Abstract
The current study examined the relationship between a prognostic indicator of vascular health, flow-mediated dilation (FMD), and working memory-related brain activation in healthy middle-aged adults. Forty-two participants underwent functional magnetic resonance imaging while completing a 2-Back working memory task. Brachial artery endothelial-dependent flow-mediated dilation (FMD) was assessed using B-mode ultrasound. The relationship between FMD and task-related brain activation in a priori regions of interest was modeled using hierarchical linear regression. Brachial FMD, was significantly related to reduced working memory-related activation in the right superior parietal lobule (β=0.338, p=0.027), independent of age, sex, systolic blood pressure, and full scale IQ (F(5,36)=2.66, p=0.038). These data provide preliminary support for the association between a preclinical marker of endothelial dysfunction and cerebral hemodynamic alterations in healthy middle-aged adults. Considering the modifiable nature of endothelial function, additional investigations on the prognostic significance of FMD on future cognitive impairment are warranted.
Keywords: working memory, magnetic resonance imaging, cardiovascular disease, endothelium, middle-aged, cognition
Introduction
Dementia currently affects over 24 million people and its prevalence is anticipated to double over the next twenty years (Qiu, De Ronchi, & Fratiglioni, 2007), making it one of the most significant and prevalent emerging public health issues. As successful treatments for dementia are lacking, identification and management of modifiable risk factors are essential. Recent reports suggest that vascular etiology contributes to over 50% of all dementia cases (Breteler, 2000). Atherosclerotic processes have been implicated in the pathogenesis of Alzheimer’s disease (Roher et al., 2003) and greater arterial stiffness has been recorded in individuals with dementia than in age-matched healthy controls (Hanon et al., 2005). Given that vascular risk factors for dementia are potentially modifiable, it is of utmost importance to find an efficacious method for identifying the early signs of vascular deterioration and their impact on cognition.
One promising approach has been to examine cardiac factors in relation to cognitive performance and brain structure integrity in non-demented older adults with cardiovascular disease (Gunstad et al., 2005; Haley et al., 2007a; Hoth et al., 2007; Moser et al., 2008; Paul et al., 2005). Using this approach, clinically significant levels of atherosclerosis and peripheral endothelial dysfunction have been associated with poorer attention-executive-psychomotor performance and greater white matter damage (Forman et al., 2008; Haley et al., 2007b; Hoth et al., 2007). While these studies have helped to elucidate the relationship between vascular health and brain function, their findings were limited to elderly adults already suffering from cardiovascular disease and exhibiting signs of vascular cognitive impairment. Considering that our best defense against dementia is prevention, it would be ideal to identify vascular markers that are associated with cerebral alterations in younger, healthier individuals.
The key challenge was to identify a vascular marker with prognostic significance for future vascular dysfunction. We chose to focus on endothelial function since it is considered an overall indicator of vascular health (Vita & Keaney Jr, 2002). Vascular endothelium plays an important role in the maintenance of vascular tone and prevention of atherosclerosis by inhibiting leukocyte and platelet adhesion to the arterial wall (Brunner et al., 2005). More importantly, endothelial dysfunction is evident before the development of clinically-defined atherosclerosis (Vita & Keaney Jr, 2002) and is considered a pathogenic factor in the development and progression of cardiovascular disease (Perticone et al., 2001).
The aim of the present study was to determine if initial signs of vascular dysfunction as indicated by poorer endothelial function are associated with the cerebral hemodynamic response during cognition among middle-aged adults free of cardiovascular disease. To accomplish our goal, we conducted functional magnetic resonance imaging (fMRI) and brachial artery flow mediated vasodilatation (FMD) measurements on cognitively normal middle-aged adults. FMRI is unique in its sensitivity to detect early alterations in brain function in individuals at risk for cognitive impairment (Bondi, Houston, Eyler, & Brown, 2005; Bookheimer et al., 2000; Haley et al., 2008) and FMD provides an overall indicator of endothelial function throughout the vasculature (Vita & Keaney Jr, 2002). Based on previous research on elderly subjects with cardiovascular disease (Irani et al., 2009; Haley et al., 2007b), we hypothesized that decreased endothelial function would be associated with lower functional response to working memory and that lower activations would be related to lower task performance.
Methods
Subjects
Right-handed adults between the ages of 40 and 60 were recruited through flyers and newspaper advertisements posted in Austin, Texas. Handedness was assessed using the Edinburgh Handedness Questionnaire (Oldfield, 1971). Individuals with a history of coronary artery disease, angina pectoris, myocardial infarctions, heart failure, and cardiac surgery were excluded. Additional exclusion criteria included history of neurological disease (e.g., stroke, Parkinson’s disease, clinically significant traumatic brain injury), major psychiatric illness (e.g. schizophrenia, bipolar disorder), substance abuse (i.e., diagnosed abuse and/or previous hospitalization for substance abuse), metabolic disorder (i.e., diabetes, thyroid disorder), smoking (within the last two years) or MRI contraindications. The study was in accordance with the Second Declaration of Helsinki and was approved by the local institutional review committee. Forty-two adults participated in the study, providing written informed consent before enrollment. Participants characteristics are presented in Table 1.
Table I.
Participant characteristics (n=42)
| Participant Characteristics |
Mean±SD |
|---|---|
| Age, y | 49.0±6.3 |
| Sex (male/female) | 16/26 |
| Education, y | 15.4±2.7 |
| Race, n, (%) | |
| Non-Hispanic White | 21 (50.0%) |
| Hispanic | 14 (33.3%) |
| Asian American | 1 (2.4%) |
| African American | 5 (11.9%) |
| Native American | 1 (2.4%) |
| Body mass index, kg/m2 | 28.5±5.3 |
|
Systolic blood pressure, mmHg |
124±15 |
|
Diastolic blood pressure, mmHg |
75±8 |
|
Flow mediated dilation, % |
4.2±4.8 |
Neuropsychological Assessment
All participants completed standard clinical neuropsychological instruments with established reliability and validity (Lezak, Howieson, Loring, Hannay, & Fischer, 2004). These measures assessed global cognitive functioning (Mini Mental Status Exam, MMSE (M. F. Folstein, S. E. Folstein, & P. R McHugh, 1975), full scale IQ (Wechsler Abbreviated Scale of Intelligence – Two Subtest, WASI (Wechsler, 1999), language (WASI Vocabulary Subtest; Category Fluency for Animals (Morris et al., 1989)) memory (California Verbal and Learning Test II, CVLT-II (Delis, Kramer, Kaplan, & Ober, 1987); Rey Complex Figure Test, RCF (J. E. Meyers & K. R. Meyers, 1995)), attention-executive functioning (Controlled Oral Word Association Test, COWAT (Ruff, Light, Parker, & Levin, 1996); Trail Making Test A & B (Reitan, 1958), Wechsler Adult Intelligence Scale-III, WAIS-III, Digit Span Subtest (Wechsler, 1997)), psychomotor speed (Grooved Pegboard (Ruff & Parker, 1993)) visual-spatial ability (RCF copy; WASI Matrix Reasoning Subtest) and emotional functioning (Beck Depression Inventory-II, BDI-II (Beck, Steer, & Brown, 1996); State Trait Anxiety Inventory, STAI (Spielberger & Gorsuch, 1970)). Tests were administered and scored by a trained research assistant using standard administration and scoring criteria.
Cardiovascular Assessment
Participants abstained from caffeine and fasted for at least four hours prior to the assessment. After fifteen minutes of rest in a supine position, brachial blood pressure was measured using a semi-automated device (Dinamap XL, Johnson & Johnson Medical Inc, Tampa, FL) Three separate recordings were made while participants sat upright in accordance with American Heart Association Guidelines (Perloff et al., 1993).
Endothelial function was assessed with brachial artery flow-mediated dilation. A B-mode Doppler ultrasound machine (iE 33 Ultrasound System, Philips, Bothell, WA) with a customized transducer holding device was used to measure brachial artery diameters and blood flow velocity. Brachial artery images were obtained in a longitudinal orientation located 5–10 cm proximal to the antecubital fossa. Baseline blood flow and diameter measurements were made after participants rested for at least fifteen minutes in the supine position. After the acquisition of baseline measurements, a blood pressure cuff placed on the ipsilateral forearm distal to the elbow was inflated to 100 mmHg above baseline systolic blood pressure for 5 minutes using a rapid cuff inflator (E20, Hokanson, Bellevue,WA). Ultrasound-derived blood velocity and diameter data were saved as DICOM format and transferred to a computer using a digital image viewing software (Access Point 2004, Freeland Systems; Westminster, CO) for later analyses. All ultrasound brachial images were subsequently analyzed by the same investigator using image analysis software (Vascular Research Tool Brachial Analyzer, Medical Imaging Applications, Coralville, IA).
FMD was expressed as the percent change in brachial artery diameters recorded during the pre and post occlusion phases and was calculated using the equation: (maximum diameter – baseline diameter)/baseline diameter × 100. The average of 10 end-diastolic brachial artery diameters before blood flow occlusion was used for baseline diameters and the average of 3 peak end-diastolic diameters during the reperfusion phase was used for maximum brachial artery diameter.
Working Memory Task Paradigm
Working memory was assessed using a verbal n-Back task (Braver et al., 1997; J. D. Cohen et al., 1997; Walter et al., 2003), consisting of alternating blocks of 0-Back, 2-Back, and rest conditions. During each 0- and 2-Back block, a series of twelve individual consonants were visually presented in random order for 500 ms each with a 2,500 ms inter-stimulus interval. Participants responded to target letters (33% in each block) using a two-button MR-compatible response box. In the 0-Back condition, the target was a prespecified letter (H) and in the 2-Back condition, the target was any letter that was identical to the one presented two stimuli earlier. Task performance was assessed by measuring mean accuracy rates and reaction time for all correct trials. During each rest block, a fixation cross appeared in the middle of the screen for 30 seconds. The task was programmed and presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA). During neuroimaging, two consecutive 6 minute runs consisting of three blocks of alternating 0-Back, 2-Back, and rest conditions were presented. Before neuroimaging, participants were given an opportunity to practice the task on laptop computer to ensure adequate performance.
Neuroimaging
MRI data for each participant were acquired in a single session on a 3T GE Signa Excite MRI scanner equipped with a standard head coil. T1-weighted anatomical scans of the entire brain in the saggital plane were collected using a high-resolution Spoiled Gradient Echo (SPGR) sequence (256 × 256 matrix, FOV = 24 cm2, 1 mm slice thickness, 0 gap). Functional imaging was preformed while participants completed the 2-Back task. The task was back-projected from a laptop onto a screen positioned at the participant’s head, and viewed through a double-mirror attached to the head coil. Functional imaging was performed using a whole brain echo-planer imaging (EPI) sequence (TR = 3000 ms, TE = 30 ms, FOV = 24 cm2, 64 × 64 matrix, 42 axial slices, 3 mm slice thickness, 0.3 mm gap).
All EPI images were processed using Analysis of NeuroImages (AFNI) software (Cox, 1996). Each time series was spatially registered to the sixth volume of the session to reduce the effects of head movement. Data pre-processing also included adjustment for differences in adjacent slice timing due to interleaved slice acquisition, temporal smoothing, spatial filtering, and transformation to standard stereotaxic space. Task-related brain activation was determined using voxel-wise multiple regression analyses with the following parameters: a 0-Back/2-Back reference waveform convolved with a gamma function and covariates accounting for instruction screens and head movement.
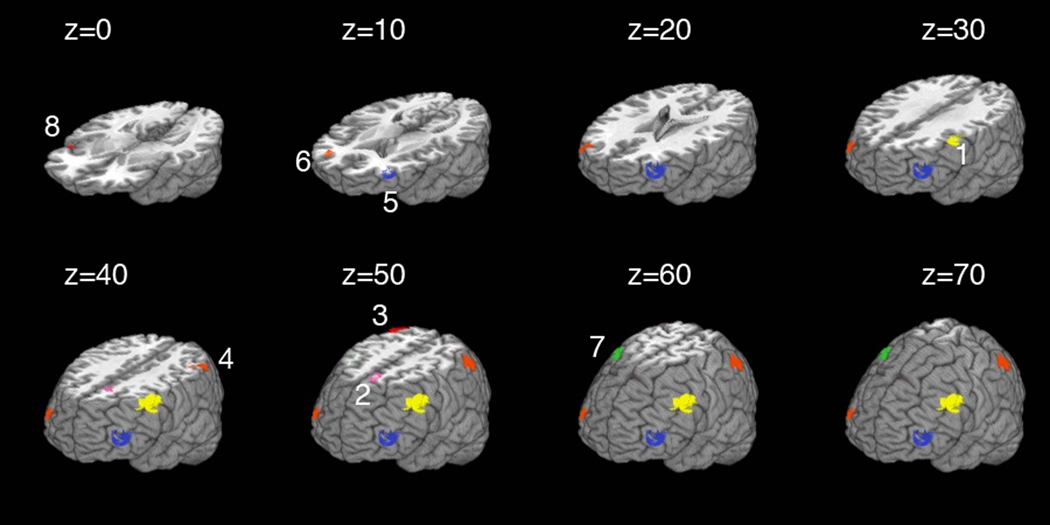
In order to elaborate upon prior research examining cerebral hemodynamics and cardiovascular function, a priori regions of interest (ROIs) were created from published coordinates that were empirically derived from a verbal 2-Back task in individuals with cardiovascular disease (Haley et al., 2007b) (Table 2, Figure 1). All sterotactic coordinates refer to Talairach space (Talairach & Tournoux, 1988). The ROIs were applied to individual data to determine mean task-related activation intensity in each region.
Table II.
A priori regions of interest
| Region | X | Y | Z |
|---|---|---|---|
| Left middle frontal gyrus | 33 | 4 | 56 |
| Left medial frontal gyrus | 5 | 19 | 44 |
| Right superior parietal lobule |
37 | −63 | 53 |
| Left inferior parietal lobule | 49 | −52 | 44 |
| Left middle frontal gyrus | 44 | 45 | 13 |
| Right superior frontal gyrus |
33 | 48 | 15 |
| Right middle frontal gyrus | 32 | 5 | 55 |
| Right inferior frontal gyrus | 47 | 14 | 3 |
Figure 1.
Statistical Analyses
Variable distributions were examined using the Shapiro-Wilk test of normality. A log transformation was used to obtain a normal distribution of the 2-Back-related activation in the right superior frontal gyrus (Shapiro-Wilk = 0.943, p = 0.035). All other outcome distributions fulfilled the assumption of normality.
The association between FMD and mean 2-Back-related activation intensity was analyzed within each a priori ROI using hierarchical linear regression. In the first block, the model was adjusted for age, sex, systolic blood pressure, and full scale IQ. FMD was entered into the second block in order to estimate its independent contribution to the variance in 2-Back-related activation. The decision to adjust the model for full scale IQ was based on research demonstrating a linear relationship between global intellectual ability and domain specific cognitive test performance. Adults with average intellectual ability tend to perform worse on neuropsychological tests than adults with above average and superior intellectual abilities, even in the absence of neurological disease (Bell & Roper, 1998; Horton, 1999; Tremont, Hoffman, Scott, & Adams, 1998). Therefore, in any search for markers of cognitive vulnerability, it is important to avoid falsely identifying adults with lower intellectual ability as showing signs of neuropsychological impairment.
In exploratory follow-up analyses, mean 2-Back accuracy and reaction time were correlated with 2-Back activation in the single ROI that was significant in the regression model. Data were analyzed using SPSS 16.0 computer software (SPSS Inc., Chicago, IL). A two-tailed alpha level of 0.05 was used as the criterion for significance for all analyses.
Results
Descriptive statistics on the results from the neuropsychological measures are presented in Table 3. Mean (±SD) accuracy on the verbal 2-Back task was 76.8±12.8% correct responses, and mean reaction time was 1185±287 ms. All participants moved less than 1.5 mm per imaging run.
Table III.
Neuropsychological test results
| Test Measures | Mean±SD |
|---|---|
| Global cognition | |
| Mini Mental Status Exam (MMSE) | 28.1±1.5 |
| Full scale IQ | |
| Weschler Abbreviated Scale of Intelligence (WASI) | 112.3±16.1 |
| Language | |
| WASI Vocabulary (scaled) | 55.0±9.2 |
| Category Fluency for Animals | 23.7±5.6 |
| Memory | |
| California Verbal Learning Testing II (CVLT-II) | |
| Immediate Recall | 10.3±3.0 |
| Delayed Recall | 11.0±3.1 |
| Recognition (Yes/No) | 3.1±0.7 |
| Rey Complex Figure Test, RCF | |
| Immediate Recall | 15.2±5.6 |
| Delayed Recall | 14.6±5.5 |
| Recognition | 19.3±2.4 |
| Attention-executive function | |
| Controlled Oral Word Association Test (COWAT) | 37.6±10.8 |
| Trail Making Test A, sec | 30.4±9.3 |
| Trail Making Test B, sec | 74.5±27.5 |
| Wechsler Adult Intelligence Scale-III (WAIS-III) | 16.5±3.9 |
| Digit Span | |
| Visual-Spatial | |
| RCF Copy | 30.6±4.2 |
| WASI Matrix Reasoning (scaled) | 57.4±7.0 |
| Psychomotor Speed | |
| Grooved Peg Board, Dominant Hand, sec | 76.7±18.4 |
| Depression | |
| Beck Depression Inventory-II, BDI-II | 5.9±5.3 |
| Anxiety | |
| STAI-Trait (scaled) | 49.8±12.6 |
The fully adjusted regression models successfully predicted the mean 2-Back-related activation intensity in the right superior parietal lobule (F(5,36)=2.66, p=0.038) and in the left inferior parietal lobule (F(5,36)=3.46, p=0.012). Male gender was significantly associated lower 2-Back-related intensity in right superior parietal lobule (β=−0.346, p=0.024) and in the left inferior parietal lobule (β=−0.431, p=0.004). The independent effects of age, systolic blood pressure, and full scale IQ did not account for any unique variance in 2-Back-related activation intensity.
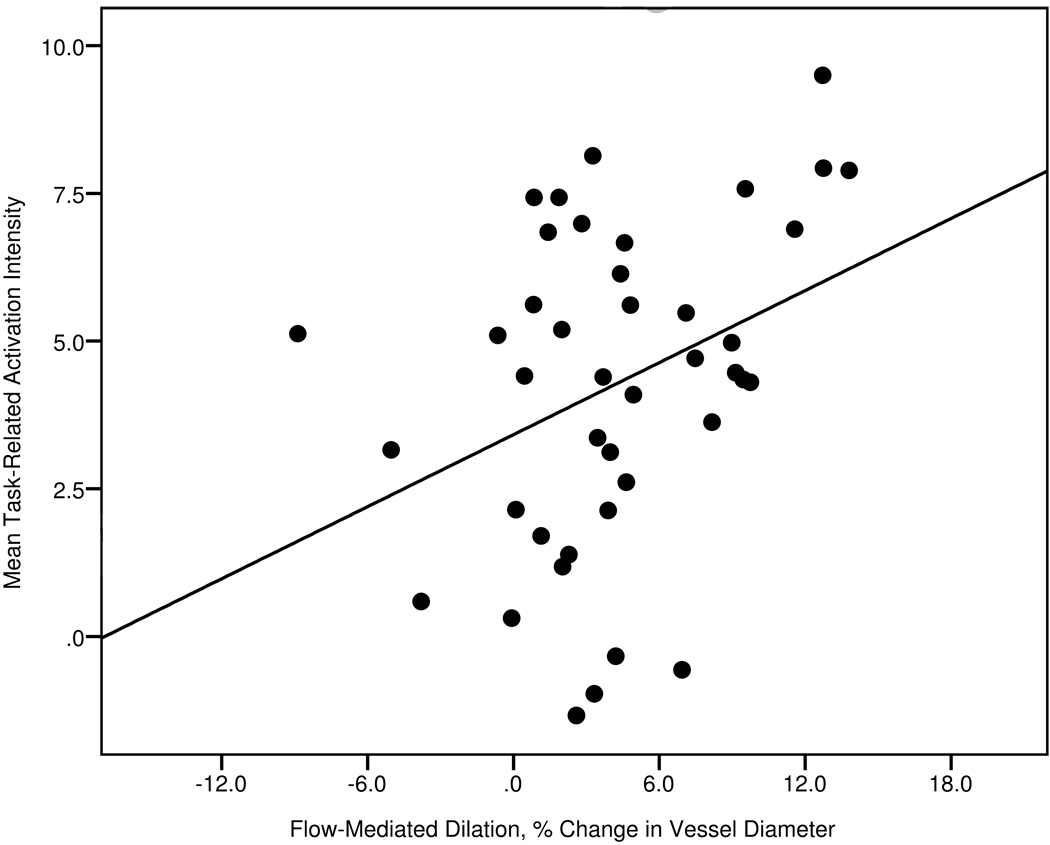
Decreased endothelial function, as measured by brachial FMD, was significantly associated with attenuated 2-Back-related activation in the right superior parietal lobule (β=0.338, p=0.027), independent of age, sex, systolic blood pressure, and full scale IQ (Figure 2). 2-Back-related activation in the same region was associated with slower reaction time (r=− 0.347, p=0.026), but not accuracy (r=0.183, p=0.252). The independent effects of FMD did not reach statistical significance in the left inferior parietal lobule (β=0.273, p=0.061).
Figure 2.
Scatterplot of Flow-Mediated Dilation and Mean Task-Related Activation Intensity in the Right Superior Parietal Lobule
Discussion
The current study investigated the relationship between endothelial function and brain activation during a verbal working memory task in healthy, middle-aged adults. We found that a sensitive measure of vascular health, FMD, was associated with reduced brain activation in the superior parietal lobule. The relationship between FMD and brain activation in this region was independent of the effects of age, sex, systolic blood pressure, and IQ. Since FMD contributes to the pathogenesis of cardiovascular disease (Perticone et al., 2001), alterations in this measure appear before clinical diagnosis (Vita & Keaney Jr, 2002). FMD’s prognostic significance, combined with the sensitivity of fMRI, may have enabled us to detect very early changes in the hemodynamic response to cognition in healthy, cognitively intact middle-aged adults.
Superior parietal activation is a common finding across working memory studies (Braver et al., 1997; J. D. Cohen et al., 1997; Walter et al., 2003). This region is considered critical for sensorimotor integration (Wolpert, Goodbody, & Husain, 1998) and responds to higher cognitive demands by tonically increasing activation in order to maintain higher levels of response readiness (Braver et al., 1997). Our findings are consistent with this interpretation as lower superior parietal lobule activation in our sample was associated with slower 2-Back reaction times. These results are of particular interest as psychomotor slowing is among the first signs of vascular cognitive decline (Looi & Sachdev, 2000). Our findings indicate that the relationship between FMD and brain activation may be sensitive enough to detect early vascular-related changes in brain function before cognition and cardiovascular function become substantially impaired. Longitudinal studies will be necessary to determine the predictive value of these findings for estimating future cognitive trajectories.
Contemplating the physiological mechanisms supporting the detected relationship between peripheral FMD and cerebrovascular response to cognition is also very interesting. Nitric oxide, which regulates endothelial dilation in FMD, is known to play an important role in the regulation of cerebral circulation (White, Vallance, & Markus, 2000). In humans, inhibition of nitric oxide release has been associated with reduced basal cerebral blood flow (White, Deane, Vallance, & Markus, 1998). These findings may bear importance on cognition since cognitive engagement enhances the demand for oxygen, thus increasing blood flow delivery to activated brain regions (Logothetis & Wandell, 2004). With severe dysfunction in the regulation of cerebral blood flow, activated brain areas may not receive adequate blood supply, inducing structural damage and impairments in cognition (Hoth et al., 2007; Rockwood, 2002). Consistent with this idea, patients with vascular dementia display attenuated cerebral blood flow at rest, suggesting hypoperfusion may play a role in the etiology of the disease (Yang et al., 2002). Additionally, brachial FMD has been associated with the severity of white matter hyperintensities in the brain in elderly cardiovascular disease patients (Hoth et al., 2007), indicating a relationship between peripheral and cerebral circulation. Future studies will be necessary to determine if brachial FMD is indeed directly related to autoregulation in the cerebral vasculature. In this context, preliminary evidence is encouraging. Prior research has found that degree and timing of blood flow autoregulation in the middle cerebral artery is closely associated with the flow through peripheral arteries such as the carotid artery (White et al., 2000).
Alternatively, reduction in task-related brain activation at normal behavioral performance levels could be an indicator of neurovascular decoupling. The blood-oxygen-level-dependent (BOLD) signal in fMRI is generated by changes in the ratio between oxygenated and deoxygenated hemoglobin in the surrounding microvasculature (Logothetis & Wandell, 2004). Engagement in cognitive tasks alters the BOLD response by influencing cerebral blood flow, cerebral blood volume, and cerebral blood oxygen consumption (D'Esposito, Deouell, & Gazzaley, 2003). In healthy individuals, the change in the BOLD response closely parallels neural activity. However, in situations where the cerebrovasculature is altered, the BOLD response may not be a reliable indicator of neural activation (D'Esposito, Deouell, & Gazzaley, 2003). Neurovascular decoupling is unlikely to be the cause for our findings, because the study population consisted of healthy, middle aged adults. Evidence for decoupling has mostly been documented in cases of severe vascular pathology such as ischemic stroke and intracranial stenosis (Girouard & Iadecola, 2006). Nonetheless, assessment of neurovascular decoupling should be addressed in future studies.
In conclusion, the current study demonstrated that poorer peripheral endothelial function was related to reduced task-related superior parietal activation independent of the effects of age, sex, systolic blood pressure, and full scale IQ. Prior research has demonstrated a relationship between vascular dysfunction and brain activation in elderly adults with cardiovascular disease (Haley et al., 2007b; Irani et al., 2009). The current findings extend the literature, by identifying a sensitive vascular factor, FMD, which was related to cerebrovascular response to cognition in a healthy, middle-aged sample. Due to its early role in the pathogenesis and progression of vascular disease (Perticone et al., 2001), FMD may be uniquely suited for identifying cognitive vulnerability at younger ages. The ability to identify individuals at risk for vascular cognitive impairment among healthy, middle-aged adults would be of paramount importance for preventive efforts, especially given the modifiable nature of endothelial function (Vita & Keaney Jr, 2002). Limitations of the current study include a relatively small sample size and a cross-sectional design. Future longitudinal research is necessary to determine the predictive validity of these findings for estimating individual cognitive trajectories. Studies of the relationship between FMD and other indicators of cerebral function such as white matter integrity, cerebral autoregularion, and cerebral neurochemistry in midlife would also help assess the value of FMD as a prognostic indicator of cognitive vulnerability. Finally, the efficacy of midlife interventions aimed at improving vascular health to delay or prevent future cognitive decline should be explored.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bell BD, Roper BL. Myths of Neuropsychology: Another View. The Clinical Neuropsychologist. 1998 Dec;:237–244. [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. The New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Breteler M. Vascular risk factors for Alzheimer's disease: An epidemiologic perspective. Neurobiology of Aging. 2000;21:153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of Hypertension. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test: Adult version. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman DE, Cohen RA, Hoth KF, Haley AP, Poppas A, Moser DJ, Gunstad J, et al. Vascular health and cognitive function in older adults with cardiovascular disease. Artery Research. 2008;2:35–43. doi: 10.1016/j.artres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. Journal of Applied Physiology. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Cohen RA, Tate DF, Paul RH, Poppas A, Hoth K, Macgregor KL, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Pressure. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley AP, Forman DE, Poppas A, Hoth KF, Gunstad J, Jefferson AL, Paul RH, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. International Journal of Cardiology. 2007a;121:148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley AP, Gunstad J, Cohen RA, Jerskey BA, Mulligan RC, Sweet LH. Neural Correlates of Visuospatial Working Memory in Healthy Young Adults at Risk for Hypertension. Brain Imaging and Behavior. 2008;2:192–199. [Google Scholar]
- Haley AP, Sweet LH, Gunstad J, Forman DE, Poppas A, Paul RH, Tate DF, et al. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. Journal of Neuroimaging. 2007b;17:227–233. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- Horton AM. Above-average intelligence and neuropsychological test score performance. International Journal of Neuroscience. 1999;99:221–231. doi: 10.3109/00207459908994326. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, Paul RH, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani F, Sweet LH, Haley AP, Gunstad JJ, Jerskey BA, Mulligan RC, Jefferson AL, et al. A fMRI Study of Verbal Working Memory, Cardiac Output, and Ejection Fraction in Elderly Patients with Cardiovascular Disease. Brain Imaging and Behavior. 2009;3:350–357. doi: 10.1007/s11682-009-9077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neuropsychological assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Reviews of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Looi JC, Sachdev PS. Vascular dementia as a frontal subcortical system dysfunction. Psychological Medicine. 2000;30:997–1003. doi: 10.1017/s003329179900269x. [DOI] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. Rey complex figure test and recognition trial: Professional manual. Odessa, Fl: Psychological Assessment Resources; 1995. [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, Van Belle G, Fillenbaum G, Mellits ED, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Moser DJ, Miller IN, Hoth KF, Correia M, Arndt S, Haynes WG. Vascular smooth muscle function is associated with initiation and processing speed in patients with atherosclerotic vascular disease. Journal of the International Neuropsychological Society. 2008;14:535–541. doi: 10.1017/S1355617708080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paul RH, Gunstad J, Poppas A, Tate DF, Foreman D, Brickman AM, Jefferson AL, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovascular Disease. 2005;20:129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Current Opinion in Psychiatry. 2007;20:380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rockwood K. Vascular cognitive impairment and vascular dementia. Journal of the Neurological Sciences. 2002;203:23–27. doi: 10.1016/s0022-510x(02)00255-1. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the Finger Tapping and Grooved Pegboard tests. Perceptual and Motor Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: Reliability and updated norms. Archives of Clinical Neuropsychology. 1996;11:329–338. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL. STAI manual for the State-trait anxiety inventory (" self-evaluation questionnaire") Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tremont G, Hoffman RG, Scott JG, Adams RL. Effect of intellectual level on neuropsychological test performance: A response to Dodrill. The Clinical Neuropsychologist. 1998;12:560–567. [Google Scholar]
- Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Walter H, Bretschneider V, Grön G, Zurowski B, Wunderlich AP, Tomczak R, Spitzer M. Special issue evidence for quantitative domain dominance for verbal and spatial working memory in the frontal and parietal cortex. Cortex. 2003;39:897–911. doi: 10.1016/s0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and scoring manual for the Wechsler Adult Intelligence Scale. Third edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- White RP, Deane C, Vallance P, Markus HS. Nitric oxide synthase inhibition in humans reduces cerebral blood flow but not the hyperemic response to hypercapnia. Stroke. 1998;29:467–472. doi: 10.1161/01.str.29.2.467. [DOI] [PubMed] [Google Scholar]
- White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clinical Science. 2000;99:555–560. [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nature Neuroscience. 1998;1:529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Yang DW, Kim BS, Park JK, Kim SY, Kim EN, Sohn HS. Analysis of cerebral blood flow of subcortical vascular dementia with single photon emission computed tomography:: Adaptation of statistical parametric mapping. Journal of the Neurological Sciences. 2002;203:199–205. doi: 10.1016/s0022-510x(02)00291-5. [DOI] [PubMed] [Google Scholar]