Abstract
We tested the hypothesis that risk of early mortality from cancers of the digestive system will be greater in men with, compared to men without, the metabolic syndrome (MetS). Participants were 33,230 men who were seen at the Cooper Clinic in Dallas, Texas and followed for 14.4 (SD=7.0) yrs. MetS was defined as having at least three of the following risk factors: abdominal obesity, fasting hypertriglyceridemia, low high-density lipoprotein cholesterol, high blood pressure, or high fasting glucose level or diabetes. MetS was associated with higher mortality (HR=1.90 [95% Confidence Interval=1.42-2.55]), and there was a graded positive association for the addition of more syndrome components (p < 0.01). Adjustment for cardiorespiratory fitness attenuated the risk estimates by 20 to 30%, but they remained significant following this adjustment. Evaluation of the independent contribution of each of the syndrome components revealed that both abdominal obesity (HR=1.89 [1.36-2.62]) and high glucose (HR=1.38 [1.02-1.87]) were independently associated with cancer mortality. Our results support the hypothesis that MetS is positively associated with mortality from cancers of the digestive system. Interventions which reduce abnormalities associated with the syndrome could reduce risk of premature death from these cancers.
1. Introduction
Cancers of the digestive system will result in more than 270,000 deaths in the United States in 2007, and this represents nearly 25% of all deaths from cancer in this period [1]. Cancers of the digestive system include those of the alimentary canal below the neck (e.g., esophagus, stomach, small and large intestines) and key digestive organs (i.e., pancreas, liver, gallbladder). While the five-year survival rates are nearly 90% or greater for breast and prostate cancer, the survival rate for digestive cancers as a group is only 45% [2]. Therefore, identification of modifiable risk factors for these more lethal cancers may provide important opportunities for reducing overall cancer mortality.
The metabolic syndrome (MetS) is a condition that has been defined as a clustering of at least three of five cardiovascular and diabetes risk factors, including: abdominal obesity, elevated fasting blood glucose, elevated blood pressure, hypertriglyceridemia, and low levels of high density lipoprotein (HDL) [3]. The prevalence of MetS in the United States is approximately 24% for all adults but is greater than 40% for those over 60 yrs of age [4], the age group in which the majority of cancers develop. The prevalence of MetS for those with diabetes is greater than 60% [5].
MetS represents the development of central adiposity and abnormalities in carbohydrate and lipid metabolism as a consequence of genetic predisposition coupled with sedentary lifestyles and poor dietary habits [3, 6]. Hallmark features associated with MetS are hyperinsulinemia and low-level, chronic inflammation [6], both of which are believed to play an important role in the development and growth of invasive cancers [7]. Indeed, recent investigations have indicated a positive association between clusters of the MetS components and adenomatous polyps [8], and incident [9-11] and fatal colon cancer outcomes [12, 13]. In addition, positive associations between pancreatic cancer and elements of the syndrome, including abdominal obesity [14, 15], hyperinsulinemia [16], and elevated glucose [16, 17] also have been reported.
There is substantial evidence that clusters of the MetS components are positively associated with risk for early mortality [18, 19], cardiovascular disease [18, 20-22], and diabetes [21, 23]. However, fewer studies have estimated risk associated with cancer outcomes using standardized definitions of MetS that are now frequently employed in clinical practice. For this reason, our current understanding of the degree to which MetS is associated with the risk of cancer outcomes remains incomplete.
Accordingly, the purpose of this investigation was to: (1) test the hypothesis that risk of early mortality from cancers of the digestive system will be greater in those with, compared to those without, MetS; and (2) to estimate risk associated with a higher number of MetS components on mortality from these cancers. Results from this study provide important translational insight into risk for cancer mortality relative to commonly measured clinical metabolic risk factors.
2. Methods
2.1. Study Population
Participants were 33,230 men aged 20-88 years who were free of known cancer, who had a baseline preventive medical examination at the Cooper Clinic, Dallas, TX between 1977 and 2003, and are enrolled in the Aerobics Center Longitudinal Study (ACLS). Study participants came to the clinic for periodic preventive health examinations and for counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Many participants were sent by their employers for the examination. Some were referred by their personal physicians. Others were self-referred. All participants in the current analysis had complete measures for each MetS component. Participants were followed from the date of their baseline examination until their date of death or until time of censoring for the current analyses, December 31, 2003. Person-years of exposure were computed as the sum of follow-up time among decedents and survivors.
2.2. Baseline Examination
Clinical examinations were performed after receiving written informed consent from each participant. These included fasting blood chemistry analyses, personal and family health history, anthropometry, resting blood pressure and electrocardiogram, and a maximal graded exercise test. Examination methods and procedures followed a standard manual of operations and have been previously described [24, 25]. Briefly, body mass index (BMI = weight[kg]/height[m]2) was computed from measured height and weight. Waist circumference was measured at the umbilicus. Resting blood pressure was recorded as the first and fifth Korotkof sounds by auscultatory methods. Serum samples were analyzed for triglycerides, HDL cholesterol, and glucose using standardized automated bioassays that met Centers for Disease Control and Prevention standards. Information on smoking habits (current smoker or not), alcohol intake (drinks per week), personal history of hypertension and diabetes, and family history of diseases (cancer, cardiovascular disease, hypertension, and diabetes) was obtained from a standardized questionnaire.
Cardiorespiratory fitness (CRF) was assessed at the baseline examination as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol [24, 26]. The duration of the test on this protocol is highly correlated with directly measured maximal oxygen uptake in men (r > 0.90) [27], an accepted measure of CRF. We used our previously published age-specific distribution of treadmill duration from the overall ACLS population to define fitness as not fit (lowest 20%), and fit (upper 80%) in order to maintain consistency in the study methods, and because we have found that not fit, defined in this way, is an independent predictor of mortality [25, 28] and morbidity [29]. The respective cut points for the fitness classifications in the not fit and fit groups have been described in detail previously [29].
2.3. Metabolic Syndrome Definition
Participants were classified as having MetS using criteria of the National Cholesterol Education Program Adult Treatment Panel III, and were based on the presence of 3 or more of the risk factors [6, 30]: 1) abdominal obesity (waist girth > 102 cm); 2) fasting hypertriglyceridemia (≥ 150 mg/dL); 3) low HDL cholesterol (<40 mg/dL); 4) high blood pressure (≥130/85 mmHg); and 5) high fasting glucose (≥110 mg/dL). History of physician-diagnosed hypertension and diabetes also were employed to identify individuals with an abnormal blood pressure and glucose level, respectively, as done in other epidemiological studies [9, 10], and in our previous report [30].
2.4. Assessment of outcomes
The National Death Index (NDI) was the primary data source for mortality surveillance, and vital status was determined for more than 95% of the cohort in this study period. The underlying cause of death was determined from the NDI report or by a nosologist’s review of official death certificates obtained from the department of vital records in the decedent’s state of residence. The NDI has been found to provide cancer mortality data that are of similar accuracy to those determined by an endpoints review committee, which reviews both death certificates and relevant medical records to make their determination. Sesso and colleagues [31] compared cause of death determined by an Endpoints Committee to those from NDI in the Physicians Health Study for deaths occurring between 1982 and 1998. For NDI, the sensitivity for cancer mortality was 89% and the specificity was 100%. Causes of cancer death were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes for deaths occurring before 1999 and Tenth Revision (ICD-10) codes (in parentheses) for deaths during 1999-2003: Our primary outcome for this analysis was death from digestive and gastrointestinal cancers, 150-159 (C15-C26); and our secondary mortality outcomes were: Colon, 153 (C18); Rectum,154 (C19-C21); Pancreas, 157 (C25); Esophagus, 150 (C15); Stomach, 151 (C16); and Liver, 155 (C22). Although the classification and rule changes between ICD-10 and ICD-9 have resulted in the shifting of deaths away from some underlying cause-of-death categories into others, the number of deaths due to cancer remained stable across revisions [32]. The study protocol was approved annually by the Institutional Review Board of the Cooper Institute.
2.5. Statistical Analysis
Characteristics of the cohort at baseline were compared by MetS status using t-tests and χ2 tests. Kaplan-Meier survival curves were generated for MetS and according to the number of prevalent MetS risk factors. Cox proportional hazard modeling was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CI), as is the convention in portraying results from epidemiologic studies such as this [33]. Tests for trend of HRs across number of MetS components were obtained by modeling the categories as continuous, ordinal variables. Three types of Cox models were fit. First, we estimated the associations adjusting only for baseline age (years). Second, we adjusted for baseline age (years), examination year (calendar year), height (inches), current smoker (yes/no), alcohol intake (≥5 drinks/wk or not), and family history of cancer (Model 1). Third, we adjusted for the covariates in Model 1, and further adjusted for cardiorespiratory fitness (treadmill test duration [min]; Model 2). Cumulative hazard plots grouped by exposure suggested no appreciable violations of the proportional hazards assumption. Because of the known correlation between BMI and several of the MetS components, we elected not to include it as a covariate in our models, but we do describe the effect of BMI on risk in our joint analyses, described below. We estimated the joint effects of MetS exposure across categories of age (< 50, ≥ 50 yrs), BMI (< 25, 25-29.9, ≥ 30 kg/m2), and CRF (fit, not fit) using a common referent group at low risk (e.g., no MetS and < 50 yrs). In addition, we tested for multiplicative interaction between MetS (yes, no) and age, BMI, and fitness (all in continuous form) by fitting each of these terms and their cross-product, along with the covariates in Model 1. We also completed our primary analyses after excluding deaths that occurred in the first five years of follow-up and after excluding men with prevalent cardiovascular disease at baseline. Similar patterns of association were observed in these analyses. Statistical analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC) software. All p values were obtained from two-sided hypothesis tests.
3. Results
At baseline, the prevalence of MetS was 27.9% (3+ components). The characteristics of participants with and without the syndrome are shown in Table 1. Compared with individuals without MetS, those with the syndrome were on average, older, less fit, had higher BMIs, drank less alcohol, and had more family history of disease, including cancer. Men with MetS were also taller and more likely to have smoked at study baseline. The mean values of each of the MetS components and the prevalence of meeting the criteria for each are provided at the bottom of Table 1.
Table 1.
Baseline characteristics according to metabolic syndrome* status
| Metabolic Syndrome† |
||
|---|---|---|
| No | Yes | |
| n | 23,962 | 9,268 |
| Age (yrs) | 44.0 ± 10.0 | 47.2 ± 9.8 |
| Fitness (Treadmill time (min)) | 19.5 ± 4.9 | 14.7 ± 4.4 |
| Height (cm) | 178.8 ± 6.5 | 179.3 ± 8.2 |
| Body Mass Index (kg/m2) | 25.4 ± 2.9 | 29.6 ± 4.5 |
| Current smoker (%) | 15.9 | 20.4 |
| ≥ 5 Alcohol drinks per week (%) | 36.9 | 30.7 |
| Family history (%) | ||
| Hypertension | 16.4 | 22.6 |
| Diabetes | 6.4 | 10.6 |
| Cardiovascular disease | 27.8 | 32.8 |
| Cancer | 23.6 | 31.0 |
| Metabolic syndrome Components | ||
| Abdominal Obesity | ||
| Waist Circumference (cm) | 90.5 ± 8.6 | 103.1 ± 12.0 |
| Abdominal Obesity (%)† | 7.2 | 55.9 |
| Blood Pressure | ||
| Systolic (mmHg) | 119 ± 13 | 128 ± 14 |
| Diastolic (mmHg) | 79 ± 9 | 86 ± 10 |
| High blood pressure (%)‡ | 32.1 | 76.8 |
| Blood Lipids | ||
| HDL (mmol/L) | 1.3 ± 0.3 | 1.0 ± 0.2 |
| Low HDL (%)§ | 19.7 | 70.3 |
| Triglycerides (mmol/L) | 1.2 ± 0.7 | 2.5 ± 2.0 |
| High triglycerides (%)¶ | 13.6 | 75.7 |
| Fasting Blood Glucose | ||
| Glucose level (mmol/L) | 5.4 ± 0.7 | 6.1 ± 1.4 |
| High glucose (%)║ | 33.6 | 76.9 |
HDL- C= high-density lipoprotein cholesterol
Data shown as mean±SD unless specified otherwise
No defined as 0 to 2 components, and Yes defined as the presence of ≥3 of the 5 components
Abdominal obesity was defined as waist girth ≥102 cm [40 in] in men
High blood pressure was defined as systolic blood pressure≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or history of physician-diagnosed hypertension
Low HDL-C was defined as HDL <1.04 mmol/L (40 mg/dL) in men
High triglycerides was defined as tiglycerides ≥1.69 mmol/L (150 mg/dL)
High glucose was defined as glucose≥6.1 mmol/L (110 mg/dL) or history of physician-diagnosed diabetes
Over an average of 14.4 (SD=7.0) yrs of follow-up, 478,512 person-years of exposure accrued and 188 digestive and gastrointestinal cancer deaths were identified. The distribution of the deaths for specific cancer types was as follows; esophageal (n=24), stomach (n=22), small intestine (n=2), colon (n=47), rectum/anus (n=10), liver (n=18), gall bladder (n=7), pancreas (n=56), and other (n=2).
Kaplan-Meier plots for overall MetS (0-2 vs. 3+ components) and according to the number of prevalent syndrome components are displayed in Figure 1. The presence of MetS was associated with earlier mortality, as was an increase in the incremental number of syndrome components (Figure 1; p < 0.01).
Fig. 1.
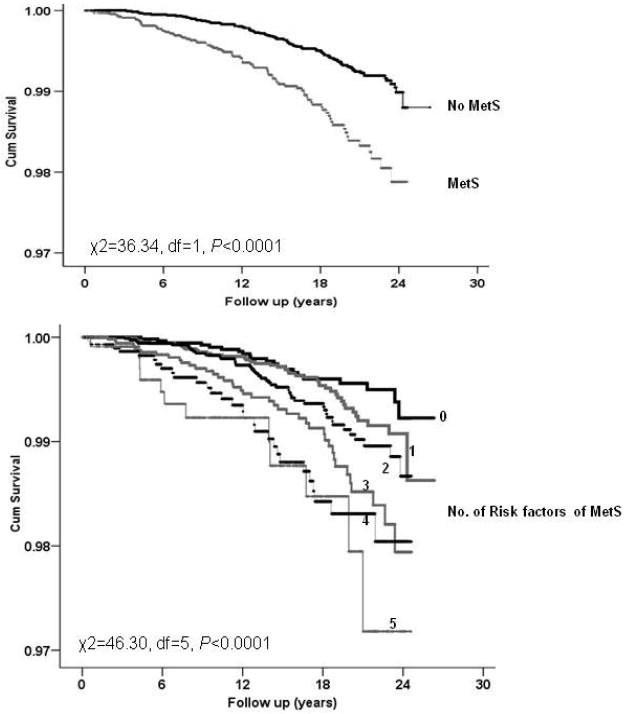
Kaplan-Meir plots of survival for cancers of the digestive system in men, the Aerobics Center Longitudinal Study (1977 to 2003). MetS=metabolic syndrome
In multivariable models, the presence of MetS (0-2 vs. 3+ components) was associated with about two-fold higher mortality from digestive cancers (HR=1.90 [1.42-2.55]), and there was a graded increase in risk associated with a greater number of prevalent MetS components (P trend < 0.01; Table 2). Compared to men with 0 components, those with three or more components were at more than twice the risk (HR=2.31 [1.42-3.76]), while men with all five components were at a three-fold higher risk (HR=3.07 [1.49-6.30]) for cancer death. Interestingly, further adjustment of Model 1 for fitness weakened these associations by 20 to 30%; but MetS remained significantly associated with cancer mortality. Additional adjustment for prevalent cardiovascular disease at baseline (myocardial infarction or stroke) did not alter the magnitude of the associations observed (data not shown).
Table 2.
Associations between metabolic syndrome, the number of metabolic components, and mortality for cancers of the digestive system, the Aerobics Center Longitudinal Study (1977 to 2003)
| Age-adjusted model |
Multivariable model 1† |
Multivariable model 2‡ |
|||
|---|---|---|---|---|---|
| N | Deaths | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Metabolic syndrome* | |||||
| No | 23962 | 106 | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) |
| Yes | 9268 | 82 | 1.94 (1.45-2.59) | 1.90 (1.42-2.55) | 1.52(1.11-2.09) |
| No. of Metabolic Syndrome Components | |||||
| 0 | 6484 | 21 | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) |
| 1 | 9489 | 41 | 1.19 (0.70-2.01) | 1.18 (0.70-2.00) | 1.11 (0.65-1.88) |
| 2 | 7989 | 44 | 1.40 (0.83-2.37) | 1.40 (0.83-2.36) | 1.22 (0.72-2.07) |
| 3 | 5214 | 41 | 2.11 (1.24-3.59) | 2.07 (1.22-3.52) | 1.62 (0.94-2.81) |
| 4 | 2952 | 29 | 2.56 (1.45-4.51) | 2.50 (1.42-4.41) | 1.82 (1.00-3.31) |
| 5 | 1102 | 12 | 3.12 (1.52-6.37) | 3.07 (1.49-6.30) | 2.05 (0.96-4.38) |
| P for linear tend | <0.0001 | <0.0001 | 0.007 | ||
HR, hazard ratios; CI, confidence interval
No defined as 0 to 2 components, and Yes defined as the presence of ≥3 of the 5 metabolic syndrome components
Adjusted for age, examination year, height, current smoking, alcohol intake, and family history of cancer
Adjusted for age, examination year, height, current smoking, alcohol intake, family history of cancer, and treadmill test duration
We also evaluated risk for overall mortality from cancers of the digestive system associated with the individual MetS components (Table 3). We found positive associations with abdominal obesity (HR=2.15 [1.58-2.92), high triglycerides (HR=1.41 [1.05-1.90]), low HDL (HR=1.45 [1.08-1.93]), and high glucose (HR=1.54 [1.15-2.07]), but not high blood pressure (HR=1.17 [0.87-1.57]), after adjustment for covariates in Model 1 (Table 3). Adjustment for additional MetS components revealed that only abdominal obesity (HR=1.89 [1.36-2.62]) and high glucose levels (HR=1.38 [1.02-1.87]) remained independently associated with mortality. Further adjustment for cardiorespiratory fitness with other covariates and additional MetS components attenuated the association with abdominal obesity somewhat (HR=1.56 [1.09-2.21]), but not the association with high glucose (HR=1.36 [1.00-1.83]).
Table 3.
Independent effects of metabolic syndrome components on risk for mortality from cancers of the digestive system, the Aerobics Center Longitudinal Study (1977 to 2003).
| Age-adjusted |
Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|
| N | Deaths | HR (95% CI) | HR (95% CI)† | HR (95% CI)‡ | |
| Abdominal obesity | 6915 | 63 | 2.16 (1.59-2.92) | 2.15 (1.58-2.92) | 1.89 (1.36-2.62) |
| High blood pressure | 14808 | 97 | 1.17 (0.87-1.56) | 1.17 (0.87-1.57) | 1.00 (0.74-1.35) |
| High triglycerides | 10284 | 73 | 1.43 (1.07-1.92) | 1.41 (1.05-1.90) | 1.08 (0.77-1.50) |
| Low HDL-C | 11245 | 84 | 1.50 (1.12-1.99) | 1.45 (1.08-1.93) | 1.24 (0.90-1.71) |
| High glucose | 15175 | 111 | 1.49 (1.11-2.01) | 1.54 (1.15-2.07) | 1.38 (1.02-1.87) |
HR, hazard ratios; CI, confidence interval
Model 1: Adjusted for age, examination year, height, current smoking, alcohol intake, and family history of cancer
Model 2: Adjusted for age, examination year, height, current smoking, alcohol intake, family history of cancer, and each of the other metabolic syndrome components
We next described the associations between MetS and site-specific cancer mortality for which there were 15 or more deaths. MetS was significantly associated with higher risk for colon, colorectal, esophageal, and liver cancer deaths, but not pancreatic or stomach cancer (Table 4). Further adjustment of Model 1 covariates for fitness attenuated the associations by about 10 to 20%, and following this adjustment, only colorectal and esophageal cancer remained significantly associated with the presence of MetS.
Table 4.
Associations between metabolic syndrome (MetS) (0-2 vs. 3+ components) and site-specific cancers of the digestive system in men, the Aerobics Center Longitudinal Study (1977 to 2003)
| Age-adjusted |
Model 1 |
Model 2 |
||
|---|---|---|---|---|
| Outcomes (anatomic site) | Deaths by MetS Category (Yes/No)* | HR (95% CI) | HR (95% CI)† | HR (95% CI)‡ |
| Colon | 20/27 | 1.72 (0.97-3.08) | 1.73 (0.97-3.11) | 1.50 (0.80-2.83) |
| Colorectal | 27/30 | 2.15 (1.27-3.62) | 2.14 (1.26-3.62) | 1.71 (0.97-3.02) |
| Pancreas | 21/35 | 1.54 (0.89-2.65) | 1.52 (0.88-2.64) | 1.38 (0.76-2.49) |
| Esophagus | 13/11 | 3.15 (1.40-7.10) | 3.02 (1.34-6.83) | 3.23 (1.32-7.90) |
| Stomach | 5/17 | 0.74 (0.27-2.01) | 0.69 (0.25-1.90) | 0.45 (0.16-1.30) |
| Liver | 10/8 | 3.25 (1.27-8.29) | 3.05 (1.19-7.84) | 1.62 (0.59-4.41) |
HR, hazard ratios; CI, confidence interval.
No defined as 0 to 2 components, and Yes defined as the presence of ≥3 of the 5 metabolic syndrome components
Adjusted for age, examination year, height, current smoking, alcohol intake, and family history of cancer.
Adjusted for age, examination year, height, current smoking, alcohol intake, family history of cancer, and treadmill test duration.
Finally, we described the joint associations between the presence of MetS and other risk factors for digestive cancer mortality. For this analysis, the referent group consisted of men who did not have MetS and were at lowest risk for the other factor (Table 5). Among men without MetS, age was associated with increased risk (HR=3.48 [2.37-5.13]; < 50 vs. ≥ 50 yrs), as was obesity (HR=2.02 [0.95-4.27]; < 25 vs. ≥ 30 kg/m2), and low fitness levels (HR=2.03 [1.18-3.47]; vs. fit). Older men (≥ 50 yrs) were at greater risk if they had MetS (HR=7.55 [5.14-11.08]). Interestingly, among normal weight men (BMI < 25), MetS was not associated with increased risk (HR=0.93 [0.40-2.18]), but it did increase risk among overweight (BMI 25-29 kg/m2: HR=1.95 [1.28-2.95]) men, and further still among obese men (BMI ≥ 30 kg/m2: HR=2.92 [1.88-4.52]) as compared with men who were normal weight and without MetS (Table 5). Compared with men who were fit and without MetS, mortality risk was higher among those with MetS who were more fit (HR=1.78 [1.26-2.51]) and in those who were unfit (HR=2.81 [1.86-4.26]). No tests for multiplicative interactions among our joint effects reached statistical significance (all p > 0.05).
Table 5.
Joint associations between metabolic syndrome (0-2 vs. 3+ components), age, body mass index, smoking, and fitness, and mortality from cancers of the digestive system in men, the Aerobics Center Longitudinal Study (1977 to 2003)
| Metabolic Syndrome* |
||||
|---|---|---|---|---|
| No | Yes | |||
| N | Deaths | HR (95% CI) | ||
| Age (years) † | ||||
| <50 | 22,959 | 70 | 1.00 | 1.62 (0.97-2.68) |
| ≥50 | 10,271 | 118 | 3.48 (2.37-5.13) | 7.55 (5.14-11.08) |
| Body mass index (kg/m2)** | ||||
| <25 | 12,458 | 57 | 1.00 | 0.93 (0.40-2.18) |
| 25-29 | 15,580 | 88 | 1.11 (0.75-1.65) | 1.95 (1.28-2.95) |
| ≥30 | 5,192 | 43 | 2.02 (0.95-4.27) | 2.92 (1.88-4.52) |
| Current Smoking§ | ||||
| No | 27,530 | 150 | 1.00 | 1.88 (1.36-2.61) |
| Yes | 5,700 | 38 | 1.26 (0.77-2.08) | 2.50 (1.52-4.11) |
| Cardiorespiratory fitness‡ | ||||
| Fit | 28,625 | 141 | 1.00 | 1.78 (1.26-2.51) |
| Not fit | 4,605 | 47 | 2.03 (1.18-3.47) | 2.81 (1.86-4.26) |
HR, hazard ratios; CI, confidence interval.
No defined as 0 to 2 components, and Yes defined as the presence of ≥3 of the 5 metabolic syndrome components
Adjusted for age, examination year, height, current smoking, alcohol intake, and family history of disease.
Adjusted for examination year, height, current smoking, alcohol intake, and family history of disease.
Adjusted for age, examination year, height, current smoking, alcohol intake, and family history of disease.
Adjusted for age, examination year, height, alcohol intake, and family history of disease.
4. Discussion
In this prospective study with an average of 14.4 (SD=7.0) years of follow-up, we found that the presence of MetS at baseline was associated with an approximate two-fold higher in risk for mortality from cancers of the digestive system among men (HR=1.90 [1.42-2.55]). We also found that MetS was associated with increased mortality risk for a number of different cancers of the digestive system, including colorectal, esophageal, and liver cancer. Central obesity and elevated fasting glucose levels were independently associated with mortality, but risk was also increased with ach additional component present, and men with all five components were at more than a three-fold higher risk (HR=3.07 [1.49-6.03]), compared to men with no components. Evaluation of the joint effects of other putative risk factors indicated that risk associated with MetS was particularly elevated for men that were over 50 yrs of age, overweight and obese, smoked, or who had low fitness levels. These findings indicate that the presence of MetS is positively associated with mortality from cancers of the digestive system among overweight and obese men, and suggest that interventions which favorably affect adiposity and other abnormalities associated with the syndrome may reduce risk of premature death from these cancers. Given that cancers of the digestive system account for nearly 25% of all cancer deaths, we believe that such interventions may represent an important opportunity for reducing overall cancer mortality.
Our findings are consistent with those from a number of recent studies that have reported positive associations between the presence of the MetS, or a clustering of its components, and adenomatous polyps [8], incident colon cancer [10, 11], and mortality from colorectal cancer [12, 13]. Fewer studies have explicitly examined associations between clustered risk factors and other kinds of digestive cancer. Notwithstanding, there is now evidence of positive associations between obesity and adenocarcinoma of the esophagus [34, 35], pancreatic [14, 15, 36], and liver cancer [37]. In particular, abdominal obesity has been linked to risk for pancreatic cancer in at least two prospective studies [14, 15]. In terms of metabolic risk factors, insulin and glucose levels have been positively associated with pancreatic cancer [16, 17], and the presence of diabetes is associated with hepatocellular carcinoma, a common form of liver cancer [37]. Our results for stomach cancer are inconsistent with that of Calle and colleagues [38], who reported a positive association between BMI and mortality from stomach cancer. The reason for this discrepancy is not immediately clear, but it may be that our cases of stomach cancer mortality were a mixture of proximal (cardia) and distal cancers, the latter of which is believed to be more strongly linked to heliobacter pylori infection than obesity [39].
It is notable that we found associations between MetS and a number of cancers of the digestive system. While each type of cancer may have a unique natural history (i.e., susceptibility genes, metabolic pathways, and environmental risk factors), several different biological mechanisms have been proposed which may explain our findings. Colorectal cancer is among the most heavily investigated digestive cancer and there are links between this outcome and central obesity [40-42] and insulin and the IGF-1 axis [43]. Similarly, pancreatic cancer also has been associated with these factors. Both esophageal and liver cancer, for which the incidence rates have increased in the last 20 years [37], also have been linked to obesity; and it has been suggested that the obesity epidemic could be partly responsible for the parallel increase in the incidence of these cancers. While it has been proposed that obesity influences risk for adenocarcinoma of the esophagus by increasing risk for gastroesophageal reflux disease, which can cause tissue damage, Barrett’s esophagus, and ultimately invasive disease [34, 44], recent reports have highlighted a potential role for abdominal obesity [44], as well as alternate mechanisms[45, 46]. Hepatocellular carcinoma accounts for a large proportion of all liver cancers, and liver damage from infection is thought to be an early event in the development of these cancers. Increasingly, non-alcoholic fatty liver disease, which is linked to obesity, also appears to contribute to liver damage, cirrhosis, and to invasive cancer [37, 47]. Our results suggest that the clustered obesity and metabolic abnormalities associated with the syndrome are adversely associated with both esophageal and liver cancer (Table 4).
The present study extends our understanding of risks associated with mortality from cancer of the digestive system in at least three important ways. First, we found that the information obtained from routinely measured cardiovascular and diabetes metabolic risk factors (i.e., MetS components) were associated prospectively with risk for cancer mortality. Thus, our results provide a direct translation of risk associated with clinically defined MetS to death from cancers of the digestive system. While we recognize the controversies surrounding definitions for MetS, and the intended use of these definitions [3, 48, 49], we believe our results provide additional insights for other clinical outcomes, beyond cardiovascular and diabetes, that are associated with these clustered risk factors. Second, we observed associations between MetS and several different cancers of the digestive system. Other recent reports have linked similar exposures to breast cancer [9] and together with our findings which suggests the potential for a more generalized effect of these metabolic risk factors on cancer outcomes. Third, our unique ability to evaluate the influence of cardiorespiratory fitness on these associations, both in multivariate modeling and joint effects analyses, provides evidence for the hypothesis that lifestyle interventions that can increase fitness and improve metabolic risk factors may help reduce risk for early mortality from digestive cancers. Results from our multivariate models revealed that inclusion of cardiorespiratory fitness in the models attenuated risk associated with MetS. For example, the presence of the MetS was associated with a 2-fold higher risk (HR=1.90 [1.42-2.55]) before further adjustment for fitness, but after adjustment, risk was reduced by about 20% (HR=1.52 [1.11-2.09]). This finding is generally consistent with our previous finding that a high level of fitness also attenuated risk for all-cause and cardiovascular mortality associated the MetS [19]. While inclusion of fitness in the models did not completely attenuate the association in this study, the magnitude of the associations were reduced considerably, which suggests an important role for physical activity and fitness in the causal pathway between MetS and mortality from digestive cancers. LaMonte and colleagues [30] and others [50] have reported that high fitness levels reduce risk of developing MetS in prospective studies, and there is a wealth of evidence indicating that increased physical activity levels can increase cardiorespiratory fitness (e.g. [51]) , and improve the metabolic profile of high-risk individuals [52]. Collectively, we believe these findings suggest that interventions designed to increase cardiorespiratory and metabolic fitness may be an important avenue for reducing risk of early death from cancers of the digestive system.
The present study also has limitations that should be carefully considered. The sample size of the ACLS cohort is modest relative to other cohorts designed to evaluate cancer outcomes, and accordingly, the number of deaths due to digestive cancers limited the precision with which we could estimate risk for site specific cancers. While we did observe statistically significant associations with a number of outcomes, caution should be employed when interpreting our results, particularly for our site-specific results. In addition, available sample size did not allow us to estimate weaker associations with much precision; therefore future studies are needed to evaluate the robustness of our finding of a 40 to 50% higher risk for pancreatic cancer among men with MetS. In terms of exposure assessment, we employed a clinical assessment of the MetS components to classify men at study enrollment, but in the present analysis we were unable to evaluate the impact of changes in these risk factors over time on our outcomes. It is possible that many men with the MetS were treated for the presence of a particular syndrome component (e.g., hypertension, high blood glucose) at some point in the follow-up interval. Additionally, others may have experienced increases in these components. Such misclassification of exposure would likely lead to and underestimate of the magnitude of the association observed in the present study. In terms of confounder adjustment, we were able to adjust for many important factors (e.g., age, smoking, alcohol intake), but we had little information on dietary behaviors, or use of non-steroidal anti-inflammatory drugs, factors which also could influence risk for our outcomes. Therefore, we cannot exclude the possibility that our lack of control for these covariates influenced our results. Finally, the ACLS cohort is predominantly white (>95%), well-educated, and from middle to upper socioeconomic status households in the United States. For this reason, our results may not be generalizable to other ethnic and socioeconomic groups with substantially different demographic characteristics. Additional studies are needed to confirm and expand on the associations we report herein and to better understand the relationship between exposure timing and these outcomes.
The strengths of this report should also be considered. First, while the relative socio-demographic homogeneity of our cohort may limit the broad generalizablity of our results, this characteristic of our cohort is likely to increase the internal validity of the present study. Men in this study elected to obtain a preventive medical examination at the Cooper Clinic, which we believe to be a good indicator of the members of this cohort’s access to health care. Thus, the potential for variation in access to cancer screening and treatment for cancer is likely small, and therefore is less likely to have produced bias in our results. Second, our study had an average of roughly 14 years of follow-up and exclusion of deaths from digestive cancers that occurred in the first five-years of follow-up did not substantively alter our primary results, indicating that the potential for preclinical disease processes to influence levels of our metabolic exposures is low. The long follow-up available in this study provides assurance that we have maintained a reasonable temporal sequence between our exposures and outcomes, and that the potential for preclinical disease processes to influence levels of our metabolic exposures is low. Finally, we had data on objectively measured cardiorespiratory fitness, and the addition of this information to our models substantially attenuated the association between MetS and gastrointestinal cancer mortality. Few other studies allow for adjustment of this exposure.
In conclusion, in this prospective study with an average of 14 years of follow-up we found that the MetS and its components were positively associated with mortality from cancers of the digestive system. The presence of the syndrome was also positively associated with colorectal, esophageal, and liver cancer. Risk appeared to be particularly increased for men that were older, heavier, smoked, or that had low fitness levels. These findings suggest that interventions which reduce abnormalities associated with the syndrome may represent an important opportunity for reducing mortality from cancers of the digestive system. Because the MetS is quite prevalent in the US population, particularly among adults over age 60, and because these cancers account for about 25% of all cancer deaths, such interventions may provide an important mechanism for reducing overall cancer mortality. It will be important in future studies to determine when, along the progression to death from these cancers that factors associated with MetS produce their effect, and the extent to which lifestyle and pharmacological treatment for these metabolic abnormalities can reduce risk.
Acknowledgments
Supported by National Institute of Health grants AG06945 and HL62508 and supported in part by an unrestricted research grant from The Coca-Cola company.
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, staff at the Cooper Institute for data entry and data management, and Gaye Christmus for editorial assistance.
Footnotes
Financial Disclosures: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures - 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2004. 2007. [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the Metabolic Syndrome Among US Adults: Findings From the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Lin SX, Pi-Sunyer EX, Lin SX, Pi-Sunyer EX. Prevalence of the metabolic syndrome among US middle-aged and older adults with and without diabetes--a preliminary analysis of the NHANES 1999-2002 data. Ethnicity & Disease. 2007;17:35–9. summary for patients in Ethn Dis. 2007 Winter;17(1):174; PMID: 17274231. [PubMed] [Google Scholar]
- 6.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA for Conference. Clinical Management of Metabolic Syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association Conference on Scientific Issues Related to Management. Circulation. 2004;109:551–6. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Lim YJ, Kim YH, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543–6. doi: 10.1158/1055-9965.EPI-07-0199. [DOI] [PubMed] [Google Scholar]
- 9.Sturmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev. 2006;15:2391–7. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 11.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–64. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 12.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal Cancer Mortality and Factors Related to the Insulin Resistance Syndrome. Cancer Epidemiology Biomarkers Prevention. 2002;11:385–91. [PubMed] [Google Scholar]
- 13.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of Insulin Resistance and Colorectal Cancer Mortality. Cancer Epidemiology Biomarkers Prevention. 2001;10:937–41. [PubMed] [Google Scholar]
- 14.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–66. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 15.Berrington de GA, Spencer EA, Bueno-De-Mesquita HB, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:879–85. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 17.Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561–7. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- 18.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 19.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–7. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 20.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164:1092–7. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 22.Malik S, Wong ND, Franklin SS, et al. Impact of the Metabolic Syndrome on Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The Metabolic Syndrome as Predictor of Type 2 Diabetes: The San Antonio Heart Study. Diabetes Care. 2003;26:3153–9. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 24.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 25.Blair SN, Kampert JB, Kohl HW, et al. Influence of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 26.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. United States Armed Forces Medical Journal. 1959;10:765–688. [PubMed] [Google Scholar]
- 27.Pollock M, Bohannon R, Cooper K, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 28.Kampert JB, Blair SN, Barlow CE, Kohl HW., 3 Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Annals of Epidemiology. 1996;6:452–7. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 29.Sui X, LaMonte MJ, Blair SN, Sui X, LaMonte MJ, BLAIR SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–23. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaMonte MJ, Barlow CE, Jurca R, et al. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 31.Sesso HD, Gaziano JM, Glynn RJ, et al. Value of an Endpoints Committee versus the use of nosologists for validating cause of death. Contemporary Clinical Trials. 2006;27:333–9. doi: 10.1016/j.cct.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RN, Minino AM, Hoyert DL, Rosenberg RM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2008;49:1–32. [PubMed] [Google Scholar]
- 33.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia, PA: Lippincott - Raven; 1998. [Google Scholar]
- 34.Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132(11 Suppl):3467S–70S. doi: 10.1093/jn/132.11.3467S. [DOI] [PubMed] [Google Scholar]
- 35.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–8. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 36.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–9. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 38.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 39.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Annals of Internal Medicine. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes & Control. 1996;7:253–63. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 42.Pischon T, Lahmann PH, Boeing H, et al. Body Size and Risk of Colon and Rectal Cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) JNCI Cancer Spectrum. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 43.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 44.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 45.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–54. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 46.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 47.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(3 Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 48.Blaha M, Elasy TA. Clinical Use of the Metabolic Syndrome: Why the Confusion? Clin Diabetes. 2006;24:125–31. [Google Scholar]
- 49.Kahn R, Buse J, Ferrannini E, Stern M. The Metabolic Syndrome: Time for a Critical Appraisal: Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 50.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low Levels of Leisure-Time Physical Activity and Cardiorespiratory Fitness Predict Development of the Metabolic Syndrome. Diabetes Care. 2002;25:1612–8. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 51.Church TS, Earnest CP, Skinner JS, BLAIR SN. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness Among Sedentary, Overweight or Obese Postmenopausal Women With Elevated Blood Pressure: A Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2007;297:2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 52.Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–39. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]


