Abstract
Effects of purinergic agonists (α,β-meATP and ATP) and cyclophosphamide-induced cystitis on bladder afferent nerve (BAN) activity were studied in an in vitro bladder-pelvic nerve preparation. Distension of the bladder induced spontaneous bladder contractions that were accompanied by multiunit afferent firing. Intravesical administration of 40 and 130 µM α,β-meATP increased afferent firing from 27 ± 3 to 53 ± 6 and 61 ± 2 spikes/s, respectively, but did not change the maximum amplitude of spontaneous bladder contractions. Electrical stimulation on the surface of the bladder elicited action potentials (AP) in BAN. α,β-meATP decreased the voltage threshold from 9.0 ± 1.2 to 3.5 ± 0.5 V (0.15-ms pulse duration) and increased the area of the APs (82% at 80-V stimulus intensity). These effects were blocked by TNP-ATP (30 µM). ATP (2 mM) applied in the bath produced similar changes in BAN activity. These effects were blocked by bath application of PPADS (30 µM). Neither TNP-ATP nor PPADS affected BAN activity induced by distension of the bladder. Cystitis induced by pretreatment of the rats with cyclophosphamide (100 mg/kg ip) increased afferent firing in response to isotonic bladder distension (10–40 cmH2O), decreased the threshold, and increased the area of evoked APs. The increase in afferent firing at 10 cmH2O intravesical pressure was reduced 52% by PPADS. These results indicate that purinergic agonists acting on P2X receptors and cystitis induced by cyclophosphamide can increase excitability of the BANs.
Keywords: evoked action potentials, bladder mechanosensitive and chemosensitive afferent nerves, purinergic receptors
Afferent activity arising in the bladder and urethra is conveyed to the central nervous system by three sets of nerves (15). The most important afferents for initiating the micturition reflex are those traveling in the pelvic nerve to the sacral spinal cord (16, 17). The afferents consist of small myelinated (Aδ) and unmyelinated (C) axons and convey impulses from tension and volume receptors as well as nociceptors in the bladder wall (15–17, 32). The afferent nerves are located in the smooth muscle, as well as the serosal and urothelial layers of the bladder (21, 22). Electrophysiological studies in cats showed that Aδ afferent fibers respond in a graded manner to passive distension as well as active contraction; however, the C-fiber afferents can be only activated by noxious stimuli (23, 24). In rats, C fiber as well as Aδ fiber afferents respond to bladder distension as well as chemical irritation (16, 32, 40). Many substances, especially irritant chemicals and inflammatory mediators, are able to activate bladder afferents and/or modulate their mechanosensitive properties (9, 23, 24, 34, 35, 37, 38, 40).
Considerable attention has been focused on ATP as a modulator of afferent nerve excitability (2, 6, 9, 11, 33, 38). Immunocytochemical studies revealed that P2X and P2Y receptors are expressed in bladder afferent nerves and urothelial cells (3, 7, 8, 31). ATP can be released from the urothelium by mechanical or chemical stimulation (2, 19, 20, 28, 43) and is thought to act on adjacent afferent nerves or in an autofeedback manner on urothelial cells to modulate sensory mechanisms in the bladder (2, 12, 18–20). There is considerable evidence suggesting that extracellular ATP modulates afferent nerve excitability by activating P2X receptors (6). Among the seven P2X receptor subtypes that have been identified, P2X2 and P2X3 subtypes have been implicated in the sensitization of peripheral afferent nerves (6, 10–12). P2X3 and P2X2/3 receptors are expressed in small-diameter afferent neurons in dorsal root ganglia (10, 12) and in afferent nerves in the suburothelial plexus in the bladder wall (3, 12). Intravesical administration of ATP activates bladder afferent fibers and enhances reflex bladder activity (34, 37). On the other hand, afferent activity induced by bladder distention is significantly reduced in P2X3 knockout mice (12) or by administration of a P2X receptor antagonist (33, 34, 38). These data indicate that purinergic receptors are involved in mechanosensory signaling in the bladder (33, 38, 47a). P2X3 receptors may also be involved in pain induced by chronic inflammation or urinary urgency (7, 11–13).
Cyclophosphamide (CYP)-induced cystitis has been used as a model to study bladder-related pain (1, 14, 30, 35, 46) and inflammation. Inflammation is accompanied by an increased release of biologically active chemicals such as ATP (7, 42) and neurotrophic factors (e.g., NGF) (47) that can sensitize afferent nerves. In the present study, we used an in vitro whole bladder-pelvic nerve preparation to compare the effects of purinergic agonists (α,β-meATP and ATP) and CYP-induced cystitis on the activity of bladder mechanosensitive afferents in male rats. Preliminary data have been presented in an abstract (49).
MATERIALS AND METHODS
Male Sprague-Dawley rats (100–170 g body wt) were anesthetized with ketamine (50 mg/kg im). The urinary bladder, urethra, prostate gland, seminal vesicles, and innervation including the pelvic nerves and the major pelvic ganglia were removed, placed in a 20-ml bath that was perfused with Krebs solution (1 ml/min) at a temperature of 27°C, and continuously bubbled with 95% O2-5% CO2. The Krebs solution had the following composition (in mM/l): 128 NaCl, 1.8 KCl, 22 NaHCO3, 1.5 KH2PO4, 1.3 MgSO4, 10 glucose, 0.4 CaCl2, 0.4 H2O2 at pH 7.4. A catheter (PE-50) was inserted into the bladder through the urethra and then connected to an infusion pump and to a pressure transducer to monitor bladder activity and to infuse Krebs solution intravesically. One pelvic nerve was placed in an adjacent chamber filled with paraffin oil and positioned on silver bipolar electrodes for recording multiunit afferent nerve activity. Standard electrophysiological methods were used to amplify and analyze the afferent nerve activity. Multiunit afferent firing induced by bladder distention was measured (spikes/s) using a pulse height discriminator-ratemeter, displayed on a rectilinear paper recorder, and also recorded on a VCR for later off-line analysis. Afferent activity was elicited by isotonic bladder distention with Krebs solution for periods of 30 s at 10, 20, 30, and 40 cmH2O or by intravesical infusion of Krebs solution at a filling rate of 0.04 ml/min. The effect of intravesical administration of α,β-meATP (40 and 130 µM in Krebs solution) was also examined. ATP was added to the bath solution to produce a final concentration of 2 mM. These concentrations of purinergic agonists were selected based on previous studies in isolated bladder (38, 49). Afferent responses were also elicited by electrical stimulation (0.15-ms pulse duration) using a pair of silver electrodes positioned on the serosal surface of the bladder close to the neck of the bladder. The distance between the stimulating electrodes was 1.5 mm. The diameter of the stimulating electrodes was 0.25 mm. The distance between stimulating and recording electrodes ranged between 11 and 13 mm. Based on the latencies of evoked action potentials in the pelvic nerve and the distance between stimulus and recording sites, the conduction velocities of the afferent nerves were calculated. In some experiments (n = 12), 100 mg/kg CYP or its vehicle (n = 10) was injected intraperitoneally 17 h before experiments to induce cystitis (34). All procedures utilized in this study were approved by University of Pittsburgh, Institutional Animal Care and Use Committee.
Data analysis
Multiunit recordings of afferent activity are presented as peak firing frequency in spikes/s recorded under isovolumic conditions for 20 min after filling the bladder with 0.32 ml of Krebs solution or during isotonic bladder distension to different pressures. The resting activity of afferent nerves was counted for 1 min before the start of bladder filling. The Lab View program (National Instrument) was used to analyze the area (µV·ms) of the action potentials evoked by electrical stimulation. All data are expressed as means ± SE. Results were evaluated using two-way ANOVA and when the test showed statistical significance (P < 0.05) it was followed by t-test (paired or unpaired for different experiments) using Prism 4 program (GraphPad Software, San Diego, CA).
Drugs
ATP, α,β-meATP, 2′,3′-o-trinitrophenyl-ATP (TNP-ATP), and pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS) (all obtained from Sigma) were diluted to final concentration in Krebs solution with the pH adjusted to 7.4. CYP (Sigma) was dissolved in distilled water at 40 mg/ml concentration for intraperitoneal injection. 2,3-Butanedione monoxime (BDM; Sigma) was dissolved in Krebs solution to the final concentrations.
RESULTS
Multiunit pelvic nerve afferent activity induced by distention of the bladder
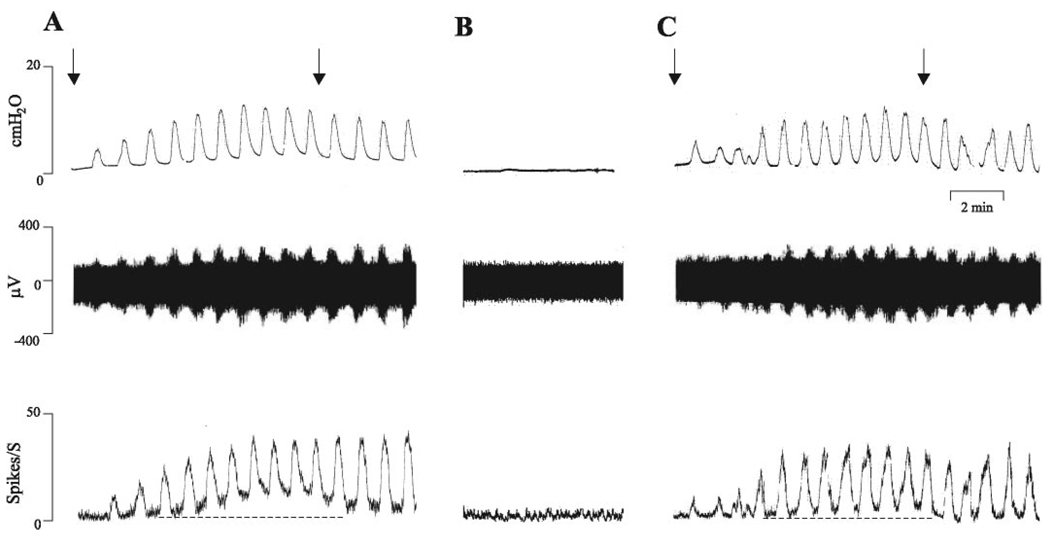
With the bladder empty, the asynchronous multiunit afferent firing on the pelvic nerve was at a very low level (<1 spike/s; Fig. 1). When the bladder was filled by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min, bladder pressure and afferent firing increased gradually (Fig. 1A) and the bladder developed spontaneous rhythmic contractions. Two components of afferent activity were identified: 1) phasic firing which occurred during bladder contractions and 2) tonic firing which occurred between bladder contractions (Fig. 1, A and C). Firing reached a peak 5–7 min after the start of bladder infusion and slowly declined after the infusion was stopped at 8 min. The maximum intravesical pressure during bladder contractions was 12.6 ± 3.0 cmH2O and the average frequency of spontaneous bladder contractions was ~1/min (n = 16). The average peak afferent firing rate was 27 ± 3 spikes/s (n = 16). After emptying the bladder, the spontaneous bladder contractions and afferent firing returned to control levels (Fig. 1B). The response to bladder distension could be repeated several times (Fig. 1C) and responses remained stable for more than 1 h.
Fig. 1.
Effects of repeated bladder distension by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min (A and C) on bladder activity and multiunit pelvic afferent nerve firing. Arrows indicate start and stop of infusion. Top traces represent bladder contractile activity measured as intravesical pressure. Middle traces represent afferent nerve firing. Bottom traces represent ratemeter recording of pelvic afferent nerve firing. The dashed lines in the bottom traces indicate basal level of afferent activity and show that phasic and tonic firing increased during intravesical infusion of Krebs solution. B: recording with bladder empty.
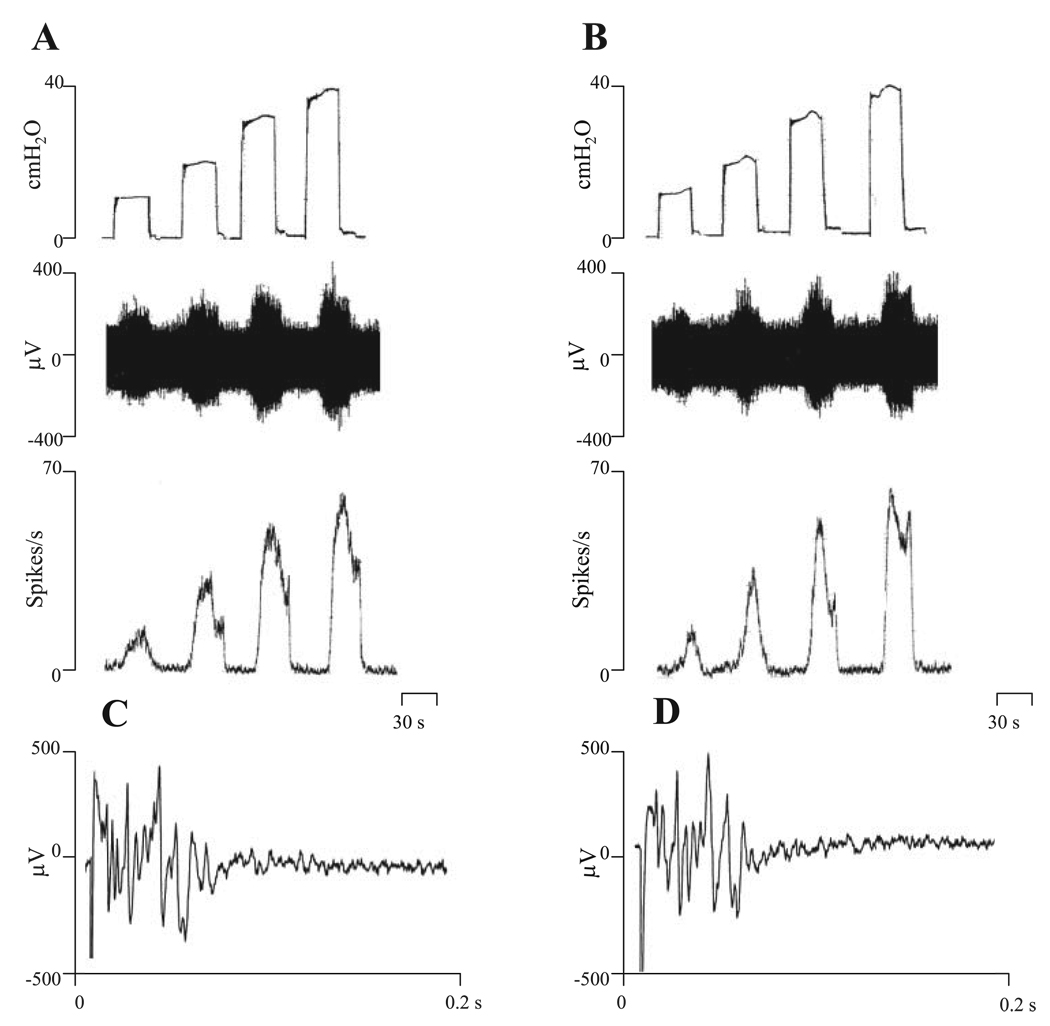
In some experiments, afferent nerve firing was elicited by isotonic distension of the bladder for 30 s at 30-s intervals with Krebs solution at 10, 20, 30, and 40 cmH2O (see Fig. 4A) to evaluate the pressure response properties of afferent activity. The maximum firing during isotonic bladder distension was 22 ± 6 spikes/s at 10 cmH2O, 46 ± 7 spikes/s at 20 cmH2O, 56 ± 8 spikes/s at 30 cmH2O, and 64 ± 9 spikes/s at 40 cmH2O (n = 6).
Fig. 4.
Effects of PPADS (30 µM), a nonselective purinergic receptor antagonist applied in the bath, on afferent nerve activity evoked by isotonic distention of bladder with Krebs solution at 10, 20, 30, and 40 cmH2O for 30 s (A and B) and action potentials in pelvic afferent nerves evoked by electrical stimulation of the bladder (C and D). A and C represent control recordings; B and D represent recording after PPADS (30 µM). Top traces in A and B show intravesical pressures during isotonic distention of the bladder. Middle traces show the afferent nerve activity. Bottom traces represent ratemeter recording of afferent nerve firing. C and D: evoked action potentials recorded on the pelvic nerve evoked by stimulation on the surface of the bladder (80 V, 0.15-ms duration, average of 5 responses) before PPADS (C) and after PPADS (D). Note that PPADS (30 µM) did not alter afferent nerve firing or evoked action potentials.
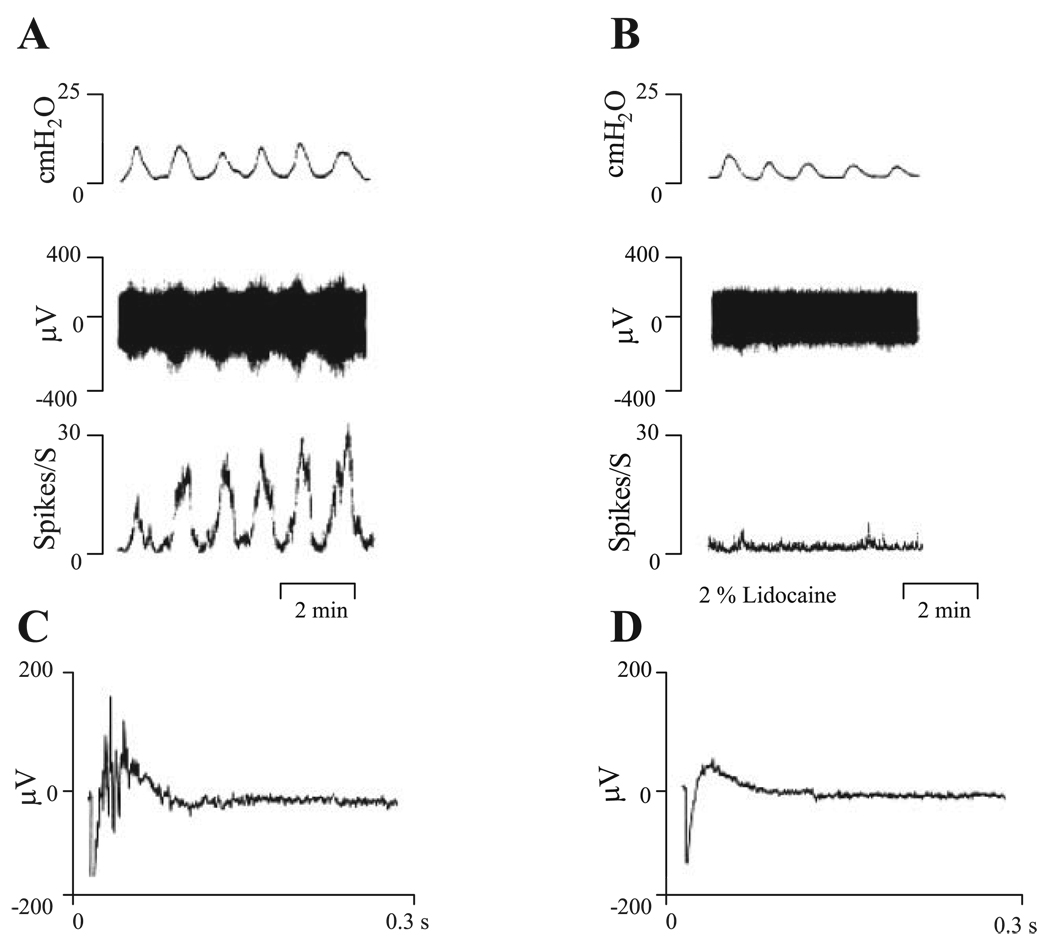
To evaluate the possibility that afferent nerve activity was contaminated by movement artifacts due to bladder contractions, we examined the effect of a local anesthetic agent, 2% lidocaine (n = 4), on bladder contractions and pelvic afferent nerve activity (Fig. 2). Intravesical injection of the local anesthetic abolished the phasic afferent nerve firing and the electrically evoked compound action potentials but did not abolish the spontaneous bladder contractions (Fig. 2, B and D). Effects of lidocaine occurred within 2–3 min after injection.
Fig. 2.
Effects of 2% lidocaine on bladder contractions and pelvic afferent nerve firing. A: responses after distention of bladder with 0.32 ml Krebs solution. B: responses after distention of bladder with 2% lidocaine in Krebs solution. Top traces represent bladder contractile activity measured as intravesical pressure. Middle traces represent afferent nerve firing. Bottom traces represent ratemeter recording of afferent nerve firing. C: evoked action potentials recorded on the pelvic nerve at the stimulus intensity of 80 V and 0.15-ms duration. D: evoked action potentials after intravesical infusion of 2% lidocaine at the same stimulation parameters as in C. Note that lidocaine blocked pelvic afferent nerve firing and evoked action potentials but did not abolish the bladder contractions. Vertical calibrations in A and B are intravesical pressure in cmH2O, nerve activity in µV, and spikes/s. Horizontal calibration represents 2 min. Vertical calibration in C and D is in µV and horizontal calibration represents 0.3 s.
Effects of purinergic agonists and antagonists on bladder activity and pelvic nerve afferent firing
The effects of two purinergic agonists, α,β-meATP administered intravesically and ATP administered in the bath, were evaluated on bladder activity and afferent firing. Intravesical administration of 40 and 130 µM α,β-meATP in a volume of 0.32 ml did not significantly change the maximum amplitude of bladder contractions (12.31 ± 2.8 cmH2O, n = 12 in 40 µM and 13.68 ± 1.7 cmH2O, n = 8 in 130 µM α,β-meATP) or the frequency of the contractions (Fig. 3, B and D), but significantly increased the peak afferent firing that occurred during spontaneous bladder contractions and the tonic afferent firing occurring between bladder contractions (Fig. 3, B and D). This study focused on the phasic firing. The effect of α,β-meATP on afferent firing occurred within 3 min after the start of infusion and reached a peak in 5–7 min and then slowly declined after the infusion was stopped at 8 min. The peak afferent nerve discharge was 53 ± 6 spikes/s at the concentration of 40 µM α,β-meATP (n = 12) and 61 ± 2.4 spikes/s at the concentration of 130 µM α,β-meATP (n = 8). The facilitatory effects of the agonist were prevented by administration of a P2X receptor antagonist, TNP-ATP (30 µM, intravesical injection; Fig. 3, C and D), or PPADS (30 µM bath concentration), a nonselective purinergic receptor antagonist. Neither TNP-ATP (30 µM, n = 6) nor PPADS (30 µM, bath concentration, n = 3) administered alone in the absence of α,β-meATP significantly altered bladder activity or afferent firing during intravesical infusion of the bladder with Krebs solution at the rate of 0.04 ml/min for 8 min or isotonic distension of the bladder with Krebs solution at 10, 20, 30, and 40 cmH2O for 30 s (Fig. 4).
Fig. 3.
Enhancement of pelvic afferent nerve firing after intravesical administration of α,β-meATP. Top traces represent bladder contractile activity measured as intravesical pressure. Middle traces represent pelvic afferent nerve activity. Bottom traces represent ratemeter recording of afferent firing. A: control recording during bladder distension induced by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min. B: intravesical infusion of 130 µM α,β-meATP. C: after pretreatment with intravesical infusion of 0.3 ml TNP-ATP (30 µM) for 10 min and then intravesical administration of 130 µM αβ-meATP combined with 30 µM TNP-ATP which suppressed the afferent firing. Vertical calibrations are the same as in Fig. 1. Horizontal calibration represents 4 min. D: intravesical infusion of α,β-meATP did not change bladder pressure (top), but enhanced pelvic afferent nerve firing (40 µM, ***P < 0.001, n = 12; 130 µM, ***P < 0.001, n = 8; bottom). This enhanced afferent nerve firing was blocked by TNP-ATP, a P2X receptor antagonist (30 µM, n = 8).
In other experiments (n = 11), the effects of 2 mM ATP administered in the bath solution to the serosal surface of the bladder were evaluated after intravesical infusion of Krebs solution into the bladder (0.32 ml). ATP elicited a large-amplitude bladder contraction and a large burst of afferent nerve firing (Fig. 5A). The effects of ATP occurred within 1 min and lasted a few minutes. Following the initial excitatory response to ATP, spontaneous bladder contractions and phasic firing were abolished (Fig. 5A).
Fig. 5.
Effects of 2,3-butanedione monoxime (BDM) on bladder contractions and afferent nerve activity induced by ATP (2 mM). Top traces represent bladder contractile activity measured as intravesical pressure with bladder distended by 0.32 ml of Krebs solution. Middle traces represent afferent nerve firing. Bottom traces represent ratemeter recording of pelvic afferent nerve firing. All records were obtained in the same preparation at 45- to 60-min intervals. A: responses to ATP (2 mM) applied in the bath. B: responses to 2 mM ATP after 60 mM BDM. Note that bladder activity was partially blocked and ATP-evoked firing was reduced by BDM. C: responses to 2 mM ATP after 80 mM BDM. Note that ATP-evoked bladder contraction but not ATP-evoked firing was blocked by BDM. D: 60 min after washout of BDM with Krebs solution the bladder spontaneous contractions and afferent nerve activity partially recovered.
Because ATP can induce a bladder contraction, it is possible that the afferent activity induced by ATP was due to indirect activation of mechanosensitive afferents by the increased tension in the bladder wall. To determine whether ATP was acting directly on the afferent nerve terminals or indirectly by inducing a smooth muscle contraction, another series of experiments was conducted to examine the effects of BDM, an ATPase and Ca2+ release inhibitor, on bladder activity (n = 6). BDM is known to block smooth muscle contractions (3). The effects of ATP on bladder activity were assessed after adding BDM to the bath in a range of concentrations (20, 40, 60, and 80 mM). At concentrations of 20, 40, and 60 mM, BDM partially blocked spontaneous bladder contractions and reduced the 2 mM ATP evoked bladder contractions (Fig. 5B). After 80 mM BDM which reversibly abolished spontaneous bladder contractions, ATP still initiated an increase in pelvic afferent nerve firing within 1 min (Fig. 5C). However, the excitatory effect of ATP was markedly reduced (<50% of control). After BDM was washed out, spontaneous bladder contractions and afferent nerve firing returned within 60 min (Fig. 5D).
Effects of purinergic agonists on evoked compound action potentials in pelvic afferent nerves
To identify the types of bladder afferent nerves affected by purinergic agonists, axons on the serosal surface of the bladder close to the bladder neck were electrically stimulated with bipolar electrodes and evoked compound action potentials were recorded on the pelvic nerve. The voltage threshold for evoking action potentials with the bladder filled with Krebs solution was 9.0 ± 1.2 V (0.15-ms pulse duration, n = 14). Action potentials were characterized by short- and long-latency components corresponding to axonal conduction velocities ranging from 10 to 0.3 m/s at 27°C. The largest amplitude action potential in the recording occurred at short latencies and was biphasic. Lower amplitude potentials occurred at slower conduction velocities ranging from 0.3 to 1.0 m/s.
After intravesical administration of α,β-meATP (130 µM), the threshold for evoked compound action potentials decreased to 3.5 ± 0.5 V (n = 8, P < 0.05; Fig. 6A) and the area of action potentials including the short- and long-latency potentials evoked at a submaximal stimulus intensity (80 V, 0.15-ms duration) increased from 4.1 ± 1.4 to 9.2 ± 3.0 µV·ms (P < 0.05; Fig. 6, B and C). The effects induced by intravesical administration of α,β-meATP were blocked by 30 µM TNP-ATP (n = 7; Fig. 6).
Fig. 6.
Effects of α,β-meATP (130 µM) administered by intravesical infusion on evoked compound action potentials. A: threshold for evoked action potentials was reduced by purinergic receptor agonist, α,β-meATP (n = 8), and this effect was blocked by P2X receptor antagonist, TNP-ATP (30 µM, n = 7), in combination with α,β-meATP. B: compound action potentials recorded in pelvic afferent nerves in response to electrical stimulation on the surface of the urinary bladder (80 V, 0.15-ms duration, average of 5 responses). Top trace represents the control response at the stimulus intensity of 80 V, 0.15 ms with the bladder distended with 0.32 ml of Krebs solution. Middle trace was recorded after bladder distention with α,β-meATP (130 µM) at the same stimulus parameters. Bottom trace represents distention of the bladder with 130 µM α,β-meATP and 30 µM TNP-ATP at the same stimulus parameters after 10-min pretreatment with intravesical administration of 30 µM TNP-ATP. Dot above each record indicates the stimulus artifact. C: area of evoked action potentials was increased by α,β-meATP (130 µM) and reversed by TNP-ATP (30 µM) in combination with α,β-meATP. *P < 0.05 in A and C.
Administration of ATP (2 mM bath concentration) produced a similar decrease in the threshold for the evoked action potential from 8.3 ± 0.9 to 4.9 ± 1.0 V (n = 7, P < 0.05; Fig. 7A) and an increase in the area of the evoked action potentials from 4.6 ± 0.7 to 10.9 ± 1.1 µV·ms (n = 7, P < 0.05). The excitatory effects of ATP were blocked by bath application of 30 µM PPADS (Fig. 7B). The effects induced by purinergic agonists could be repeated several times after more than 60-min washout with Krebs solution.
Fig. 7.
Effects of ATP on electrically evoked action potentials. A: threshold of evoked action potentials was reduced by ATP (2 mM, bath concentration, n = 7) and this effect was reversed by PPADS (30 µM, bath concentration, n = 6). B: compound action potentials recorded in pelvic afferent nerves in response to electrical stimulation on the surface of the urinary bladder (80 V, 0.15-ms stimulation duration, average of 5 responses). Top trace represents the control response with the bladder distended with Krebs solution. Middle trace was obtained after application of 2 mM ATP (bath concentration). Bottom trace shows responses under the same conditions in the presence of 2 mM ATP (bath concentration) after pretreatment with 30 µM PPADS in the bath. Dot under each record indicates the stimulus artifact. C: area of evoked action potentials was increased by ATP (2 mM) and reversed by PPADS (30 µM). *P < 0.05 in A and **P < 0.01 in C.
Pelvic afferent nerve activity after CYP-induced chemical cystitis
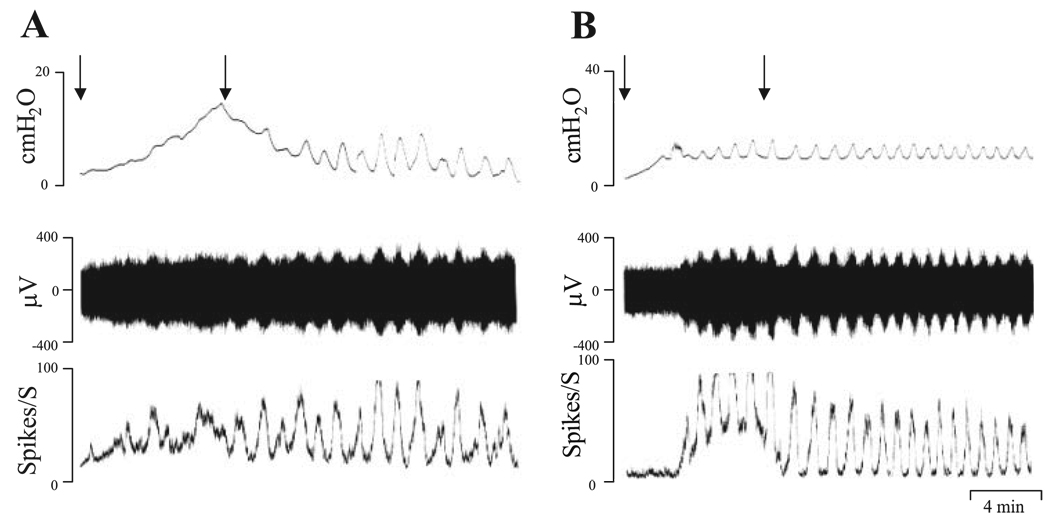
The bladder was irritated by pretreating with CYP (100 mg/kg ip, n = 12), 17 h before the experiments (35, 49). In these rats, the urine was pink or red indicating the presence of hemorrhagic cystitis and in some preparations (n = 4) the pattern of the bladder contractions during intravesical infusion was different than in vehicle-treated preparations. In these experiments during the initial period of intravesical infusion, the bladder pressure increased markedly, but declined after the infusion was completed, leading to the emergence of phasic contractions (Fig. 8A). In the remaining experiments, the bladder activity was similar to that in vehicle-treated preparations (n = 8; Fig. 8B). After filling the bladder with Krebs solution at the rate of 0.04 ml/min for 8 min, the maximal pressure of the rhythmic bladder contractions was not changed after CYP pretreatment (maximal pressure 12.4 ±1.4 cmH2O in CYP pretreatment preparations and 13 ± 1.0 cmH2O in vehicle-treated preparations). However, the pelvic nerve afferent firing was larger in CYP-pretreated experiments (69 ± 16 spikes/s) than in vehicle-treated preparations (26 ± 5 spikes/s, P < 0.05, n = 9). It should be noted that the magnitude of the peak afferent firing in CYP-treated experiments showed considerable variability ranging from 27 to 188 spikes/s in different experiments. This may be due to the variable irritation induced by CYP treatment in different animals or a variable number of active fibers in each multiunit afferent fiber recording. In some experiments, afferent nerve activity was induced by isotonic distention of the bladder with Krebs solution at 10, 20, 30, and 40 cmH2O for 30 s (Fig. 9A). The peak afferent firing was 29 ± 3, 67 ± 9, 82 ± 4, and 91 ± 5 spikes/s (n = 4) at bladder pressures of 10, 20, 30, and 40 cmH2O, respectively. After intravesical administration of TNP-ATP (30 µM), the afferent nerve firing induced by 10 cmH2O bladder pressure was significantly decreased more than 62%, from 29 ± 3 to 11.3 ± 4.0 spikes/s (P < 0.05, n = 4). TNP-ATP also decreased by 25–45% the afferent nerve firing induced by higher bladder pressures (20–40 cmH2O), but these changes were not statistically significant (P < 0.05, n = 4). After bath application of PPADS (30 µM), the afferent nerve firing significantly decreased 52% at the bladder pressure of 10 cmH2O (P < 0.05, n = 5; Fig. 9, B and E), but the decrease in afferent nerve firing at bladder pressures of 20, 30, and 40 cmH2O was not statistically significant (P > 0.05).
Fig. 8.
Pelvic nerve afferent firing induced by intravesical infusion of Krebs solution at the rate of 0.04 ml/min in cyclophosphamide-pretreated preparations (100 mg/kg ip, 17 h before experiments). Top traces represent bladder contractile activity measured as intravesical pressure. Middle traces represent pelvic nerve afferent firing. Bottom traces represent ratemeter recording of afferent firing. A and B recordings in different experiments showing different types of bladder contractions in cyclophosphamide-pretreated preparations. A: hyperactive noncompliant bladder that generates a large intravesical pressure during the initial stage of bladder filling. Tonic pressure declined after filling was stopped and spontaneous contractions emerged. B: bladder activity that was similar to activity in vehicle-treated bladder. However, peak afferent firing in both preparations was higher than in vehicle-treated preparations. Arrows represent start and stop of infusion of the Krebs solution.
Fig. 9.
Effects of PPADS (30 µM, n = 5), a nonselective purinergic receptor antagonist applied in the bath, on afferent nerve activity in cyclophosphamide (100 mg/kg ip, 17 h before the experiments)-pretreated preparations. Top traces in A and B show isotonic distention of the bladder with Krebs solution at 10, 20, 30, and 40 cmH2O for 30 s. Middle traces show the afferent nerve activity. Bottom traces show ratemeter recording of afferent nerve firing. A: before PPADS. B: after PPADS (30 µM). C and D: action potentials evoked by a stimulus intensity of 80 V and 0.15-ms pulse duration (average of 5 responses) before PPADS (C) and after PPADS (30 µM; D) in a cyclophosphamide-pretreated preparation. Note that PPADS reduced afferent nerve firing. E: summary of 5 experiments conducted using the protocol illustrated in A and B showing that PPADS (30 µM) applied in the bath reduced afferent nerve firing induced by isotonic distention of the bladder at 10, 20, 30, and 40 cmH2O for 30 s (n = 5). However, the effect of PPADS was only statistically significant during the 10 cmH2O pressure stimulus (n = 5, P < 0.05). F: area of action potentials evoked by a stimulus intensity of 80 V and 0.15-ms pulse duration as shown in C and D was reduced by PPADS (30 µM, n = 5, P < 0.05). *P < 0.05 in E and F.
The threshold for evoked action potentials in CYP-pretreated preparations was significantly lower (3.1 ± 0.5 V, n = 12, P < 0.05) compared with vehicle-pretreated preparations (8.8 ± 0.2 V). The area of evoked action potentials at a submaximal stimulation intensity (80 V, 0.15 ms) was also increased from 4.0 ± 0.8 µV·ms in vehicle-treated preparations (n = 9) to 9.0 ± 2.0 µV·ms in CYP-treated preparations (Table 1), but this change was not statistically significant (P > 0.05). TNP-ATP increased the threshold for evoked action potentials by 75% (P < 0.05, n = 5) and the area of evoked action potentials decreased 46% (P < 0.05, n = 5; Table 2). After bath application of PPADS (30 µM), the threshold for evoked action potentials increased 80% (P < 0.05, n = 5), and the area of evoked action potentials decreased (P < 0.05, n = 5; Fig. 9, C, D, and F, and Table 2).
Table 1.
Effects of purinergic receptor agonists on evoked action potentials in normal bladders and effect of CYP pretreatment
| α,β-meATP (130 µM) |
ATP (2 mM) |
CYP |
||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Vehicle | CYP | |
| Threshold, V | 9.0±1.2 | 3.5±0.5* | 8.3±0.9 | 4.9±1.0* | 8.8±0.2 | 3.1±0.5* |
| Area, µV·ms | 4.1±1.4 | 9.2±3* (8) | 4.6±0.7 | 10.9±1.1† (7) | 4.0±0.8 (9) | 9±2 (12) |
Values are means ± SE. The number in brackets represents number of experiments. Measurements are significantly different before and after agonists or between vehicle controls and CYP treatment (*P < 0.05) and †P < 0.01). The area of evoked action potentials was measured at submaximal stimulation (80 V, 0.15 ms). CYP, cyclophosphamide.
Table 2.
Effects of purinergic receptor antagonists on evoked action potentials in normal and CYP-treated preparations
| Normal Preparations |
CYP-treated Preparations |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | After TNP-ATP | Control | After PPADS | Control | After TNP-ATP | Control | After PPADS | |
| Threshold, V | 8.7±0.5 | 7.4±0.6 | 8.8±1 | 8.7±0.8 | 2.7±0.5 | 4.8±0.7* | 3.5±0.4 | 6.3 ±0.6* |
| Area, µV·ms | 3.6±1.1 | 3.7±1 (6) | 3.9±0.5 | 4.9±0.4 (6) | 8.6±1.2 | 4.6±2* (5) | 8.8±2.0 | 4.9±1* (5) |
Values are means ± SE. The number in brackets represents number of experiments. Measurements are significantly different before and after antagonists in CYP-treated preparations (*P < 0.05). The area of evoked action potentials was measured at submaximal stimulation (80 V, 0.15 ms).
DISCUSSION
The present study used a rat in vitro whole bladder-pelvic nerve preparation to compare the effects of purinergic agonists (α,β-meATP and ATP) and chemically induced cystitis on the activity of mechanosensitive bladder afferent nerves. The results indicate that purinergic agonists can increase the peak firing of pelvic afferent nerves elicited by distension and/or contraction of the urinary bladder, decrease the threshold for evoked compound action potentials, and increase the area of the action potentials elicited by submaximal electrical stimulation. The effects of purinergic agonists were blocked by TNP-ATP, a P2X receptor antagonist, or PPADS, a nonselective purinergic receptor antagonist, suggesting that purinergic receptors are involved in these responses. CYP-induced chemical cystitis produced effects on bladder afferent nerve activity that mimicked the sensitizing effect of purinergic agonists. These effects were also reduced by TNP-ATP or PPADS, suggesting that purinergic mechanisms contribute to the afferent sensitization induced by CYP.
In the urinary bladder of both humans and animals, afferent nerve terminals have been identified suburothelially in a dense nerve plexus that lies immediately beneath and extending into the urothelium (21, 22, 48) as well as in the smooth muscle (16, 17, 32, 41, 48). These afferents monitor the volume of the bladder and intramural tension as well as the chemical environment (15–17). Immunohistochemical studies showed that P2X3-immunoreactive sensory fibers terminate in the suburothelial layer of the bladder wall in the mouse (12), rat, and cat (3, 17). It has been proposed that ATP released by urothelial cells in response to bladder distention activates afferent nerves via an interaction with P2X3 or P2X2/3 receptors and in turn facilitates the micturition reflex or induces painful bladder sensations (11, 33). In this study we could not detect a significant decrease in the afferent firing induced by bladder distention after application of TNP-ATP or PPADS in normal bladders. This suggests that release of the endogenous transmitter ATP under the conditions of our experiments was not involved in triggering mechanosensitive pelvic afferent nerve activity even though exogenously applied ATP or α,β-meATP did increase afferent firing. A similar finding has been reported in the guinea pig bladder (50, 51).
In our study, bipolar silver electrodes were positioned on the serosal surface of the bladder close to the neck to directly excite the afferent axons. Stimulation in this area consistently evoked an action potential in the pelvic nerves. Since the stimulation electrode was positioned on the serosal surface of the bladder, the electrical current could excite axons on the bladder surface but also might pass through the bladder wall to excite intramural axons or even the afferent nerve terminals near the urothelium. While the site for activation of afferent nerves is uncertain, it is nevertheless noteworthy that purinergic agonists increased the excitability at that site, as evidenced by a lower electrical threshold and increased area of the evoked action potentials. Modulation of peripheral axonal excitability in the rat vagus and sciatic nerves by neurotransmitters has also been described (29, 39). Using a combination of threshold tracing and confocal Ca2+ imaging techniques, Irnich et al. (27, 29) showed that ATP affects both unmyelinated C fiber axons and Schwann cells in peripheral nerves. In our study, administration of purinergic agonists onto the serosal surface of the bladder or intravesical infusion into the bladder decreased the electrical threshold for evoking action potentials in afferent nerves, while the areas of evoked action potential at submaximal stimulus intensities were increased. These results imply that purinergic agonists might affect receptors distributed along bladder afferent axons as they pass through the bladder wall, as well as afferent terminals near the urothelium. Thus afferent purinergic receptors might be responsive to ATP released at all sites in the bladder wall and thereby increase afferent nerve firing.
In the present study, the firing of pelvic afferent fibers induced by bladder distention was facilitated by intravesical application of the P2X receptor agonist, α,β-meATP. It seems that the increased afferent firing was not due to the effects of the purinergic agonist on bladder smooth muscle because neither the intravesical pressure nor the amplitude of bladder contractions increased significantly after intravesical administration of α,β-meATP, suggesting that this agent applied intravesically was affecting the afferent nerves and not smooth muscle. Since the increased afferent activity following application of α,β-meATP could be blocked by intravesical TNP-ATP, a P2X receptor antagonist, it is reasonable to conclude that pelvic afferent nerve sensitization induced by intravesical application of α,β-meATP was mediated by P2X receptors located in suburothelial afferent nerves and/or on the urothelial cells which express purinergic receptors and could in turn release ATP or other transmitters (18, 20, 43). Thus intravesical administration of α,β-meATP in this study could influence afferent nerve activity via multiple mechanisms: 1) direct action on P2X receptors in urothelial cells, 2) direct action on P2X receptors in afferent nerve terminals, or 3) combined action on P2X receptors in urothelial cells and afferent nerve terminals.
In rat and human urinary bladder smooth muscle, activation of P2X purinoceptor subtypes evokes bladder contractions (8, 36, 44). Our results show that ATP applied to the serosal surface of the bladder can increase pelvic nerve firing as well as the amplitude of bladder contractions, indicating that ATP activated mechanosensitive afferent nerve terminals as well as smooth muscles. To distinguish between a direct effect of ATP on afferent nerves and an indirect effect due to bladder smooth muscle contractions that in turn activate afferent nerve firing, we used an excitation-contraction uncoupler, butanedione monoxime (BDM), to abolish the bladder contractions. BDM is a reversible myosin ATPase inhibitor with phosphatase-like properties. Because of its ability to uncouple skeletal and cardiac muscle contraction, BDM has been used to produce cardioplegic arrest (4). BDM in a concentration of 60 mM abolished the spontaneous bladder contractions completely. However, serosal administration of 2 mM ATP still induced a small bladder contraction. A higher concentration of BDM (80 mM) abolished the ATP-evoked bladder contractions completely but did not completely block the ATP effect on afferent firing. These results indicate that part of the pelvic nerve afferent firing induced by administration of ATP to the serosal surface is directly mediated by P2X receptors in afferent nerves but partly mediated by evoking bladder contractions.
Many studies indicated that ATP is involved in peripheral pain signaling by actions on P2X receptors, particularly P2X3 receptors (7, 13, 26). There is also evidence for an enhanced role for ATP in inflammation (25, 26). In this study, we compared the effects of purinergic agonists, ATP and α,β-meATP, either infused intravesically or applied to the serosal surface with the effects of CYP pretreatment on the activity of bladder afferent nerves. Our previous study indicated that 100 mg/kg ip CYP 17 h before experiments can cause cystitis and increased voiding frequency (35). In the present study, we found that the threshold for evoked action potentials was decreased in CYP-pretreated preparations, while the peak afferent nerve firing to distention of the bladder and the area of the evoked action potential were significantly increased. These results indicate that CYP sensitized and/or activated mechanosensitive low threshold Aδ afferent fibers in rat urinary bladder. These results were similar to the effect of the purinergic agonists ATP or α,β-meATP on bladder afferent nerves. The peak afferent nerve firing induced by isotonic distension of the bladder at 10 cmH2O was significantly reduced by purinergic receptor antagonists TNP-ATP or PPADS in CYP-pretreated preparations, suggesting that purinergic receptors are tonically activated in CYP-irritated preparations and are involved in the cystitis-induced increased afferent nerve excitability. However, the P2X receptor antagonists did not significantly change afferent firing in nonirritated bladders. This finding indicates that an endogenously released purinergic agent has a tonic facilitatory effect on afferent nerves after CYP-induced cystitis but not in normal bladders. Smith et al. (42) reported that stretched evoked release of ATP from the urothelium is enhanced by CYP-induced cystitis, whereas the resting release of ATP was not changed. Other studies (5) revealed that bladder afferent neurons in the L6-S1 dorsal root ganglia from rats with CYP-induced cystitis exhibited a downregulation of P2X3 and/or P2X2/3 receptors. This was attributed to the massive release of ATP from the inflamed bladder tissue. It is not known whether a similar downregulation of P2X receptors occurs at afferent nerve terminals in the bladder wall with CYP-induced cystitis. However, in feline interstitial cystitis, a condition in which ATP release from the urothelium is also increased (2), P2X3 receptor immunoreactivity in the urothelium was not changed, but P2X1 receptor immunoreactivity was markedly reduced (3).
In summary, this study provides evidence that purinergic agonists acting on P2X receptors in the urothelium or directly on suburothelial afferent axons can increase the excitability of bladder afferent nerves. Cystitis induced by CYP pretreatment can mimic the sensitizing effect of purinergic agonists, indicating that ATP may be involved in nociceptive mechanisms in the urinary bladder.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health Grant DK-49430.
REFERENCES
- 1.Ahluwalia A, Maggi CA, Santicioli A, Lecci A, Giuliani S. Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol. 1994;111:1017–1022. doi: 10.1111/j.1476-5381.1994.tb14845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 4.Borlak J, Zwadlo C. The myosin ATPase inhibitor 2,3-Butanedione monoxime dictates transcriptional activation of ion channels and Ca2+-handling proteins. Mol Pharmacol. 2004;66:708–717. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 5.Borvendeg-Khrasani M, Rubini P, Fischer W, Allgaier C, Wirkner K, Himmel HM, Gillen C, Illes P. Subsensitivity of P2X but not vanilloid 1 receptors in dorsal root ganglia of rats caused by cyclophosphamide cystitis. Eur J Pharmacol. 2003;474:71–75. doi: 10.1016/s0014-2999(03)02003-x. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. P2X receptors in sensory neurons. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Purinergic signalling in lower urinary tract. In: Abbrachio MP, Williams M, editors. Handbook of Experimental Pharmacology. Berlin: Springer-Verlag; 2001. pp. 423–515. [Google Scholar]
- 9.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 11.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2000;53:553–568. [PubMed] [Google Scholar]
- 12.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 13.Cook SP, Vulchanova L, Hargreaves KM, Eide R, McClesky EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 14.Cox PJ. Cyclophosphamide cystitis identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- 15.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System, Vol. 3, Nervous Control of The Urogenital System. London: Harwood Academic Publishers; 1993. pp. 227–289. [Google Scholar]
- 16.de Groat WC, Downie JW, Levin RM, Long Lin AT, Morrison JFB, Nishizawa O, Steers WD, Thor KB. Basic neurophysiology and neuropharmacology. Chapter 7. Sponsored by the World Health Organization; 1st International Consultation on Incontinence. 1999:105–154.
- 17.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50 Suppl 6A:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 18.de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64 Suppl 6A:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson DR. Urothelial function. Br J Urol. 1999;84:235–242. doi: 10.1046/j.1464-410x.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- 21.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 22.Gosling JA, Dixon JS. Sensory nerves in the mammalian urinary tract. An evaluation using light and electron microscopy. J Anat. 1974;117:133–144. [PMC free article] [PubMed] [Google Scholar]
- 23.Häbler HJ, Jänig W, Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agents Actions. 1988;25:219–221. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- 24.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgsia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irnich D, Burgstahler R, Bostock H, Grafe P. ATP affects both axons and Schwann cells of unmyelinated C fibers. Pain. 2001;92:343–350. doi: 10.1016/S0304-3959(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 28.Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 29.Lang PM, Sippel W, Schmidbauer S, Irnich D, Grafe P. Functional evidence for P2X receptors in isolated human vagus nerve. Anesthesiology. 2003;99:232–235. doi: 10.1097/00000542-200307000-00038. [DOI] [PubMed] [Google Scholar]
- 30.Lantéri-Minet M, Bon K, de Pommery J, Michiels JF, Menetréy D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-fos and krox-24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- 32.Morrison JFB. The activation of bladder wall afferent nerves. Exp Physiol. 1999;84:131–136. doi: 10.1111/j.1469-445x.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 33.Namasivayam S, Eardley L, Morrison JF. Purinergic sensory neuron-transmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- 34.Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology. 2005;65:1332–1337. doi: 10.1016/j.urology.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 36.Palea S, Pietra C, Trist DG, Artibani W, Calpista A, Corsi M. Evidence for the presence of both pre- and postjunctional P2-purinoceptor subtypes in human isolated urinary bladder. Br J Pharmacol. 1994;114:35–40. doi: 10.1111/j.1476-5381.1995.tb14902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- 38.Rong W, Spyer KM, Burnstock G. Activation and sensitization of low and high threshold afferent fibers mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer SK, Reeh PW, Bove GM. Noxious heat-induced CGRP release from rat sciatic axons in vitro. Eur J Neurosci. 2001;14:1203–1208. doi: 10.1046/j.0953-816x.2001.01741.x. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 41.Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- 42.Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2007;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27–C34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Kokubun S. Subtypes of purinoceptors in rat and dog urinary bladder smooth muscle. Br J Pharmacol. 1994;112:117–122. doi: 10.1111/j.1476-5381.1994.tb13039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 47a.Vlaskovska M, Kasakov L, Rong WF, Bodin P, Bardini M, Cockayne DA, Ford APD, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2004;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi Y, Tomoyoshi T, Fujimiya M, Arai R, Maeda T. Substance P-containing axon terminal in the mucosa of the human urinary bladder: pre-embedding immunohistochemistry using cryostat sections for electron microscopy. Histochemistry. 1993;100:401–407. doi: 10.1007/BF00267819. [DOI] [PubMed] [Google Scholar]
- 49.Yu YB, de Groat WC. Sensitization of pelvic afferent nerve in the in vitro urinary bladder-pelvic nerve preparation of the rat by purinergic agonists or by cyclophosphamide (CYP) pretreatment; 34th Annual Meeting of the Society for Neuroscience; 2004. Abstract. [Google Scholar]
- 50.Zagorodnyuk VP, Costa M, Brookes SJH. Major classes of sensory neurons to the urinary bladder. Auton Neurosci. 2006;126–127:390–397. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJH, Gregory SJ. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]