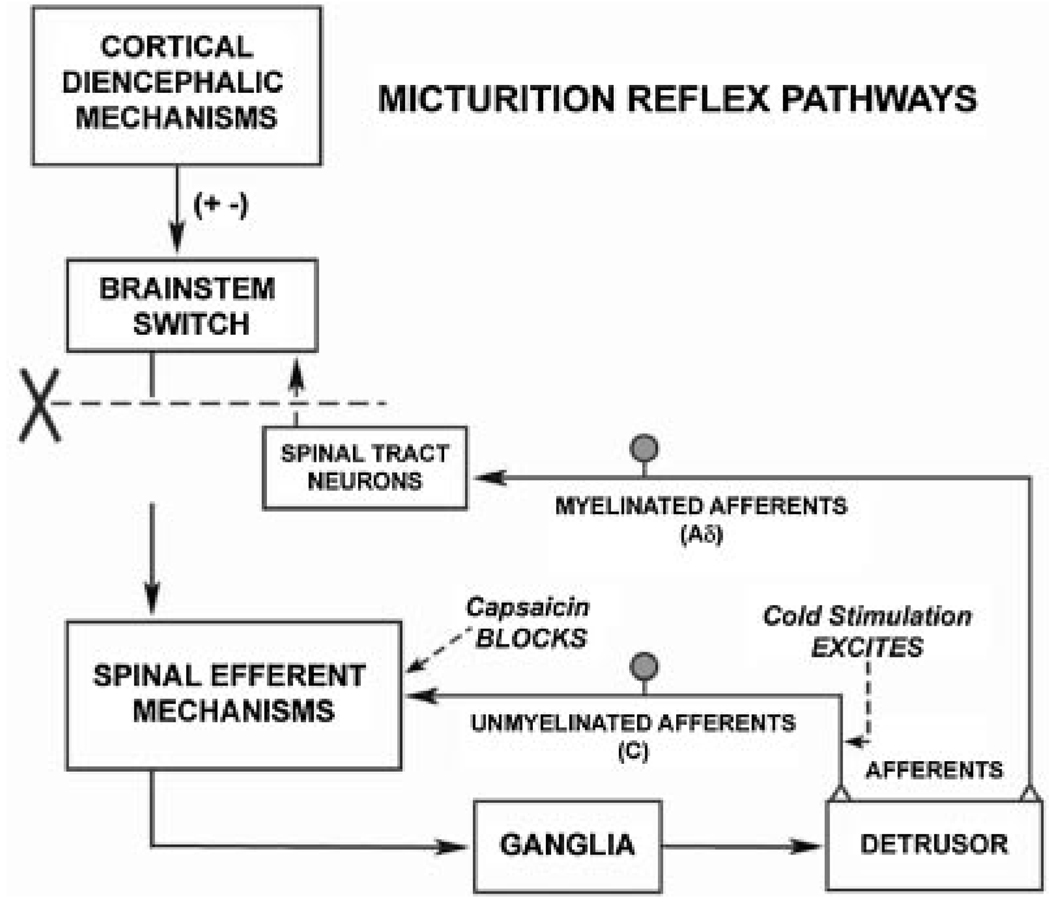
Fig. 6.
Diagram showing the organization of the parasympathetic excitatory reflex pathway to the detrusor muscle. Scheme is based on electrophysiologic studies in cats. In animals with an intact spinal cord, micturition is initiated by a supraspinal reflex pathway passing through a center in the brain stem. The pathway is triggered by myelinated afferents (Aδ-fibers), which are connected to the tension receptors in the bladder wall. Injury to the spinal cord above the sacral segments (X) interrupts the connections between the brain and spinal autonomic centers and initially blocks micturition. However, over a period of several weeks following spinal cord injury, a spinal reflex mechanism emerges, which is triggered by unmyelinated vesical afferents (C-fibers); the A-fiber afferent inputs are ineffective. The C-fiber reflex pathway is usually weak or undetectable in animals with an intact nervous system. Stimulation of the C-fiber bladder afferents by instillation of ice water into the bladder (cold stimulation) activates voiding responses in patients with spinal cord injury. Capsaicin (20–30 mg, subcutaneously) blocks the C-fiber reflex in chronic spinal cord injured cats, but does not block micturition reflexes in intact cats. Intravesical capsaicin also suppresses detrusor hyperreflexia and cold-evoked reflexes in patients with neurogenic bladder dysfunction.
EMERGENCE OF A C-FIBER AFFERENT-MEDIATED MICTURITION REFLEX AFTER SPINAL CORD INJURY
Spinal-cord injury (SCI) rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention, followed by a slow development of automatic micturition and bladder hyperactivity mediated by spinal reflex pathways.90 However, voiding is commonly inefficient due to simultaneous contractions of the bladder and urethral sphincter, that is, DSD.
Electrophysiologic studies in cats revealed that the recovery of bladder function after SCI is mediated by a change in the afferent limb of the micturition reflex pathway and remodeling of synaptic connections in the spinal cord.8,88,90–92 In chronic spinal-cord injured cats, unmyelinated C-fiber afferents rather than Aδ afferents initiate voiding (Fig. 6), and the spinal micturition reflex occurs with a short central delay (15 msec) in contrast to the long central delay (60 msec) of the reflex in cats with an intact spinal cord.88 These findings are supported by pharmacologic studies showing that subcutaneous administration of capsaicin, a C-fiber neurotoxin, completely blocks reflex bladder contractions induced by bladder distention in chronic spinal-cord injured cats (Fig. 6), whereas capsaicin has no inhibitory effect on reflex bladder contractions in spinal-cord intact cats.91,92 Thus it is plausible that C-fiber bladder afferents, which usually do not respond to bladder distention (i.e., silent C-fibers),73 become mechanosensitive and initiate automatic micturition after SCI.
In the rat, both Aδ and C-fiber afferents are involved in evoking bladder reflexes after SCI.90 Capsaicin treatment reduces nonvoiding contractions during cystometry but does not alter voiding contractions.90 Thus C-fiber afferents are necessary for generating neurogenic detrusor overactivity (NDO) during the storage phase, but Aδ afferents initiate voiding.
In humans with NDO, intravesical administration of C-fiber neurotoxins (capsaicin or resiniferatoxin, RTX) increases bladder capacity and decreases the frequency and number of episodes of urinary incontinence.5,6 In several randomized controlled trials in spinal cord-injured patients in which capsaicin was compared to RTX, both agents were effective in improving both urodynamic and clinical parameters.4,5
Chronic spinal injury in animals and humans also causes the emergence of an unusual bladder reflex that is elicited by infusion of cold water into the bladder (the Bors Ice Water Test).93,94 The response to cold water does not occur in normal adults but does occur in: (1) infants, (2) patients with suprasacral cord lesions, (3) patients with multiple sclerosis and Parkinson’s disease, and (4) elderly patients with hyperactive bladders. Studies in animals indicate that cold temperature activates TRPM8 and possibly other temperature-sensitive receptors in bladder C-fiber afferents (Fig. 6) and/or urothelial cells.93,95 Intravesical administration of capsaicin to spinal cord-injured patients blocks cold-induced bladder reflexes,96 indicating that they are mediated by C-fiber afferents, as has been demonstrated by the reflex activity of cat urinary bladders mediated by C-fiber afferents following cold stimulation.97,98 Cold stimulation of the rat bladder also induces DSD, and capsaicin pretreatment prevents this response.99 The presence of the cold reflex in infants, its disappearance with maturation of the nervous system, and its reemergence under conditions in which higher brain functions are disrupted suggest that it may reflect a primitive spinal involuntary voiding reflex activated by C-fiber afferents.
MORPHOLOGIC AND CHEMICAL PLASTICITY OF AFFERENT NEURONS INDUCED BY SPINAL CORD INJURY
Changes in Bladder Afferent Nerves
Studies in patients with NDO resulting from multiple sclerosis or various types of SCI have revealed increased TRPV1, P2X3, and pan-neuronal marker (PGP9.5) staining in suburothelial nerves and increased TRPV1 staining in the basal layer of the urothelium.100–102 Treatment of NDO patients with intravesical capsaicin or resiniferatoxin reduces the density of TRPV1, P2X3, and PGP9.5 immunoreactive nerve fibers and urothelial TRPV1 immunoreactivity in the subpopulation of these patients exhibiting symptomatic improvement.101–103 Injections into the bladder wall of botulinum neurotoxin type A (BoNT/A), an agent that blocks the release of neurotransmitters from urothelial cells and from afferent and efferent nerves, also reduces NDO104–106 and reduces the density of TRPV1- and P2X3-immunoreactive nerves but does not alter TRPV1- and P2X3-staining in the urothelium.100,103,104 These results suggest that an abnormality of the C-fiber afferent innervation contributes to NDO.
Changes in DRG Neurons and in Spinal Afferent Pathways
In the sacral spinal cord of the cat where VIP is expressed exclusively in C-fiber afferent neurons,107 the VIP-IR C-fiber afferent projections expand and reorganize after SCI (Fig. 4).42,108 This is evident as (1) a wider distribution of VIP-IR axons in the lateral lamina I of the dorsal horn (the LCP), forming an almost continuous band of axons in the rostrocaudal direction, in contrast to a discontinuous distribution in normal cats, (2) the appearance of rostrocaudal axons in this region where they are not normally present, and (3) a more extensive projection to lateral lamina VII, which contains bladder preganglionic neurons (area 4 in Fig. 4).108 These observations raise the possibility that C-fiber bladder afferents sprout and contribute to the synaptic remodeling in the spinal micturition reflex pathway that occurs after SCI. The pharmacologic effect of VIP on bladder activity is also changed after SCI. Intrathecal administration of VIP, which suppresses reflex bladder activity in cats with an intact spinal cord, enhances or unmasks reflex bladder activity in chronic SCI cats.92
Pudendal afferent projections in the region of the sacral dorsal horn and sacral autonomic nucleus are also increased in chronic SCI cats.108 Afferent axons labeled by transganglionic transport of horseradish peroxidase exhibited an expanded distribution in certain areas of the dorsal horn ipsilateral and contralateral to the labeled nerve. The change is most obvious in lateral lamina I where the afferents have a periodic distribution in the rostrocaudal axis in spinal intact animals (Fig. 4) but have a continuous distribution in chronic SCI animals. The width of this afferent projection also increases in the transverse axis and projects more densely into the region of bladder preganglionic neurons in lateral lamina VII.
Changes in morphology, neuropeptide expression, and function of C-fiber afferents have also been detected in the rat after SCI. The changes include: (1) somal hypertrophy of bladder afferent neurons (45–50% increase in cross-sectional area) in the L6-S1 DRG;35,83,109 (2) elimination of bladder afferent neuron hypertrophy by urinary diversion, which prevented bladder overdistension and bladder hypertrophy;109 (3) an increase in expression of PACAP-IR in bladder DRG neurons and expansion of PACAP-IR afferent axons in the lumbosacral spinal cord;39,110 (4) expansion of CGRP- and IB4-containing primary afferent fibers in the spinal cord prior to the recovery of reflex bladder activity;48,111,112 (5) association of CGRP and IB4 staining with GAP-43 staining in afferent fibers in SCI rats, indicating that afferents were sprouting;48,112 (6) neurotoxin damage with IB4-saporin treatment decreasing IB4 afferent staining in the spinal cord and improving voiding efficiency in SCI rats;48 (7) an increase in Fos protein expression in the spinal cord in response to bladder distension;31 and (8) an increase in nNOS-IR, galanin-IR, TrkA-IR, and TrkB-IR in bladder DRG neurons and in the region of the lumbosacral parasympathetic nucleus.39
Involvement of PACAP-Containing C-Fiber Afferents in Plasticity After SCI
Six weeks after SCI in the rat, PACAP-IR is markedly increased in spinal segments and DRG (L1, L2, L6, S1) involved in micturition reflexes, but no changes occur in adjacent spinal segments (L4-L5).110 PACAP-IR increases in the superficial laminae (I–II) of the relevant spinal segments and in a fiber bundle extending ventrally from Lissauer’s tract in lamina I along the lateral edge of the dorsal horn to the sacral parasympathetic nucleus (SPN) in the L6-S1 spinal segments. This is the same region in which VIP-IR increases in the cat after SCI.108 After SCI, the percentage of bladder afferent cells expressing PACAP-IR significantly increases in the lumbosacral DRG.
The pharmacologic effects of PACAP on voiding function raise the possibility that it may have a role as a neurotransmitter in bladder afferent pathways. Intrathecal administration of PACAP-27113 or PACAP-38114,115 in spinal-cord intact unanesthetized rats decreases bladder capacity, decreases micturition volume, and increases micturition pressure. PACAP-38114,115 also reduces EUS EMG activity. Thus in spinal-cord intact rats, PACAP-38 seems to facilitate micturition by enhancing the parasympathetic excitatory pathway to the bladder and inhibiting the somatic excitatory pathway to the EUS.
The effects of PACAP-38 in chronic SCI rats are somewhat different. During continuous infusion cystometrograms (CMGs) in SCI rats, intrathecal injection of PACAP-38 decreases the amplitude of bladder contractions and suppresses EUS EMG activity.115 These responses have been attributed to the combined effect of PACAP-38 on bladder and sphincter, where the excitatory effect of PACAP-38 on bladder activity is masked by a simultaneous inhibitory effect on the EUS that in turn blocks DSD and reduces urethral outlet resistance. This would indirectly lower intravesical pressure during voiding.
The physiologic role of PACAP in the control of bladder function in chronic SCI rats was examined by administering PACAP6–38, a PAC1 receptor antagonist, during continuous infusion CMGs in awake SCI rats.116 Intrathecal administration of the antagonist reduces premicturition contractions during bladder filling and reduces maximal voiding pressure, suggesting that activation of PAC1 receptors by endogenous PACAP contributes to the micturition reflex and bladder hyperreflexia.
The site and mechanism of action of PACAP on spinal micturition reflex pathways were explored using patch clamp recording in lumbosacral parasympathetic preganglionic neurons in neonatal rat spinal-cord slice preparations.117 The experiments revealed that PACAP-38 has direct excitatory effects on the preganglionic neurons and enhances excitatory input to the neurons, suggesting that it might act at several sites in the spinal micturition reflex pathway. In parasympathetic preganglionic neurons, PACAP-38 decreases the electrical threshold for triggering action potentials, increases the number of action potentials induced by depolarizing current pulses, increases input resistance, and suppresses a 4-aminopyridine-sensitive outward current. PACAP-38 also induces spontaneous firing and increases the frequency of spontaneous excitatory postsynaptic potentials in the presence of tetrodotoxin. Thus, PACAP-38 could act presynaptically to enhance the firing of excitatory interneurons, enhance glutamate release from interneuronal terminals, or act postsynaptically directly on parasympathetic neurons.
CHANGES IN THE ELECTROPHYSIOLOGIC PROPERTIES OF BLADDER AFFERENT NEURONS AFTER SPINAL CORD INJURY
Changes in Action Potentials
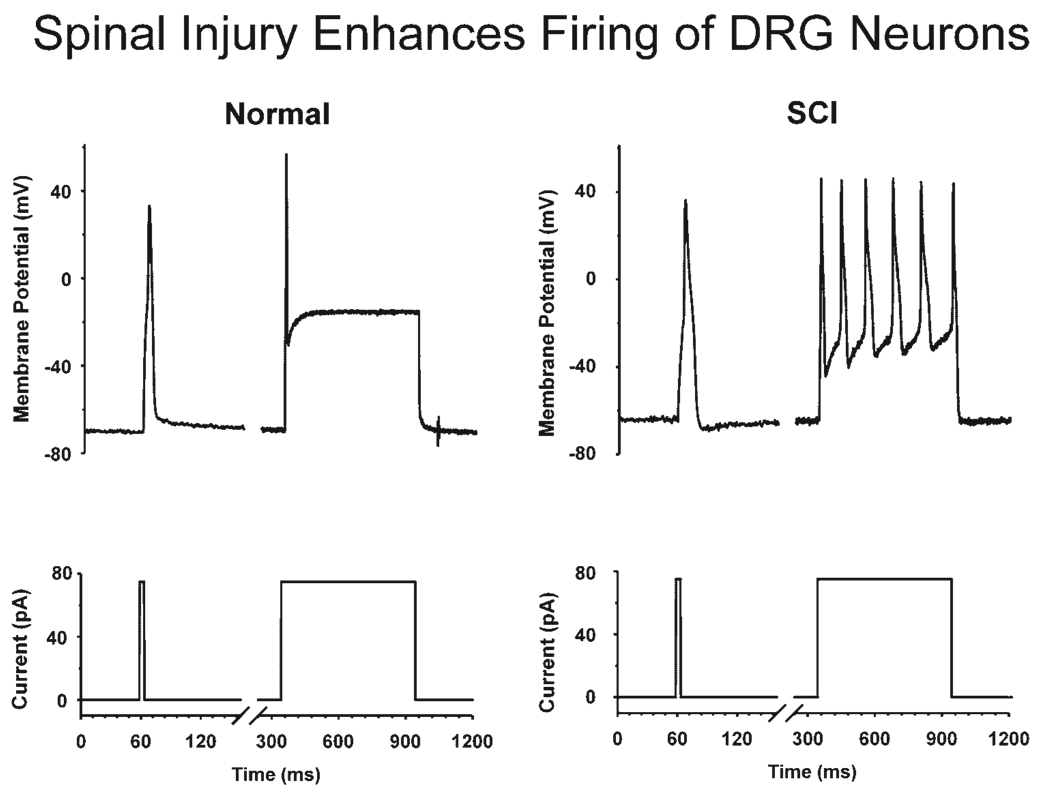
The ionic mechanisms underlying the hyperexcitability of C-fiber bladder afferents after SCI were investigated using whole-cell patch clamp recording in bladder DRG neurons.83,118 Dissociated bladder DRG neurons from chronic SCI in rats were larger in size and had increased input capacitance. This is consistent with results from histologic studies showing that bladder afferent neurons in the L6-S1 DRG undergo somal hypertrophy (45–50% increase in cross-sectional area) in SCI rats.109 The action potentials in bladder afferent neurons were also different after SCI in rats and cats.83,118 In contrast to neurons from spinal intact rats where the majority (approximately 70%) of bladder afferent neurons exhibit high-threshold TTX-resistant action potentials,86 60% of bladder afferent neurons from chronic SCI rats exhibit low-threshold TTX-sensitive action potentials. In SCI cats, bladder afferent neurons also exhibit multiple action potentials (tonic firing) in response to long depolarizing current pulses, whereas in cats with an intact spinal cord, the neurons usually respond with one or two action potentials (phasic firing) (Fig. 7).119
Fig. 7.
Effect of chronic spinal cord injury on the firing of a bladder sacral dorsal root ganglion neuron. Records on the left side, which are from an afferent neuron from a cat with an intact spinal cord, show action potentials (top trace) elicited by short (10 msec) and long (600 msec) duration depolarizing current pulses (bottom trace). This neuron is typical of small diameter bladder neurons which exhibit phasic firing. Records on the right side show tonic firing during a long duration depolarizing current pulse in a small diameter bladder neuron from a spinal cord injured (SCI) cat.
Plasticity in Sodium and Potassium Channels After Spinal Cord Injury
The alteration of electrophysiologic properties in bladder afferent neurons after SCI was also reflected in changes in Na+ current distribution.83 Consistent with the increment in the proportion of neurons with TTX-sensitive spikes, the number of bladder afferent neurons that predominantly express TTX-sensitive Na+ currents (60–100% of total Na+ currents) also increases. The density of TTX-sensitive Na+ currents in bladder afferent neurons significantly increased from 32.1 to 80.6 pA/pF, while TTX-resistant current density decreased from 60.5 to 17.9 pA/pF following SCI. In addition, an increase in TTX-sensitive Na+ currents was detected in some bladder afferent neurons that still retained a predominance of TTX-resistant currents (>50% of total Na+ currents) after SCI. These data indicate that SCI induces a switch in expression of Na+ channels from the TTX-resistant to the TTX-sensitive type. Since TTX-sensitive Na+ currents have a lower threshold for activation than TTX-resistant currents, it is reasonable to assume that these changes in expression of Na+ channels in bladder afferent neurons after SCI contribute to a low threshold for spike activation in these neurons.
Bladder afferent neurons with TTX-sensitive spikes in chronic SCI rats stimulated by low-intensity depolarizing current pulses do not exhibit membrane potential relaxation, which is mediated by an A-type K+ channel.90 Furthermore, the voltage responses induced by current injections are not altered, as in neurons from control animals, by application of 4-aminopyridine, an A-type K+ channel blocking agent.87 Therefore it is likely that following SCI, A-type K+ channels are suppressed in parallel with an increased expression of TTX-sensitive Na+ currents, thereby increasing excitability of C-fiber bladder afferent neurons. If the changes occurring in afferent cell bodies also occur at peripheral receptors in the bladder or in the spinal cord, these changes could contribute to the emergence of the C-fiber-mediated spinal micturition reflex following SCI.
ROLE OF NERVE GROWTH FACTOR IN AFFERENT PLASTICITY AFTER SPINAL CORD INJURY
Neurotrophic factors, including nerve growth factor (NGF), have been implicated as chemical mediators of pathology-induced changes in C-fiber afferent nerve excitability and reflex bladder activity.119–121 After SCI in rats, the levels of NGF increase in the bladder,39,120 in the lumbosacral spinal cord, and in the DRG.122 In the bladder, NGF could originate from multiple sites, including smooth muscle and urothelial cells. The stimulus for increased levels of NGF may be overdistension of the bladder due to DSD and decreased voiding efficiency (Fig. 8), because NGF levels also increase in the bladder of rats after partial obstruction of the urethral outlet.123,124
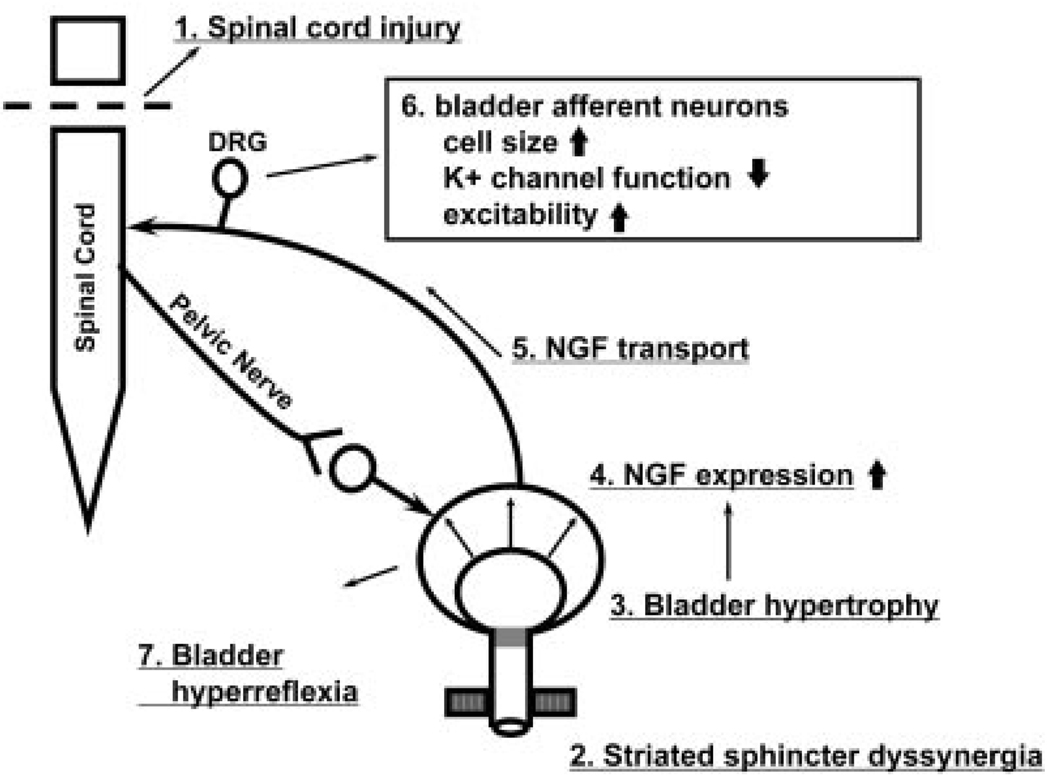
Fig. 8.
Diagram showing hypothetical mechanisms inducing lower urinary tract dysfunction following spinal cord injury (SCI). Injury to the spinal cord (1) causes detrusor-sphincter-dyssynergia (DSD) (2), which leads to functional urethral obstruction, reduced voiding efficiency, urinary retention, and bladder hypertrophy (3), resulting in increased levels of NGF in the bladder wall (4). NGF is taken up by afferent nerves and transported to the dorsal root ganglion cells (5) where it changes gene expression, which leads to increased cell size; regulation of ion channels, including a decrease in K+ channel function; and increased neuronal excitability (6). The levels of NGF also increase in the spinal cord after SCI. Hyperexcitability of bladder afferent pathways causes or enhances neurogenic detrusor overactivity and DSD. Intrathecal application of NGF antibodies reduces NGF levels in DRGs and suppresses neurogenic detrusor overactivity and DSD.
Intravesical administration of NGF in rats acutely increases bladder reflex activity.59 Chronic administration of NGF into the spinal cord122,125 or the bladder wall of rats126,127 induces bladder hyperactivity, increases the firing frequency of dissociated bladder afferent neurons (Fig. 9), and enhances Fos expression and CGRP-IR in the spinal cord.121,122,125–127 NGF might act by multiple mechanisms, because it is known that it upregulates PACAP and TRPV1 expression in DRG neurons124,128,129 and downregulates A-type K+ channel currents.125
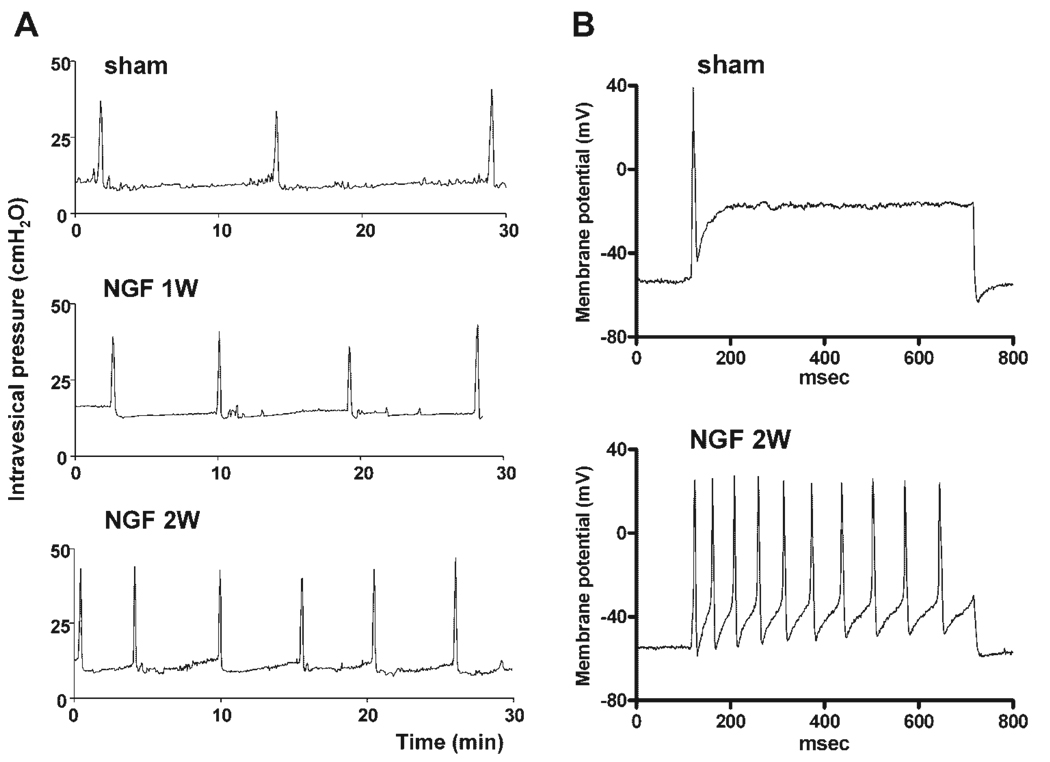
Fig. 9.
The effect of nerve growth factor (NGF) treatment on voiding function (A) and bladder afferent neuron firing pattern (B). NGF (2.5 µg/µl) was continuously infused into the intrathecal space at the L6-S1 level of the spinal cord for 1–2 weeks using osmotic minipumps (0.5 µl/hr). A: Cystometrograms showing continuous infusion cystometry in awake vehicle-treated (sham) and NGF-treated animals one week (1 w) and two weeks (2 w) after the start of treatment. Note the increased frequency of voiding after infusion of NGF. B: Firing patterns in response to 600 msec duration depolarizing current pulses in bladder afferent neurons, with TTX-resistant spikes from sham- and NGF-treated (2 w) rats. The current intensity was set to the threshold value for inducing single spikes with 10 msec current pulses. Note NGF-induced tonic firing, whereas the sham animal exhibited phasic firing as noted for C-fiber neurons from normal untreated animals (see Fig. 5).
Endogenous NGF seems to contribute to LUT dysfunction after SCI because intrathecal application of NGF antibodies, which neutralize NGF in the spinal cord, suppresses detrusor hyperreflexia and DSD in SCI rats.130,131 This treatment with NGF antibodies produces effects similar to the effect of desensitizing C-fiber afferents with capsaicin or resiniferatoxin.90,132 Intrathecal administration of NGF antibodies also blocks autonomic dysreflexia induced by bladder or distal bowel distension in SCI rats.111
In humans with NDO or IDO, increased levels of NGF have been detected in bladder tissue133 and in urine.134 After treatment with BoNT/A injections into the bladder wall, patients that exhibited reduced symptoms also had reduced NGF levels.133,134 Thus it has been proposed 134 that NGF may be a sensitive biomarker for the diagnosis of NDO and IDO and may be a useful tool for evaluating the therapeutic effect of BoNT/A injections. In addition, NGF and its receptors in the bladder and/or the spinal cord may be important targets for new therapies to reduce voiding dysfunction after SCI.
BLADDER AFFERENT NERVES AND URETHRAL OUTLET OBSTRUCTION
The probable contribution of DSD and bladder overdistension to afferent nerve plasticity after SCI raises the possibility that a similar plasticity of bladder afferent fibers may be involved in the irritative symptoms accompanying bladder outlet obstruction (BOO) resulting from benign prostatic hyperplasia (BPH). Evidence obtained from ice water cystometry, which elicits a C-fiber–dependent spinal micturition reflex, suggests considerable C-fiber upregulation in symptomatic subjects with BOO.135,136 Among BOO patients with a positive ice-water test, the incidence of detrusor overactivity (DO) is significantly greater in those who report nocturia equal to or greater than three times per night than in those who reported fewer episodes.136
Histologic and electrophysiologic confirmation of neural plasticity in bladder afferent and bladder reflex pathways was obtained using a rat model of partial BOO. A significant increase in the size of bladder afferent23 and postganglionic efferent137 innervation in the enlarged bladder was documented in animals following 6 weeks of partial urethral obstruction. Remodeling of the spinal cord components of the micturition reflex pathway was also evident following experimental BOO. Using axonal labeling to identify afferent axonal projections to the spinal cord, it was demonstrated that bladder afferent terminals expand to cover a larger area (60% increase) in the lateral dorsal horn and in the region of the sacral parasympathetic nucleus in rats with BOO.23,119,124 Electrophysiologic experiments revealed that a spinal micturition reflex mechanism is unmasked in rats with BOO.138 Immunohistochemical analysis of the distribution and density of growth-associated protein-43 (GAP-43) shows that this protein is increased in the spinal cord in the region of the sacral parasympathetic nucleus in BOO rats.124,139 Because this protein is a marker for axonal sprouting, its upregulation provides further indirect support for morphologic plasticity in afferent pathways after BOO.
Subsequent experiments using the same BOO rat model revealed that hypertrophied bladder tissue contained significantly greater amounts of NGF protein than normal bladders.123 To determine if the changes induced by BOO were due to an action of NGF, BOO was carried out in NGF-immune animals in which endogenous NGF antibody prevents access of NGF to nerves. BOO in the NGF-immune animals does not elicit hypertrophy of bladder sensory neurons, increase in afferent projections in the spinal cord, or increase in GAP-43 expression in afferent pathways.139 Removal of the urethral obstruction in BOO rats causes a partial reversal of both the elevated NGF levels in the bladder and the neuronal hypertrophy; however, bladder overactivity persists in the presence of the elevated NGF levels.
The stimulus for NGF production in the bladder is due in part to urinary retention and stretching of the bladder after BOO (Fig. 8). Stretching bladder smooth muscle cells in vitro increases mRNA for NGF and stimulates the secretion of NGF.124 Protein synthesis inhibitors suppress the stretch-evoked secretion. NGF levels also increase in the urothelium of BOO rats. These results indicate that mechanical stretch activates cellular machinery for the production and secretion of NGF, which in turn acts on sensory nerves in the bladder to enhance afferent input to the spinal cord and enhance reflex bladder activity.
Patch-clamp recordings from bladder sensory neurons in BOO rats have explored the mechanisms underlying the changes in afferent neuron excitability. The neurons exhibit increased amplitude and altered kinetics of TTX-sensitive Na+ currents that result in lowered firing thresholds.124 An experimental drug that preferentially blocks TTX-sensitive currents reduces bladder overactivity in BOO rats.
Human bladder tissue obtained from subjects undergoing suprapubic prostatectomy for outlet obstruction had more than twice the level of NGF than tissue obtained by cystoscopy from patients who were being evaluated for conditions other than obstruction.124 Increased levels of urinary NGF have also been detected in BOO patients exhibiting overactive bladder (OAB) symptoms.140 Total urinary NGF levels were low in controls and in patients with BOO without OAB symptoms, but considerably higher in patients with BOO and OAB symptoms or BOO and DO.140 Following successful medical treatment with a combination of an alpha adrenergic blocking agent and a 5-alpha reductase inhibitor that reduced symptoms, the urinary NGF levels were reduced to normal levels. It was concluded that urinary NGF levels can be used as a biomarker for OAB and BOO and as a method for assessing successful therapies.140
GENE THERAPY TO INCREASE GABAERGIC INHIBITION IN BLADDER AFFERENT PATHWAYS
GABA, which is synthesized from glutamate by glutamic acid decarboxylase (GAD), is known to have an important role in the inhibitory regulation of micturition in spinal intact rats. In SCI rats, both muscimol and baclofen (GABAA- and GABAB-receptor agonists, respectively) produce a dose-dependent inhibition of the C-fiber-mediated nonvoiding bladder contractions and a decrease in micturition pressure.141 The effects of muscimol and baclofen are antagonized by bicuculline and saclofen, GABAA and GABAB antagonists, respectively. Decreased activation of GABA inhibitory receptors due to hypofunction of GABAergic mechanisms in the spinal cord could contribute to the development of NDO because GAD67 mRNA levels are decreased by 55% in the spinal cord of SCI rats 4 weeks after spinal cord transection.141 Therefore, stimulation of spinal GABAergic mechanisms could be effective for the treatment of NDO after SCI. Baclofen, the GABAB-receptor agonist, is reportedly effective for treatment of DO in SCI patients,142 but this agent has not been widely used, because its therapeutic window is modest, and the dose is limited by side effects.
Viral-mediated gene delivery targeting bladder afferent nerves has been evaluated in an attempt to develop a more selective approach for increasing GABA inhibitory functions in the lumbosacral spinal cord with fewer side effects (Fig. 10).143,144 The study used herpes simplex virus (HSV), which is taken up by peripheral nerve terminals of small diameter sensory nerves and transported to the cell bodies in sensory ganglia. Three weeks after a replication-defective HSV vector encoding the GAD gene (HSV-GAD) was injected into the bladder of SCI rats, the number and amplitude of nonvoiding bladder contractions were significantly decreased compared with the nonvoiding contractions in untreated or HSV-LacZ (control vector)-treated SCI rats. These effects occurred without a significant change in the amplitude of bladder contractions during voiding.143,144 Similar changes were observed in chronic SCI rats after C-fiber afferent desensitization induced by systemic capsaicin administration.132 Thus GAD gene delivery using HSV vectors appears to inhibit NDO by suppressing C-fiber bladder afferents without affecting the voiding contractions triggered by Aδ bladder afferents. On the other hand, intrathecal administration of GABA receptor agonists in SCI rats decreases nonvoiding contractions as well as voiding contractions.141 Therefore, GABA gene therapy using nonreplicating HSV vectors that can restore urine storage function without affecting voiding function might be more beneficial than drug therapy for the treatment of urinary problems in SCI patients.
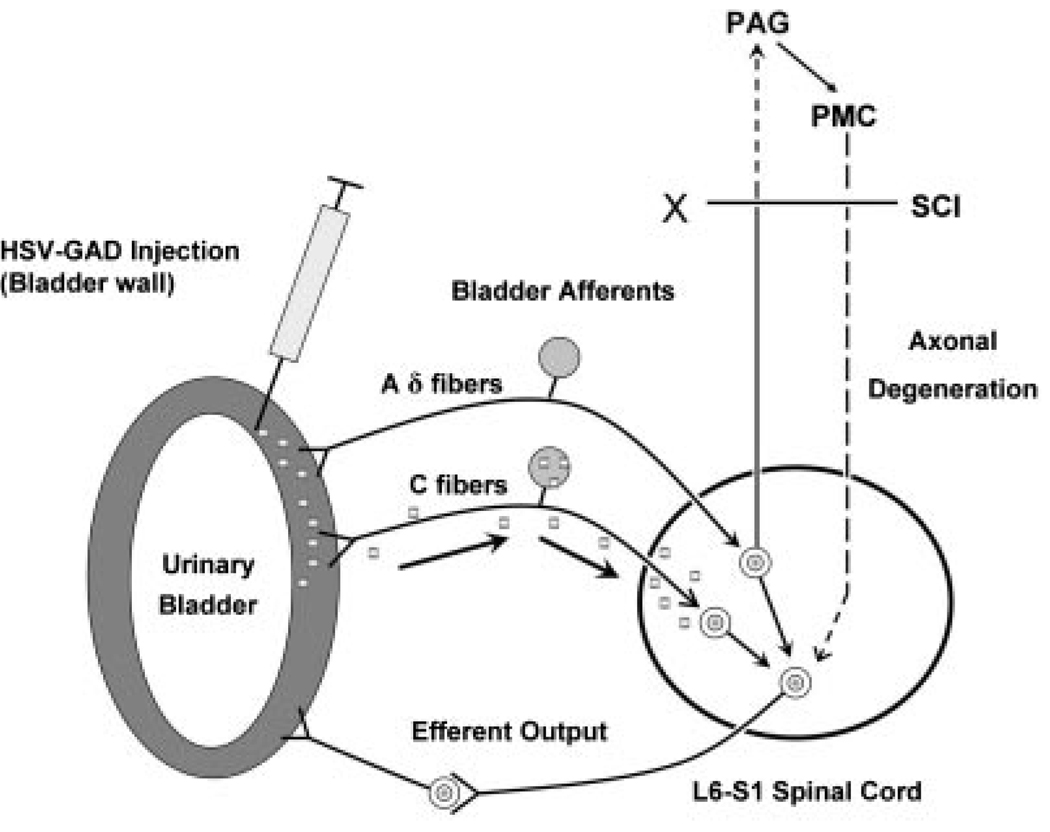
Fig. 10.
Experimental gene therapy using a herpes simplex viral vector to increase the expression of glutamic acid decarboxylase (GAD) in the afferent pathways to the bladder. GAD is an enzyme involved in the synthesis of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the spinal cord. Following injection of HSV-GAD into the bladder wall in SCI rats, the HSV-GAD is transported to the L6-S1 bladder afferents in the dorsal root ganglia and increases GABA synthesis and release from afferent terminals in the spinal cord. Three weeks after the injection, cystometry in awake rats revealed decreased number and amplitude of non-voiding contractions compared to the measurements in SCI rats receiving control vector (HSV-LacZ). HSV-GAD treatment did not alter the amplitude of voiding contractions. Since the effect of HSV-GAD is similar to the effect of capsaicin pretreatment, which targets C-fiber afferent pathways, it is possible that HSV-GAD selectively suppresses reflex responses induced by capsaicin-sensitive C-fiber afferents and does not affect Aδ-fiber-evoked reflexes which elicit voiding reflexes in spinal cord intact and chronic SCI rats.
CONCLUSIONS
Studies in animals and humans indicate that the emergence of reflex bladder activity and development of NDO after complete spinal cord injury rostral to the lumbosacral segments is due in part to plasticity in bladder C-fiber afferent nerves.5 Because spinal lesions do not directly damage the C-fiber afferent neurons, plasticity must be induced indirectly by pathologic changes in the peripheral or central nervous system. Thus multiple mechanisms might be involved in afferent neuron plasticity. For example disruption of descending pathways to the lumbosacral spinal cord results in denervation of segmental spinal neurons that could in turn lead to local release of neurotrophic factors that induce changes in the properties of the afferent neurons, afferent nerve sprouting, and remodeling of spinal synaptic connections. NGF may play a role in plasticity because NGF levels increase in the spinal cord of SCI rats.122 A peripheral mechanism of afferent neuron plasticity is very likely related to changes in the function of the LUT after SCI. DSD after SCI leads to decreased voiding efficiency, bladder overdistension, and hypertrophy, as well as to increased levels of NGF in the bladder and the urine.39,133,134 Administration of exogenous NGF to the bladder or the spinal cord can induce DO, and also increase bladder afferent neuron excitability, by altering the expression of neuronal ion channels.125 Because intrathecal administration of antibodies to NGF reduces NDO and DSD in SCI rats,130,131 it seems reasonable to conclude that endogenous NGF is an important factor in the development of LUT dysfunction after SCI. Thus therapies to target NGF overexpression, receptors, or signaling mechanisms may be effective in reducing bladder symptoms in individuals with SCI.
Acknowledgments
Grant sponsor: NIH; Grant numbers: DK49430, DK 57267, P01 HD 39768.
Footnotes
Conflicts of interest: none.
REFERENCES
- 1.Barrington FJF. The effect of lesion of the hind-and mid brain on micturition in the cat. Q J Exp Physiol. 1925;15:81–102. [Google Scholar]
- 2.Chancellor MB, Yoshimura N. Physiology and pharmacology of the bladder and urethra. In: Wein AJ, editor. Campbell-walsh urology. 9th edition. Philadelphia, PA, USA: B. Saunders Elserion; 2006. pp. 1922–1972. Chapter 56. [Google Scholar]
- 3.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. Nervous control of the urogenital system. London, UK: Harwood Academic Publishers; 1993. pp. 227–290. [Google Scholar]
- 4.Everaerts W, Gevaert T, Nilius B, et al. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 5.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison J, Birder L, Craggs M, et al. Neural control. In: Abrams P, Wein A, editors. Incontinence. Plymouth, UK: Health Publications; 2005. pp. 363–422. [Google Scholar]
- 7.Torrens M, Morrison JFB. The physiology of the lower urinary tract. Berlin, Germany: Springer-Verlag; 1987. [Google Scholar]
- 8.Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- 10.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 11.Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- 12.Uemura E, Fletcher T, Dirks V, et al. Distribution of sacral afferent axons in cat urinary bladder. Am J Anat. 1973;136:305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- 13.Birder L, de Groat W, Apodaca G. Physiology of the urothelium. In: Corcos J, Schick E, editors. Textbook of the neurogenic bladder. London, UK: Informa Healthcare; 2008. pp. 19–39. [Google Scholar]
- 14.Uemura E, Fletcher TF, Bradley WE. Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anat Rec. 1975;183:579–587. doi: 10.1002/ar.1091830408. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie JI, Markerink-van Ittersum M, de Vente J. Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: Evidence for intramural neural circuits. Cell Tissue Res. 2006;325:33–45. doi: 10.1007/s00441-006-0166-8. [DOI] [PubMed] [Google Scholar]
- 16.Chai TC, Steers WD, Broder SR, et al. Characterization of laterality of innervation of the rat bladder. Scand J Urol Nephrol Suppl. 1996;179:87–92. [PubMed] [Google Scholar]
- 17.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 18.Christianson JA, Liang R, Ustinova EE, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 20.Malykhina AP, Qin C, Greenwood-van Meerveld B, et al. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: Mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 21.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Jancsó G, Maggi CA. Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res. 1987;418:371–376. doi: 10.1016/0006-8993(87)90106-5. [DOI] [PubMed] [Google Scholar]
- 23.Steers WD, Ciambotti J, Etzel B, et al. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991;310:401–410. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- 24.Morgan C, deGroat WC, Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986;243:23–40. doi: 10.1002/cne.902430104. [DOI] [PubMed] [Google Scholar]
- 25.Appelbaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cells that innervate the bladder. J Comp Neurol. 1980;192:203–209. doi: 10.1002/cne.901920202. [DOI] [PubMed] [Google Scholar]
- 26.Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 27.Thor KB, Morgan C, Nadelhaft I, et al. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 28.Ueyama T, Mizuno N, Nomura S, et al. Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol. 1984;222:38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]
- 29.Birder LA, de Groat WC. Induction of c-fos gene expression of spinal neurons in the rat by nociceptive and non-nociceptive stimulation of the lower urinary tract. Am J Physiol Regul Integr Comp Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 30.Birder LA, Roppolo JR, Erickson VL, et al. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999;834:55–65. doi: 10.1016/s0006-8993(99)01546-2. [DOI] [PubMed] [Google Scholar]
- 31.Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- 32.Hulsebosch CE, Coggeshall RE. An analysis of the axon populations in the nerves to the pelvic viscera in the rat. J Comp Neurol. 1982;211:1–10. doi: 10.1002/cne.902110102. [DOI] [PubMed] [Google Scholar]
- 33.Uvelius B, Gabella G. The distribution of intramural nerves in urinary bladder after partial denervation in the female rat. Urol Res. 1998;26:291–297. doi: 10.1007/s002400050060. [DOI] [PubMed] [Google Scholar]
- 34.Lawson SN, Perry MJ, Prabhakar E, et al. Primary sensory neurones: Neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura N, Erdman SL, Snider MW, et al. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- 36.de Groat WC. Neuropeptides in pelvic afferent pathways. In: Polak JM, editor. Regulatory Peptides Basel. Switzerland: Birkhauser Verlag AG; 1989. pp. 334–361. [Google Scholar]
- 37.Maggi CA. The dual, sensory and efferent function of the capsaicin-sensitive primary sensory nerves in the bladder and urethra. In: Maggi CA, editor. Nervous control of the urogenital system. London, UK: Harwood Academic Publishers; 1993. pp. 383–422. [Google Scholar]
- 38.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 39.Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- 40.Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424:577–587. [PubMed] [Google Scholar]
- 42.Kawatani M, Erdman SL, de Groat WC. Vasoactive intestinal polypeptide and substance P in primary afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1985;241:327–347. doi: 10.1002/cne.902410307. [DOI] [PubMed] [Google Scholar]
- 43.Kawatani M, Nagel J, de Groat WC. Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. J Comp Neurol. 1986;249:117–132. doi: 10.1002/cne.902490109. [DOI] [PubMed] [Google Scholar]
- 44.Kawatani M, Suzuki T, de Groat WC. Corticotropin releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Syst. 1996;61:218–226. doi: 10.1016/s0165-1838(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 45.Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 46.Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klinger MB, Vizzard MA. The role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–F1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zinck ND, Downie JW. IB4 afferent sprouting contributes to bladder dysfunction in spinal rats. Exp Neurol. 2008;213:293–302. doi: 10.1016/j.expneurol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Bennett DL, Dmietrieva N, Priestley JV, et al. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 50.Streng T, Axelsson HE, Hedlund P, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844:174–187. doi: 10.1016/s0006-8993(99)01936-8. [DOI] [PubMed] [Google Scholar]
- 52.Zhong Y, Banning AS, Cockayne DA, et al. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura N, Seki S, Erickson KA, et al. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Averill S, McMahon SB, Clary DO, et al. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrade EL, Ferreira J, Andre E, et al. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006;72:104–114. doi: 10.1016/j.bcp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Avelino A, Cruz C, Nagy I, et al. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 57.Birder LA, Kanai AJ, de Groat WC, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 59.Chuang YC, Fraser MO, Yu Y, et al. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- 60.Du S, Araki I, Yoshiyama M, et al. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007;70:826–831. doi: 10.1016/j.urology.2007.06.1110. [DOI] [PubMed] [Google Scholar]
- 61.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- 62.Nishiguchi J, Hayashi Y, Chancellor MB, et al. Detrusor overactivity induced by intravesical application of adenosine 5’-triphosphate under different delivery conditions in rats. Urology. 2005;66:1332–1337. doi: 10.1016/j.urology.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa T, Kamo I, Pflug BR, et al. Differential roles of peripheral and spinal endothelin receptors in the micturition reflex in rats. J Urol. 2004;172:1533–1537. doi: 10.1097/01.ju.0000139540.56916.0e. [DOI] [PubMed] [Google Scholar]
- 64.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- 65.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–550. [PubMed] [Google Scholar]
- 66.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studeny S, Torabi A, Vizzard MA. P2×2 and P2×3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1155–R1168. doi: 10.1152/ajpregu.00234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beckel JM, Kanai A, Lee SJ, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kullmann FA, Artim DE, Birder LA, et al. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masuda H, Kim JH, Kihara K, et al. Inhibitory roles of peripheral nitrergic mechanisms in capsaicin-induced detrusor overactivity in the rat. BJU Int. 2007;100:912–918. doi: 10.1111/j.1464-410X.2007.07099.x. [DOI] [PubMed] [Google Scholar]
- 71.Ozawa H, Chancellor MB, Jung SY, et al. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 72.Birder LA, de Groat WC. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bahns E, Ernsberger U, Janig W, et al. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- 75.Morrison J, Wen J, Kibble A. Activation of pelvic afferent nerves from the rat bladder during filling. Scand J Urol Nephrol Suppl. 1999;201:73–75. [PubMed] [Google Scholar]
- 76.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 77.Shea VK, Cai R, Crepps B, et al. Sensory fibers of the pelvic nerve innervating the rat’s urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 78.Chuang YC, Fraser MO, Yu Y, et al. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin and capsaicin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1302–R1310. doi: 10.1152/ajpregu.2001.281.4.R1302. [DOI] [PubMed] [Google Scholar]
- 79.Maggi CA, Giuliani S, Conte B, et al. Prostanoids modulate reflex micturition by acting though capsaicin-sensitive afferents. Eur J Pharmacol. 1988;145:105–112. doi: 10.1016/0014-2999(88)90221-x. [DOI] [PubMed] [Google Scholar]
- 80.Yu Y, Fraser MO, de Groat WC. Effects of ZD6169, a KATP channel opener, on neurally-mediated plasma extravasation in the rat urinary bladder induced by chemical or electrical stimulation of nerves. Brain Res. 2004;996:41–46. doi: 10.1016/j.brainres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- 82.Black JA, Cummins TR, Yoshimura N, et al. Tetrodotoxin-resistant sodium channels Nav1.8/SNS and Nav1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res. 2003;963:132–138. doi: 10.1016/s0006-8993(02)03957-4. [DOI] [PubMed] [Google Scholar]
- 83.Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurons innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshimura N, Seki S, Novakovic SD, et al. The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci. 2001;21:8690–8696. doi: 10.1523/JNEUROSCI.21-21-08690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshimura N, White G, Weight FF, et al. Patch-clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques. J Auton Nerv Syst. 1994;49:85–92. doi: 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 87.Yoshimura N, White G, Weight FF, et al. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurons innervating the rat urinary bladder. J Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Groat WC, Nadelhaft I, Milne RJ, et al. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 89.de Groat WC, Roppolo JR, Yoshimura N, et al. Neural control of the urinary bladder and colon. In: Taché Y, Wingate D, Burks T, editors. Proceedings of the second international symposium on brain-gut interaction. Boca Raton, FL: CRC Press; 1993. pp. 167–190. [Google Scholar]
- 90.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 91.Cheng CL, Liu JC, Chang SY, et al. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol Regul Integr Comp Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- 92.de Groat WC, Kawatani M, Hisamitsu T, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 93.Fall M, Lindström S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geirsson G, Lindstrom S, Fall M, et al. Positive bladder cooling test in neurologically normal young children. J Urol. 1994;151:446–448. doi: 10.1016/s0022-5347(17)34984-4. [DOI] [PubMed] [Google Scholar]
- 95.Stein RJ, Santos S, Nagatomi J, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004;172:1175–1178. doi: 10.1097/01.ju.0000134880.55119.cf. [DOI] [PubMed] [Google Scholar]
- 96.Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyper reflexia. J Urol. 1995;154:1825–1829. [PubMed] [Google Scholar]
- 97.Jiang CH, Mazieres L, Lindstrom S. Cold- and menthol-sensitive C afferents of cat urinary bladder. J Physiol. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazieres L, Jiang C, Lindstrom S. The C fibre reflex of the cat urinary bladder. J Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng CL, Chai CY, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am J Physiol Regul Integ Comp Physiol. 1997;41:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- 100.Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2×3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 101.Brady CM, Apostolidis A, Yiangou Y, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 102.Brady CM, Apostolidis AN, Harper M, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- 103.Apostolidis A, Brady CM, Yiangou Y, et al. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Apostolidis A, Fowler CJ. The use of botulinum neurotoxin type A (BoNTA) in urology. J Neural Transm. 2008;115:593–605. doi: 10.1007/s00702-007-0862-x. [DOI] [PubMed] [Google Scholar]
- 105.Popat R, Apostolidis A, Kalsi V, et al. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol. 2005;174:984–989. doi: 10.1097/01.ju.0000169480.43557.31. [DOI] [PubMed] [Google Scholar]
- 106.Schurch B, Denys P, Kozma CM, et al. Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur Urol. 2007;52:850–858. doi: 10.1016/j.eururo.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 107.Morgan CW, Ohara PT, Scott DE. Vasoactive intestinal polypeptide in sacral primary sensory pathways in the cat. J Comp Neurol. 1999;407:381–394. doi: 10.1002/(sici)1096-9861(19990510)407:3<381::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 108.Thor K, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger ME, Gorio A, Murray M, editors. Development and plasticity of the mammalian spinal cord. Padova, Italy: Liviana Press; 1986. pp. 65–80. [Google Scholar]
- 109.Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 110.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 111.Weaver LC, Marsh DR, Gris D, et al. Autonomic dysreflexia after spinal cord injury: Central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–263. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- 112.Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 113.Ishizuka O, Alm P, Larsson B, et al. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 114.Yoshiyama M, de Groat W. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J Mol Neurosci. 2008;36:227–240. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide on lower urinary tract functions in rats with intact or transected spinal cords. Exp Neurol. 2008;211:449–455. doi: 10.1016/j.expneurol.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zvara P, Braas KM, May V, et al. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI) Ann NY Acad Sci. 2006;1070:622–628. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- 117.Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2001;895:223–232. doi: 10.1016/s0006-8993(01)02112-6. [DOI] [PubMed] [Google Scholar]
- 118.Sculptoreanu A, Birder L, Buffington A, et al. Different mechanisms contribute to sensitization of C-fiber primary afferent neurons from cats with feline interstitial cystitis and spinal cord injury. J Urol. 2003;169:40. [Google Scholar]
- 119.Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4:S7–S18. [PMC free article] [PubMed] [Google Scholar]
- 120.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 121.Yoshimura N. Bladder afferent pathway and spinal cord injury: Possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 122.Satoshi S, Sasaki K, Igawa Y, et al. Detrusor overactivity induced by increased levels of nerve growth factor in bladder afferent pathways in rats. Neurourol Urodyn. 2003;22:375–377. [Google Scholar]
- 123.Steers WD, Kolbeck S, Creedon D, et al. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991;88:1709–1715. doi: 10.1172/JCI115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steers WD, Tuttle JB. Mechanisms of disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3:101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- 125.Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 127.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jongsma WH, Danielsen N, Johnston JM, et al. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–282. doi: 10.1046/j.0953-816x.2001.01641.x. [DOI] [PubMed] [Google Scholar]
- 129.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–348. [PubMed] [Google Scholar]
- 130.Seki S, Sasaki K, Fraser MO, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 131.Seki S, Sasaki K, Igawa Y, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 132.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 133.Giannantoni A, di Stasi SM, Nardicchi V, et al. Botulinum A toxin injection into the detrusor decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol. 2006;175:2341–2342. doi: 10.1016/S0022-5347(06)00258-8. [DOI] [PubMed] [Google Scholar]
- 134.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol. 2008;56:700–707. doi: 10.1016/j.eururo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 135.Chai TC, Gray ML, Steers WD. The incidence of a positive ice water test in bladder outlet obstructed patients: Evidence for bladder neural plasticity. J Urol. 1998;160:34–38. [PubMed] [Google Scholar]
- 136.Hirayama A, Fujimoto K, Matsumoto Y, et al. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62:909–913. doi: 10.1016/s0090-4295(03)00588-0. [DOI] [PubMed] [Google Scholar]
- 137.Steers WD, Ciambotti J, Erdman S, et al. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci. 1990;10:1943–1951. doi: 10.1523/JNEUROSCI.10-06-01943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988;140:864–871. doi: 10.1016/s0022-5347(17)41846-5. [DOI] [PubMed] [Google Scholar]
- 139.Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- 140.Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008;72:104–108. doi: 10.1016/j.urology.2008.01.069. [DOI] [PubMed] [Google Scholar]
- 141.Miyazato M, Sasatomi K, Hiragata S, et al. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steers WD, Meythaler JM, Haworth C, et al. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;148:1849–1855. doi: 10.1016/s0022-5347(17)37048-9. [DOI] [PubMed] [Google Scholar]
- 143.Miyazato A, Sugaya K, Ogawa Y, et al. Herpes simplex virus vector-mediated delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:349. doi: 10.1038/gt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miyazato M, Sugaya K, Chancellor MB, et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector-mediated glutamic acid decarboxylase gene delivery in spinal cord injures rats. Neurourol Urodyn. 2008;27:603–604. [Google Scholar]