Abstract
We performed a single-arm, open-label pilot trial of the anti-inflammatory drug pentoxifylline to reduce systemic inflammation and improve endothelial function, measured by flow-mediated dilation of the brachial artery, in HIV-infected patients not requiring antiretroviral therapy. Pentoxifylline significantly reduced circulating levels of vascular cell adhesion molecule-1 and interferon-gamma-induced protein and significantly improved endothelial function during the 8 week trial. Pentoxifylline may reverse HIV-related endothelial dysfunction by directly inhibiting the endothelial leukocyte adhesion pathway.
Keywords: HIV-1, Endothelium, Inflammation, Pentoxifylline
Treatment interruption studies [1] suggest that discontinuing combination antiretroviral therapy (cART) is associated with an increase in cardiovascular events in HIV-infected patients. In this population, higher levels of the inflammatory markers hsCRP and IL-6 are associated with a greater risk of cardiovascular disease [2]. These findings suggest that untreated HIV infection, perhaps through increased systemic inflammation, contributes to cardiovascular disease.
We previously demonstrated that the anti-inflammatory drug salsalate improved in vivo endothelial function in HIV-infected persons not receiving cART [3]. However salsalate usage in this study was associated with hepatotoxicity. Pentoxifylline (PTX), a phosphodiesterase inhibitor, has been shown to reduce inflammation [4]. On cellular levels, PTX blocks TNF-α production in monocytes by inhibiting nuclear factor-kappa B [5]. At doses of 1200 mg/day, PTX reduces TNF-α expression and serum triglycerides in patients with AIDS [6].
Therefore, we performed a prospective, open-label, single-arm pilot study of pentoxifylline 400mg orally thrice daily. Brachial artery flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NTGMD) were measured according to established guidelines [7] at baseline and after four and eight weeks of PTX. Additional plasma and serum samples for exploratory inflammatory, metabolic, and endothelial activation markers of interest were obtained after a 12 hour fast on the mornings of the study visits. PTX levels were measured using a custom in-house modified Liquid Chromatography-Mass Spectrometry/Mass Spectrometry assay. Changes in FMD and laboratories from baseline to weeks 4 or 8 were assessed using the Wilcoxon Signed-Rank test. All tests were 2-sided with p-values less than 0.05 considered significant.
All subjects were at least 18 years of age, had documented HIV infection with a CD4 cell count ≥350/μL within one month of screening, and had been free of antiretroviral treatment for at least six months prior to screening. Subjects were excluded for known vascular disease, diabetes, hypertension, pro-inflammatory conditions (besides hepatitis B or C co-infection), or history of malignancy; estimated creatinine clearance <50mL/min, hemoglobin <9.0g/dL, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >3 times upper limit normal, or total bilirubin >2.5 times upper limit normal at screening; fever or recent treatment of infection at screening or any main study visit; or receipt of other anti-inflammatory, investigational, or lipid-lowering drugs within 28 days prior to screening. This study was approved by the Clarian (Indiana University School of Medicine) Institutional Review Board. All participants provided written, informed consent.
Of the 9 subjects who enrolled in the trial [median age 40 (IQR, 23–57) years; 6 men; 3 non-Hispanic black; median CD4 cell count 552 (IQR, 356–645)/μL; median HIV-1 RNA 4.5 (IQR, 2.7–5.7) log10copies/mL; 4 active smokers; median body mass index 20.6 (IQR, 17.3–26.5) kg/m2; 0 with hepatitis B and 2 with hepatitis C co-infections], 1 subject was removed at week 2 for initiation of a steroid inhaler for bronchitis and an additional subject was removed at week 5 for initiation of cART. Mild diarrhea was experienced by six participants, but in none was this treatment limiting. No laboratory or clinical toxicities, including graded liver abnormalities, occurred during the study.
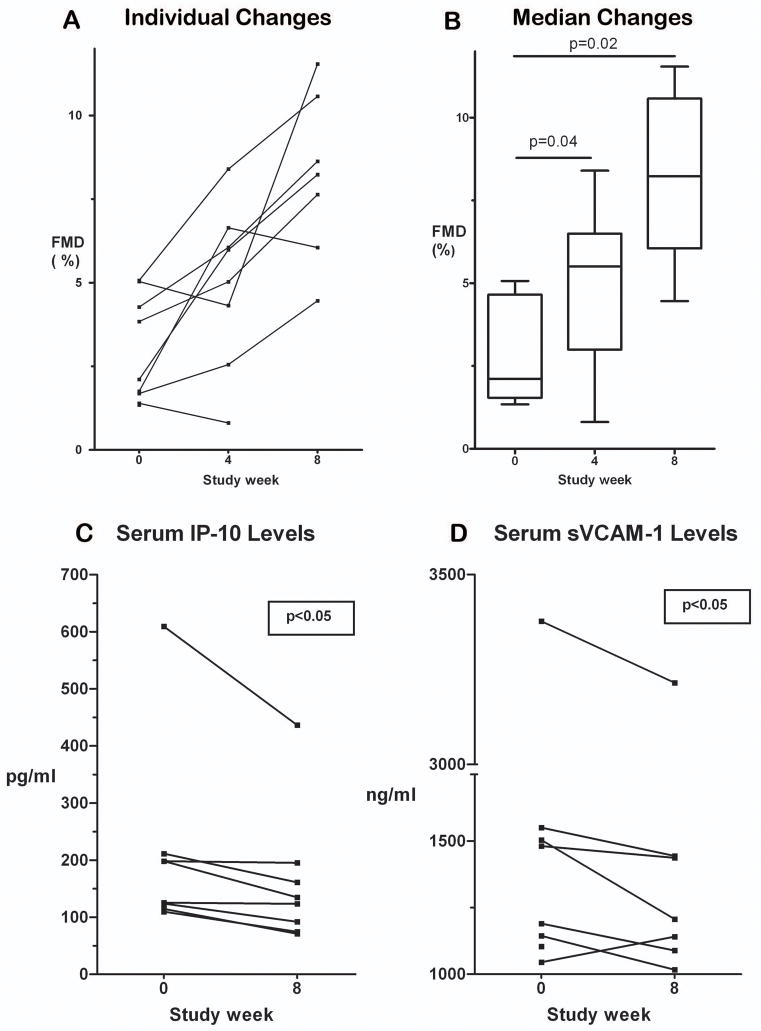
The individual changes in FMD with PTX in this trial are shown in Figure 1A. The median (range) baseline FMD was impaired at 2.1% (1.3%, 5.1%) when compared to values in healthy volunteers [8]. As shown in Figure 1B, FMD improved significantly from baseline after 4 weeks [N=8; absolute median (range) increase, 1.5% (−0.7%, 4.9%); p=0.04] and at 8 weeks [N=7; absolute median (range) increase, 4.4% (2.8%, 6.5%); p=0.02]. The median (range) baseline NTGMD was 20.8% (3.9%, 34.1%), which did not change during the trial.
Figure 1. Changes in flow-mediated dilation (FMD), interferon-gamma-induced protein (IP-10), and soluble vascular cell adhesion molecule-1 (sVCAM-1) with use of pentoxifylline (PTX) in HIV-infected subjects not requiring cART.
(A) FMD increased nearly uniformly from baseline in 7 of 8 subjects who completed the 8 week trial. (B) Changes in overall FMD levels as shown as boxplots (medians represented as horizontal lines within the boxes; bottoms and tops of boxes represent 25th and 75th percentiles, respectively; lower and upper horizontal lines outside of boxes represent minimum and maximum levels, respectively). Median absolute FMD significantly increased from baseline/week 0 (N=9; 2.1%) to study weeks 4 (N=8; 5.5%; p=0.04) and 8 (N=7; 8.2%; p=0.02). (C) IP-10 levels decreased significantly (P<0.05) over 8 weeks in the 7 subjects who completed the trial. (D) sVCAM-1 levels also decreased significantly (P<0.05) over 8 weeks in the 7 subjects who completed the trial.
Median plasma PTX concentrations at weeks 4 and 8 were 73 ng/mL (270 nM) and 97 ng/mL (340 nM), respectively. No concentration-response relationship between plasma PTX levels and FMD was observed. Blood pressure, lipid fractions, homeostatic model assessment-insulin resistance levels, and HIV-1 viral loads did not significantly change with PTX.
We then measured changes in the levels of circulating serum biomarkers [using a custom multiplex assay (SearchLight (Endogen) Human Analytes; Rockford, IL] reflecting endothelial activation [soluble vascular cell adhesion molecule-1 (VCAM-1), soluble intercellular adhesion molecule-1, E-selectin)] and inflammation [high sensitivity C-reactive protein (hsCRP), monocyte chemoattractant protein-1, soluble tumor necrosis factor receptor 1, soluble tumor necrosis factor receptor 2, macrophage inflammatory protein 1-beta, interferon-gamma-induced protein (IP-10), tumor necrosis factor-alpha (TNF-α)] in the seven subjects who completed the trial. As shown in Figure 1C and 1D, only circulating levels of IP-10 and sVCAM-1 decreased significantly (p<0.05 for both) with use of PTX. As expected, circulating TNF-α levels were below the limit of detection at entry and at trial completion in 6 of these 7 relatively healthy subjects with high CD4 counts.
Eight weeks of treatment with PTX safely and significantly improved endothelial function in HIV-infected patients not receiving cART. The absolute improvement of 4.4% in FMD that we observed has been associated with a reduced risk of future cardiovascular events in the general population [9, 10]. If a similar relationship holds for HIV-infected patients, then PTX could potentially reduce cardiovascular events in this high-risk population. Because of the lack of a control group, we cannot exclude the possibility of a ‘regression to the mean’ effect. However, the magnitudes of improvement in FMD were relatively uniform (Figure 1A), suggesting that the results were not dependent on the severity of initial endothelial dysfunction.
PTX significantly reduced levels of circulating sVCAM-1 and IP-10. These two molecules are produced by endothelial cells to promote emigration and adhesion of leukocytes as part of the normal vascular inflammatory process and are implicated in the development of atherosclerosis [11, 12]. We have previously demonstrated in an endothelial cell culture model that PTX, at concentrations similar to those achieved in our clinical trial, reduced TNF-α-, IFN-γ-, and HIV TAT-stimulated endothelial gene expression of VCAM-1 and IP-10 [13]. These in vitro results strongly corroborate our in vivo findings. However, we cannot formally exclude the possibility that these biomarker results were not due to effects of changes in alcohol usage on the liver since we did not assess for alcohol ingestion during this trial.
Randomized, placebo-controlled trials are now underway to investigate the effects of PTX on in vivo endothelial function both in HIV-infected patients not requiring cART (ClinicalTrials.gov identifier NCT00796822) as well as in those initiating cART (NCT00864916). If these trials confirm that PTX improves in vivo endothelial function, then PTX may provide an inexpensive and safe modality to reduce the risk of future cardiovascular events in the HIV-infected population.
Acknowledgments
Sources of Support: This research was supported by grants from the National Institutes of Health (K23 HL073682, R01 HL095149, R01 HL072711, M01 RR00750) and the Indiana University Research Support Funds Program.
We thank the study participants for their generosity and contributions to this study. We also thank Mr. Jeffrey Waltz, RDCS for performance of the brachial artery reactivity studies and also Dr. Chul Kim and Mr. Brian Taylor for assistance in preparation of the manuscript.
This study was designed and coordinated by S.K.G., R.M.J., K.J.M., M.C., J.R., and M.P.D. Analysis and interpretation of data were done by S.K.G., R.M.J., J.R., C.S., M.C., and M.P.D. Data were collected by S.K.G., R.M.J., and Z.D. The manuscript was drafted by S.K.G. with critical revision by R.M.J., J.R., M.C., and M.P.D.
Footnotes
Conflicts of Interest: No conflicts of interest reported for all authors.
Note: This work was presented at the 15th Conferences on Retroviruses and Opportunistic Infections (Boston, MA; 2008).
References
- 1.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 2.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta SK, Johnson RM, Saha C, Mather KJ, Greenwald ML, Waltz JS, et al. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. AIDS. 2008;22:653–655. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 4.Fossat C, Fabre D, Alimi Y, Bienvenu J, Aillaud MF, Lenoble M, et al. Leukocyte activation study during occlusive arterial disease of the lower limb: effect of pentoxifylline infusion. J Cardiovasc Pharmacol. 1995;25 (Suppl 2):S96–100. doi: 10.1097/00005344-199500252-00021. [DOI] [PubMed] [Google Scholar]
- 5.Heinkelein M, Schneider-Schaulies J, Walker BD, Jassoy C. Inhibition of cytotoxicity and cytokine release of CD8+ HIV-specific cytotoxic T lymphocytes by pentoxifylline. Journal of Acquired Immune Deficiency Syndromes & Human Retrovirology. 1995;10:417–424. doi: 10.1097/00042560-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dezube BJ, Lederman MM, Spritzler JG, Chapman B, Korvick JA, Flexner C, et al. High-dose pentoxifylline in patients with AIDS: inhibition of tumor necrosis factor production. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. J Infect Dis. 1995;171:1628–1632. doi: 10.1093/infdis/171.6.1628. [DOI] [PubMed] [Google Scholar]
- 7.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 9.Suessenbacher A, Frick M, Alber HF, Barbieri V, Pachinger O, Weidinger F. Association of improvement of brachial artery flow-mediated vasodilation with cardiovascular events. Vasc Med. 2006;11:239–244. doi: 10.1177/1358863x06075006. [DOI] [PubMed] [Google Scholar]
- 10.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 11.Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, et al. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Tempel D, van Haperen R, de Boer HC, Segers D, Huisman M, et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J Clin Invest. 2007;117:616–626. doi: 10.1172/JCI28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clauss M, Zhang Y, Rajashekhar G, Rehman J, Park I-W, He J, et al. The In Vitro Effects of HIV Proteins, Inflammatory Mediators, HIV-Infected T Cells, and Pentoxifylline on Endothelial Cell Inflammation. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); Montreal, Canada. February 8–11 2009. [Google Scholar]