INTRODUCTION
The autonomic nervous system and its sympathetic arm play important roles in the regulation of blood pressure.1,2,3 Their role in the short-term regulation of blood pressure, especially in responses to transient changes in arterial pressure, via baroreflex mechanisms is well known.4 However, the role of the sympathetic branch in longer term (days, months, years) blood pressure regulation has been a focus of debate since at least the 1970s.1,4 Our goal in this “Hypertension Highlights” is to summarize and integrate our ideas on the role of the sympathetic nervous system in long-term blood pressure regulation in humans.1,2,3,5,6,7,8,9 We will focus primarily on information from studies conducted in humans and use data from animal studies to emphasize key points. In this context, we want to address four key questions. The first three focus on our recent work. The final issue is an emerging one and more speculative.
What is the role of the sympathetic nervous system in long-term blood pressure regulation in young (18–40 year old) normotensive men?
Does the role of the sympathetic nervous system in long-term blood pressure regulation change as a function of age in men?
Does sex influence the role of the sympathetic nervous system in long-term blood pressure regulation, and are sex differences modified by aging?
Are we entering an era of sympathetically driven hypertension?
Before we address these questions, a few thoughts about how to assess the overall activity of sympathetic nerves in humans.
Assessment of sympathetic activity in humans
Various approaches used to assess sympathetic activity in humans have recently been reviewed by Grassi.10 We focus primarily on studies that use direct measurements of muscle sympathetic nerve activity (MSNA) as an overall marker of sympathetic outflow in humans; and to a lesser extent on studies use whole body and regional norepinephrine spillover, and also plasma norepinephrine (NE). There are advantages and disadvantages with each of these approaches that have been reviewed in detail.2,10
In resting humans these three approaches are typically well-correlated, and conclusions made with one technique are generally supported by studies using one of the other two approaches.11,12,13,14 There are two major caveats to this point. First, the correlations between MSNA and other indices of sympathetic activity have been most clearly demonstrated in young healthy men.11 Second, during non-resting conditions (e.g., exercise, mental stress etc.) there can be highly specific changes in sympathetic activity to selected tissues with no changes in other tissues. For example, MSNA following arousal stimuli (such as startling the subject with a loud noise) either does not change or falls for a few bursts in some subjects, and MSNA can fall during mental stress.15,16,17,18 However, skin sympathetic activity (SSNA) increases during both arousal and mental stress.19 SSNA also increases at the onset of static exercise before any rise in MSNA.20
MSNA as an index of overall sympathetic activity in resting young men has a number of attractive features
Most of the studies from our laboratories and those of many colleagues have used MSNA as the primary index of sympathetic activity in humans. MSNA is a direct measure of vasoconstrictor neural activity to skeletal muscle, a vascular bed whose sheer size and makes it central to hemodynamic control both at rest and during daily activities. MSNA is also reproducible in a given subject over time.21 However, MSNA increases with age, such that measurements in a given individual increase after a period of several years21,22. As noted above, MSNA also correlates well with other markers of sympathetic neural activity at rest, at least in young men. Most importantly from our perspective, measurements of MSNA can be combined with other hemodynamic measurements to paint an overall picture of the relationship between sympathetic neural activity and blood pressure regulation. Additionally, because it has both rapid time resolution and is also stable within a given subject from day to day, MSNA can be used to address questions about both short and long term blood pressure regulation.
What is the role of the sympathetic nervous system in long term blood pressure regulation in young normotensive men?
In normotensive young men MSNA measured during rest can vary 5- to 10-fold, and there is substantial overlap in the range of MSNA values seen in normotensive and hypertensive subjects.3,5,13,23,24,25 This observation would seem to be a powerful argument against a major role for the sympathetic nervous system in long-term blood pressure regulation. For example, if MSNA can be very low in one individual, and very high in another an individual with a similar blood pressures, then how can MSNA be important to blood pressure regulation? This variability was also frustrating to those who developed the technique and hoped to use it to diagnose sympathetically driven hypertension.19 However, it has become clear in recent years that the striking inter-individual variability in MSNA provides mechanistic information about the role of the sympathetic nervous system in long term blood pressure regulation. In this context, why isn’t MSNA more directly related to blood pressure?
Our initial hypothesis was that in normotensive subjects with high levels of baseline MSNA, a relatively low value for cardiac output (CO) would offset the influence of higher sympathetic nerve activity and vascular resistance (i.e., MAP = CO x TPR) on blood pressure. To test this hypothesis, we conducted studies in healthy young men to understand how normotension is maintained in subjects with a range of sympathetic activities.5,6,7,8,9 Our main findings (summarized in Figure 1) are that in young men there is an inverse relationship between MSNA and cardiac output.
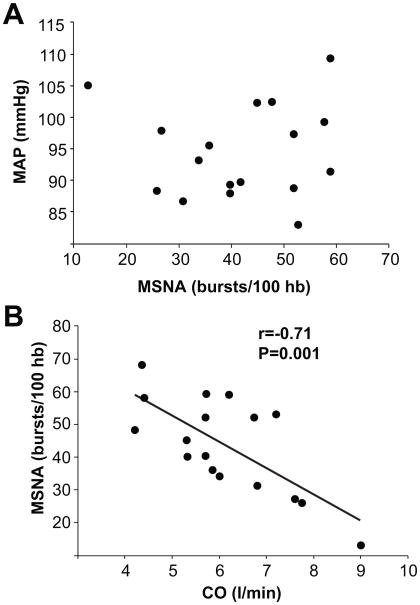
Figure 1.
Panel A demonstrates the relationship between muscle sympathetic nerve activity (MSNA) and mean arterial pressure in a group of young healthy males. Panel B demonstrates the reciprocal relationship between MSNA and cardiac output in the subjects. This relationship helps explain why blood pressure is not consistently higher in subjects with high levels of MSNA. (Figure from ref 5)
This means that subjects with high levels of MSNA have lower cardiac outputs and vice versa. At first we were surprised by the range of cardiac outputs we observed, but review of the literature indicated that the values we observed were similar to those found using invasive techniques by Julius and Conway in their classic paper from 1968.26
Importantly we also found that in young men total peripheral resistance (TPR) is highly correlated with resting MSNA.5 This observation is also consistent with studies in men showing that fall in blood pressure seen during ganglionic blockade with trimethaphan (so-called “autonomic support” of blood pressure) is proportional to resting MSNA and plasma NE concentrations.14
Next we demonstrated that the impact of high levels of MSNA on blood pressure in young men is blunted by reduced vasoconstrictor responsiveness to NE.7 Whether the inverse relationship between baseline MSNA and adrenergic sensitivity reflects receptor down regulation in response to high MSNA, or whether high levels of MSNA are a compensatory response to low number or responsiveness of post-junctional receptors is not known. If the former were responsible for the inverse relationship between MSNA and adrenergic sensitivity, this would suggest that baroreflex control of MSNA might be blunted in subjects with high levels of MSNA. In the latter case, it would suggest that the high levels of MSNA were an appropriate baroreflex-mediated response to an inherently lower level of adrenergic receptors.
Another aspect of our original hypothesis regarding inter-individual variability in MSNA was that the absence of a relationship between MSNA and blood pressure in young men was due to tonic NO release from the vascular endothelium. The idea was that NO release (and subsequent vasodilation) proportional to the level of sympathetic activity offsets the vasoconstriction caused by the MSNA. The best evidence for this came from studies showing plasma nitrate levels are correlated with baseline MSNA.27 To test this hypothesis, we performed systemic dose-response studies with the nitric oxide synthase inhibitor L-NMMA in normotensive volunteers. We found that the rise in BP was greater in subjects with high levels of baseline MSNA.7 This was especially marked with the lower doses of L-NMMA.
Interestingly, the hemodynamic mechanisms for the greater increase in blood pressure in the high MSNA group were related to cardiac output because total peripheral resistance responses to NOS inhibition were similar between high and low MSNA groups. The high MSNA group had a lower cardiac output to begin with and showed a smaller decline in CO during NOS inhibition. In this context, these data suggest that humans with high levels of baseline MSNA are at higher risk for the development of hypertension if they experience an even modest reduction in endothelial function. A major limitation to this interpretation is that systemic administration of L-NMMA, causes potentially confounding increase in blood pressure and a baroreflex mediated inhibition of MSNA.
An important final point is that the sources or cause of the inter-individual variability in MSNA are unknown. Identical twins demonstrate similar values suggesting a strong genetic component, but neither the physiological nor potential genetic mechanisms responsible for the inter-individual variability in MSNA have been identified.28
Does the role of the sympathetic nervous system in long term blood pressure regulation change in aging males in the absence of diseases and conditions known to affect blood pressure?
Whole body sympathetic neural activity increases with aging.9,14,21,22 This is reflected by increases in MSNA, whole body norepinephrine spillover, and increases in plasma norepinephrine levels.9,14,29 Additionally, indices of sympathetic activity, especially MSNA, become more linked to blood pressure as a person ages.25 In general, MSNA increases about one burst per minute per year, starting around age 30 (Figure 3). This means that MSNA in 60–70 year olds is roughly twice as high as it is in 20–30 year olds, but a wide range of MSNA is still seen in older subjects.
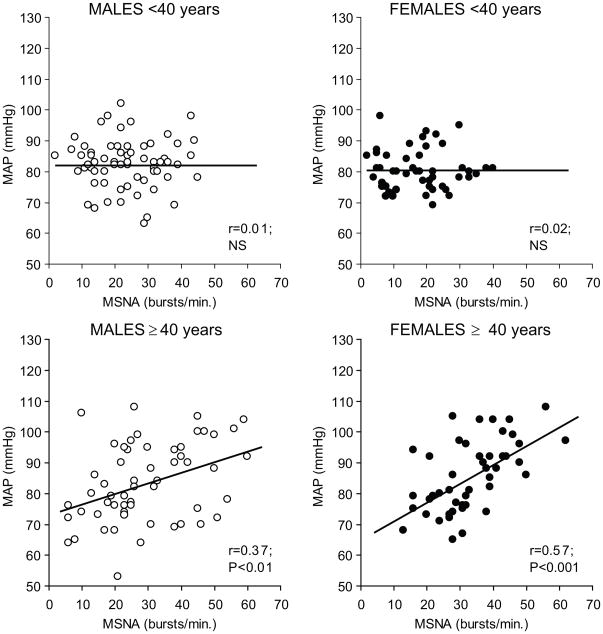
Figure 3.
Relationship between baseline MSNA and mean arterial pressure in a large group of young males, young females, older males and older females. In both men and women <40 no relationship between MSNA and mean arterial pressure was seen. By contrast, in the older men there was a modest relationship between MSNA and blood pressure. This positive relationship was more pronounced in the older women. (Figure from ref 25)
In healthy older subjects free of coexisting disease, there are several possible explanations for the rise in sympathetic activity with aging. First, there might be a loss of central inhibitory pathways in the brainstem. Central sympathetic disinhibition is clearly seen in animal models of diseases like congestive heart failure and but it is unclear if this occurs with healthy older humans without overt cardiovascular disease.30,31 However, there is evidence for increased central NE spillover in aging humans.32 Second, the large blood vessels become less distensible with aging and this might cause a given level of blood pressure to evoke fewer baroreflex mediated afferent signals and less reflex inhibition of sympathetic outflow.33,34 Third, aging (even in the absence of weight gain), is associated with increases in body fatness and there is evidence that visceral adiposity is associated with increased levels of sympathetic activity.35,36 This might be due to inflammatory mediators or substances released by visceral fat cells that stimulate sympathetic outflow at the level of the central nervous system.36 However, all of these potential mechanisms are speculative and there is at least some evidence for all of them in humans and animal models.
In spite of the increased sympathetic activity with aging, the relationship between MSNA and blood pressure remains modest in older men. One factor that limits the impact of the increased levels of sympathetic activity on blood pressure is age-related blunting of adrenergic sensitivity. In older men, there is clear evidence in the forearm and leg that vasoconstrictor responsiveness (sensitivity) to adrenergic stimulation is reduced and post-junctional alpha-2 sensitivity appears reduced more than alpha-1 sensitivity.38,39 Additionally, infusions of phenylephrine after ganglionic blockade (to block baroreflex buffering of blood pressure) cause blood pressure to rise about 50% less in older versus younger males.40
We attempted to study the individual relationships between MSNA, cardiac output and total peripheral resistance in healthy older males to determine if the relationships we saw in younger men were similar or altered in a systematic way with aging.9 In contrast to younger subjects, we could find no overall pattern of relationships between MSNA, cardiac output, vascular resistance and/or vascular adrenergic responsiveness in older men, indicating that the balances seen in younger men were absent in healthy men. However, it is interesting to note that autonomic support of blood pressure is greater in older males compared to younger males. While some of this is due to a lower intrinsic heart rate in older men, it also suggests that the increase in sympathetic activity is not completely offset by age-related reductions in adrenergic sensitivity.14 Another possible factor is that healthy older males have reduced blood volume which might lead to a larger fall in blood pressure after ganglionic blockade, and they can also have reduced endothelial function which might further modify the relationships between MSNA, cardiac output and TPR.41,42 Taking into account all available information, we speculate that if aging affects different mechanisms in different subjects, systematic relationships between and among factors are more difficult to detect.
Does sex influence the role of the sympathetic nervous system in long term blood pressure regulation, and are any sex differences modified by aging?
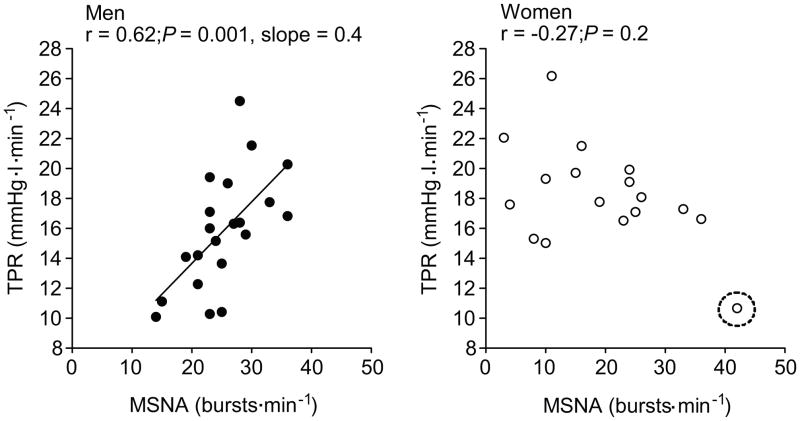
With our observations in men as a background, an important question was whether or not the relationships (or lack thereof) between MSNA, cardiac output, total peripheral resistance, and adrenergic sensitivity are the same or different in women. When we studied young healthy women we again found a wide range of MSNA, but there was no relationship between MSNA and blood pressure.8 There was also no relationship between MSNA and cardiac output and, most surprisingly, no relationship between MSNA and total peripheral resistance (Figure 4). This suggests that the physiological “strategy” used to regulate blood pressure in young women is fundamentally different from that in young men.
Figure 4.
Relationship between MSNA and total peripheral resistance (TPR) in a group of normotensive young male subjects (left panel) and a group of normotensive young female subjects (right panel). The relationship between MSNA and TPR seen in the young men was absent in the young women. (Figure from ref 8)
Additionally, the lack of relationship between MSNA and TPR in young women is consistent with observations suggesting that autonomic support of blood pressure is lower in young women than young men.43 Unfortunately, there are no clear data on the relationship between MSNA and autonomic support of blood pressure in young women.44 However, based on the range of values typically seen in young women, and assuming a relatively normal distribution of MSNA, it is reasonable to speculate that the inter-individual relationship between MSNA and autonomic support of blood pressure are blunted or absent in young women compared to young men.
What might explain the absence of a relationship between MSNA and total peripheral resistance in young women?8 One explanation is that postjunctional β2 receptors on the vascular smooth muscle and vascular endothelium in younger women are stimulated by norepinephrine and blunt the alpha-adrenergic vasoconstrictor effects of the sympathetic nerves. Indeed, Kneale et al showed that the vasoconstrictor responses to brachial artery infusions of norepinephrine are blunted in young women in comparison to young men.44 However, when norepinephrine dose-response curves were performed after β-adrenergic blockade with brachial artery administration of propranolol, vasoconstrictor responses to NE were augmented in the women and unchanged in the men. Thus, the forearm vasoconstrictor responses to NE were similar in men and women after propranolol. In this context, β2 vasodilation in the forearm has both an endothelial and non-endothelial component and about 30–40% of the vasodilator effects are due to endothelial release of nitric oxide.45
What is the situation in older women? As is the case for older men, MSNA rises with age and the relationship between MSNA and blood pressure becomes stronger with aging.25 Additionally, the strength of this relationship is greater in older women than older men (figure 4). However, there is little definitive data on why blood pressure is more strongly related to sympathetic activity in older women than men. This is important because it is well known that the incidence of hypertension rises after menopause in women.46 However, it is not known if autonomic support of blood pressure is greater in older women than younger women or in their male counterparts. Additionally, there are no data on adrenergic sensitivity in older women and there are no comprehensive measurements of cardiac output and MSNA in older women.
However, it is tempting to speculate that the loss of endothelial function seen post-menopause contributes to the more robust relationship between MSNA and blood pressure in older women.47 Additionally, if aging is associated with a loss of β2-mediated vasodilator function in women and these receptors normally blunt the relationship between MSNA and vascular resistance in young women, their loss could contribute to the positive relationship between MSNA and blood pressure in older women.
In summary, the relationship between MSNA and blood pressure differs in younger and older women and in comparison to their male counterparts. Understanding these differences may help explain why younger women are more prone to orthostatic intolerance and why older women are more subject to hypertension.
Are we entering an era of sympathetically driven clinical hypertension?
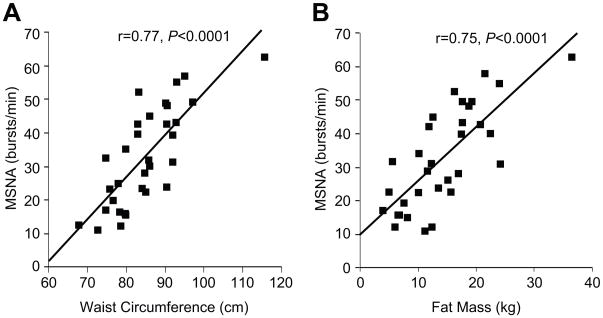
Obesity and weight gain are conditions associated with increased MSNA (figure 5).36,48,49,50 Obesity is also a major risk factor for Obstructive Sleep Apnea (OSA), and OSA appears associated with increases in both blood pressure and MSNA.51,52,53 Additionally, similar results are found when NE spillover techniques are used to assess sympathetic activity in these populations.59 These factors appear to add to and perhaps amplify any age-related increases in MSNA. They are also associated with increased vascular stiffness and reduced endothelial function which would limit baroreflex buffering of MSNA and NO-mediated buffering of vasoconstriction mediated by MSNA.1,34,42 This constellation of conditions, which is increasing in both developed countries and countries with emerging economies, is also associated with physical inactivity and/or metabolic disorders such as type II diabetes and changes in blood lipids. Importantly, these factors would tend to reinforce the changes in MSNA and vascular function highlighted above and lead to higher blood pressure.1 Additionally, there is evidence suggesting that blood pressure reactivity and chronic mental or social stress is related to the long term risk of hypertension in humans.54,55
Figure 5.
Relationship between waist circumference (left panel) or fat mass (right panel) and baseline MSNA in a large group of young and old male subjects. (Figure from ref 36)
There is also emerging evidence that obese humans with mild hypertension have increased autonomic support of blood pressure.56,57,58 This general idea is also supported by the NE spillover data reviewed by Esler and colleagues59 showing that sympathetic outflow to skeletal muscle and kidney is increased 2–3 fold in obese subjects. It is also supported by data from overfeeding studies in dogs showing that suppression of sympathetic neural activity in obese hypertensive animals by prolonged baroreflex stimulation lowers blood pressure, and similar data is emerging in humans with so-called resistant hypertension.60,61
There is also evidence that the increased sympathetic activity seen in resistant hypertension is related in part to the kidney because radiofrequency denervation of the kidney lowers blood pressure in patients with resistant hypertension.62 In this context, if MSNA reflects renal sympathetic nerve activity (RSNA) then increases in the latter may play a causal role in promoting hypertension by increasing sodium retention by the kidneys. While increases in RSNA would be expected to increase arterial pressure, this might not happen if there were reductions in renal adrenergic vascular responsiveness, increased renal NO production, or other offsetting mechanisms. However, the interactions between the sympathetic nervous system and kidney in the long term regulation of blood pressure in both normotensive and hypertensive humans remains unclear.63,64,65
Blood pressure increases with salt loading in rats are amplified by barodenervation arguing for a reinforcing interaction65 between increased sympathetic outflow and renal sodium retention. By contrast, renal denervation does not blunt the sustained reductions in arterial pressure caused by long term activation of baroreflexes.63,64 Clearly, the interactions and cross-talk between multiple redundant regulatory responses makes it especially challenging to design definitive experiments on this topic in humans. It is also possible that vascular beds that are hard to study with current approaches contribute to the relationship between sympathetic neural outflow and blood pressure. For example the splanchnic bed is a potential volume reservoir and long-term changes in vascular tone in this region could influence blood pressure.
Summary and Future Directions
We have attempted to summarize and highlight key elements of our recent thinking on the role of the sympathetic nervous system in long-term blood pressure regulation in humans. Our goal has been to use the marked inter-individual variability in MSNA seen in humans to begin to explore this topic. In normotensive young men MSNA is proportional to total peripheral resistance but the effects of this relationship on blood pressure are limited by a reciprocal relationship between MSNA and cardiac output and the fact that adrenergic sensitivity is blunted in subjects with high levels of MSNA. In young women these relationships are absent and there is some evidence that β2-adrenergic receptor mediated vasodilation limits the relationship between sympathetic activity and vascular resistance.
In older men the average level of MSNA is increased and modestly related to blood pressure, but there is still wide inter-individual variability in MSNA and no clear relationships between MSNA, cardiac output and vascular resistance. In older women the average level of MSNA is also increased and more strongly related to blood pressure. This suggests that any effect of reproductive hormones and β2- adrenergic receptor mediated vasodilation that limits the impact of high levels of MSNA on vascular resistance in young women is lost with aging. These potential age-related changes in women might explain the accelerated incidence of hypertension after menopause. However, the data on these relationships in women in general and older women in specific is limited, but clearly deserving of additional focus.
When we consider our findings and those of others in the context of emerging demographic trends for conditions like obesity, sleep apnea, physical inactivity and perhaps “social stress”; we propose that an era of sympathetically driven hypertension exacerbated by the factors discussed above is here.
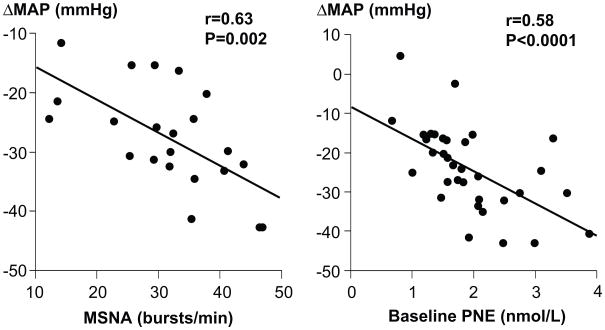
Figure 2.
Relationship between several indices of sympathetic neural activity and the fall in blood pressure during ganglionic blockade in a combined cohort of young and old males. The left panel shows that individuals with higher baseline levels of MSNA experience a larger fall in blood pressure during ganglionic blockade. The right panel is a similar comparison between the change in blood pressure and plasma norepinephrine (PNE). Together these data show that individuals with high levels of baseline sympathetic activity have increased autonomic support of their blood pressure. (Figure adapted from ref 14)
Acknowledgments
The authors would like to thank the many subjects who have participated in their studies and the superb technical support and nursing staff that made them possible. We would also like to thank John T. Shepherd who continues to have a profound influence on our thinking and laid the foundation for our collaboration many years ago.
Sources of Funding
Our work together on this topic has been supported by National Institutes of Health grantHL083947 and Swedish Medical Council grant 12170, NIH CTSA-UL-1-RR-024150, and the Caywood Professorship via the Mayo Foundation.
Footnotes
Disclosures
None
References
- 1.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- 3.Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009;84:822–830. doi: 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowley AW, Jr, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- 5.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz N, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009a;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 11.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 14.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- 15.Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG. Inhibition of human muscle sympathetic activity by sensory stimulation. J Physiol. 2002;544:285–292. doi: 10.1113/jphysiol.2002.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donadio V, Karlsson T, Elam M, Wallin BG. Interindividual differences in sympathetic and effector responses to arousal in humans. J Physiol. 2002;544:293–302. doi: 10.1113/jphysiol.2002.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol. 2009;296:H847–H853. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol. 1997;504:211–220. doi: 10.1111/j.1469-7793.1997.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallbo AB, Hagbarth K-E, Wallin BG. Microneurography: how the technique developed and it role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 20.Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympathoexcitation in healthy humans. J Physiol. 2009;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 22.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson EA, Kinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1998;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 26.Julius S, Conway J. Hemodynamic studies in patients with borderline blood pressure elevation. Circulation. 1968;38:282–288. doi: 10.1161/01.cir.38.2.282. [DOI] [PubMed] [Google Scholar]
- 27.Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol. 1997;501:671–675. doi: 10.1111/j.1469-7793.1997.671bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallin BG, Kunimoto MM, Sellgren J. Possible genetic influence on the strength of human muscle nerve sympathetic activity at rest. Hypertension. 1993;22:282–284. doi: 10.1161/01.hyp.22.3.282. [DOI] [PubMed] [Google Scholar]
- 29.Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol Regul Integr Comp Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 30.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Liu XF, Cornish KG, Zucker IH, Patel KP. Effects of nNOS antisense in the paraventricular nucleus on blood pressure and heart rate in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H205–H213. doi: 10.1152/ajpheart.00497.2004. [DOI] [PubMed] [Google Scholar]
- 32.Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–R916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- 33.Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol. 1998;275:H1768–H1772. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- 34.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 35.Jones PP, Davy KP, Alexander S, Seals DR. Age-related increase in muscle sympathetic nerve activity is associated with abdominal adiposity. Am J Physiol. 1997;272:E976–E980. doi: 10.1152/ajpendo.1997.272.6.E976. [DOI] [PubMed] [Google Scholar]
- 36.Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- 37.Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neuorsci Biobehav Rev. 2009;33:89–94. doi: 10.1016/j.neubiorev.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- 39.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 41.Davy KP, Seals DR. Total blood volume in healthy young and older men. J Appl Physiol. 1994;76:2059–2062. doi: 10.1152/jappl.1994.76.5.2059. [DOI] [PubMed] [Google Scholar]
- 42.Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:746–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- 43.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 44.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 45.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- 46.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 47.Gilligan DM, Badar DM, Panza JA, Quyyumni AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 49.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M, Lambert G. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens. 2007;25:1411–1419. doi: 10.1097/HJH.0b013e3281053af4. [DOI] [PubMed] [Google Scholar]
- 50.Gentile CL, Orr JS, Davy BM, Davy KP. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1834–R1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 51.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 52.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 53.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 54.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA Study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 55.Granado NS, Smith TC, Swanson GM, Harris RB, Shahar E, Smith B, Boyko EJ, Wells TS, Ryan AK for the Millennium Cohort Study Team. Newly reported hypertension after military combat deployment in a large population-based study. Hypertension. 2009;54:966–973. doi: 10.1161/HYPERTENSIONAHA.109.132555. [DOI] [PubMed] [Google Scholar]
- 56.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 57.Grassi G, Seravalle G, Dell’Oro R, Arenare F, Facchetti R, Mancia G. Reproducibility patterns f plasma norepinephrine and muscle sympathetic nerve traffic in human obesity. Nutr Metab Cardiovasc Dis. 2009b;19:469–475. doi: 10.1016/j.numecd.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 59.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 60.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007a;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 61.Mohaupt MG, Schmidli J, Luft FC. Management of uncontrollable hypertension with a carotid sinus stimulation device. Hypertension. 2007;5:825–828. doi: 10.1161/HYPERTENSIONAHA.107.099416. [DOI] [PubMed] [Google Scholar]
- 62.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 63.Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007b;49:373–379. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- 64.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 65.Osborn JW, Hornfeldt BJ. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am J Physiol Heart Circ Physiol. 1998;275:H1558–H1566. doi: 10.1152/ajpheart.1998.275.5.H1558. [DOI] [PubMed] [Google Scholar]